Abstract
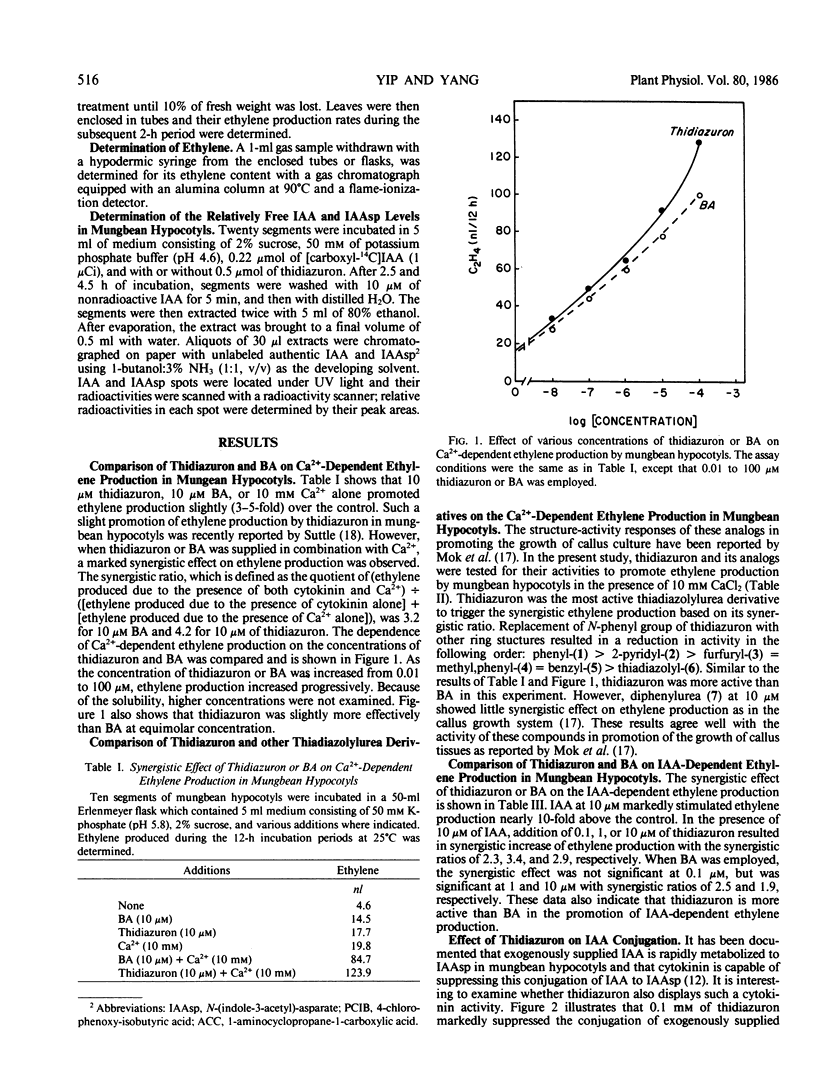
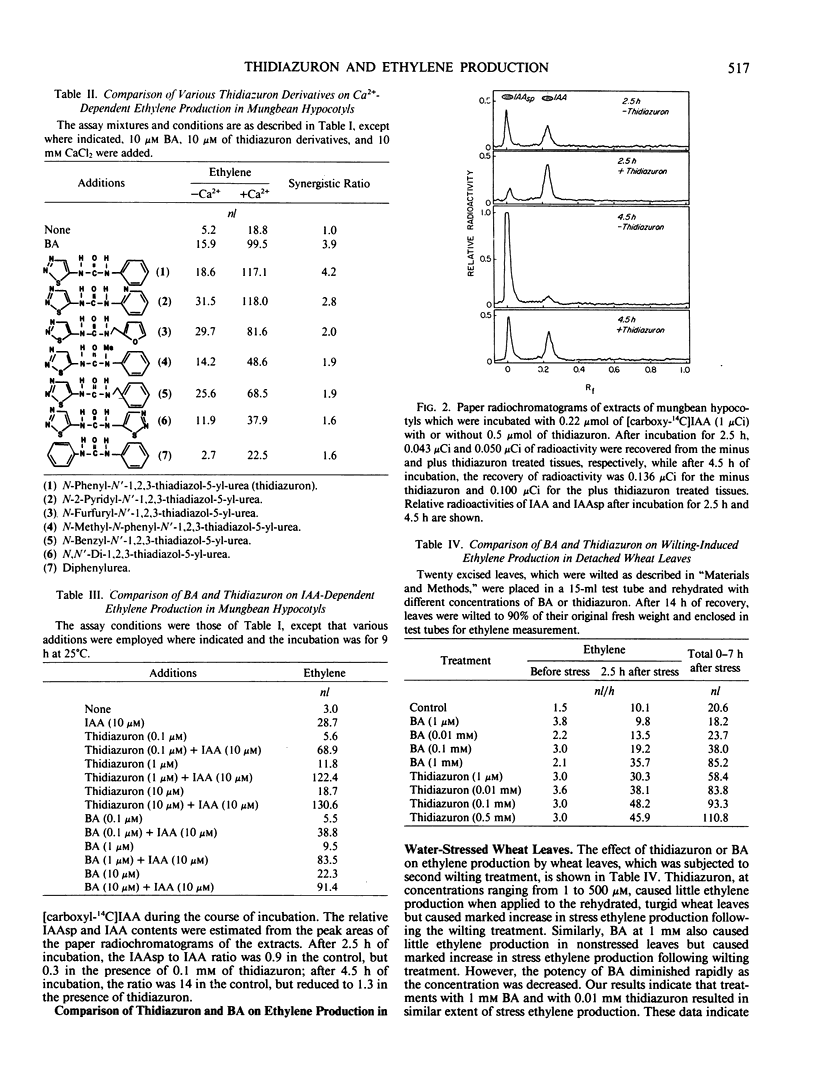
Cytokinins are known to stimulate ethylene production in mungbean hypocotyls synergistically with indoleacetic acid (IAA), in mungbean hypocotyls synergistically with Ca2+, and in wilted wheat leaves. Thidiazuron, a substituted urea compound, mimicked the effect of benzyladenine (BA) in all three systems. In the Ca2+ + cytokinin system and the IAA + cytokinin systems of mungbean hypocotyls, thiadiazuron was slightly more active than BA at equimolar concentration. In mungbean hypocotyls exogenously applied IAA was rapidly conjugated into IAA asparate, and this conjugation process was effectively inhibited by thidiazuron, as by cytokinins. In the wilted wheat leaves system, 10 micromolar thidiazuron exerted stress ethylene production equal to that exerted by 1 millimolar BA, indicating that thidiazuron is more active than BA by two orders. The structure-activity relationship of thidiazuron and its thiadiazolylurea analogs in stimulating Ca2+-dependent ethylene production in mungbean hypocotyls was found to agree well with the structure-activity relationship of these derivatives in promoting the growth of callus tissues. These results indicate that thidiazuron and its derivatives are highly active to mimic the adenine-type cytokinin responses in promoting ethylene production and that the structure-activity relationship in promoting the growth of callus and in promoting ethylene production is similar.