Abstract
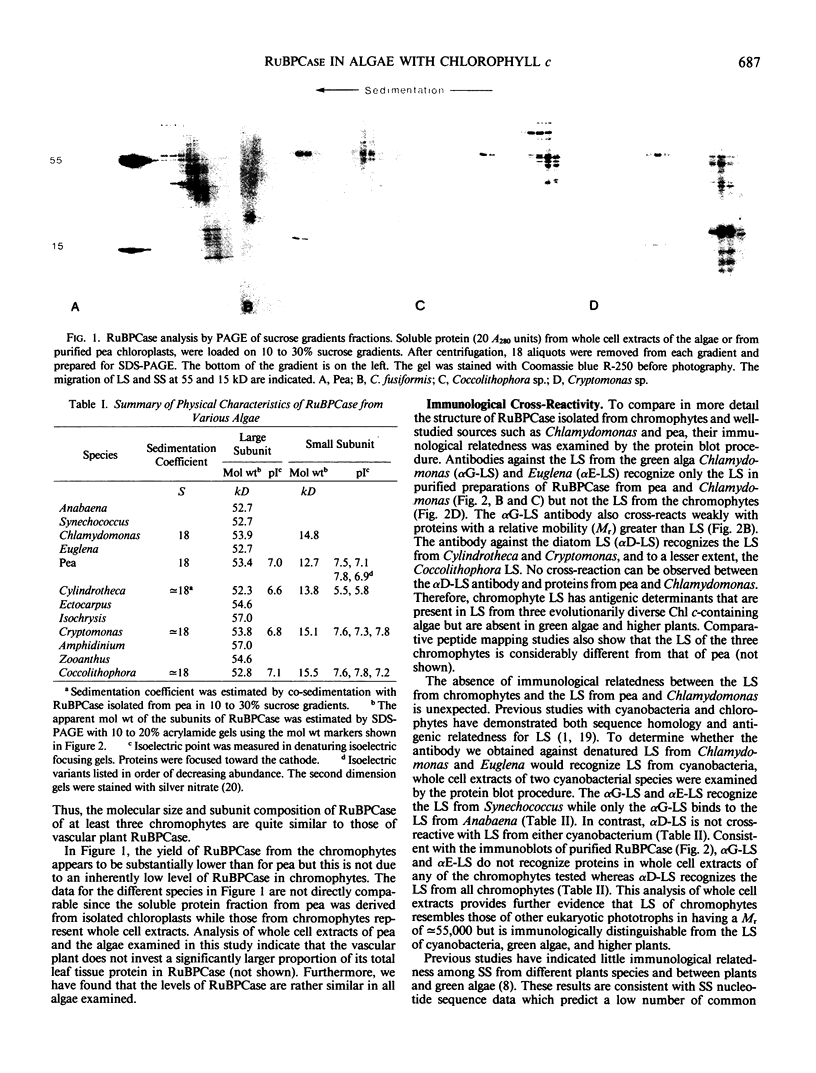