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apelbaum A., Yang S. F. Biosynthesis of stress ethylene induced by water deficit. Plant Physiol. 1981 Sep;68(3):594–596. doi: 10.1104/pp.68.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelle S. C., Mok D. W., Kirchner S. C., Mok M. C. Effects of Thidiazuron on Cytokinin Autonomy and the Metabolism of N-(Delta-Isopentenyl)[8-C]Adenosine in Callus Tissues of Phaseolus lunatus L. Plant Physiol. 1983 Nov;73(3):796–802. doi: 10.1104/pp.73.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Erion J. L. A cytokinin binding protein from higher plant ribosomes. Biochem Biophys Res Commun. 1975 May 19;64(2):694–700. doi: 10.1016/0006-291x(75)90376-9. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Interaction of kinetin and calcium in relation to their effect on stimulation of ethylene production. Plant Physiol. 1975 Apr;55(4):738–740. doi: 10.1104/pp.55.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Mechanism of a Synergistic Effect of Kinetin on Auxin-induced Ethylene Production: Suppression of Auxin Conjugation. Plant Physiol. 1973 Jun;51(6):1011–1014. doi: 10.1104/pp.51.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle J. C. Effect of the defoliant thidiazuron on ethylene evolution from mung bean hypocotyl segments. Plant Physiol. 1984 Aug;75(4):902–907. doi: 10.1104/pp.75.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Auxin-induced Ethylene Production and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion. Plant Physiol. 1979 Dec;64(6):1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]


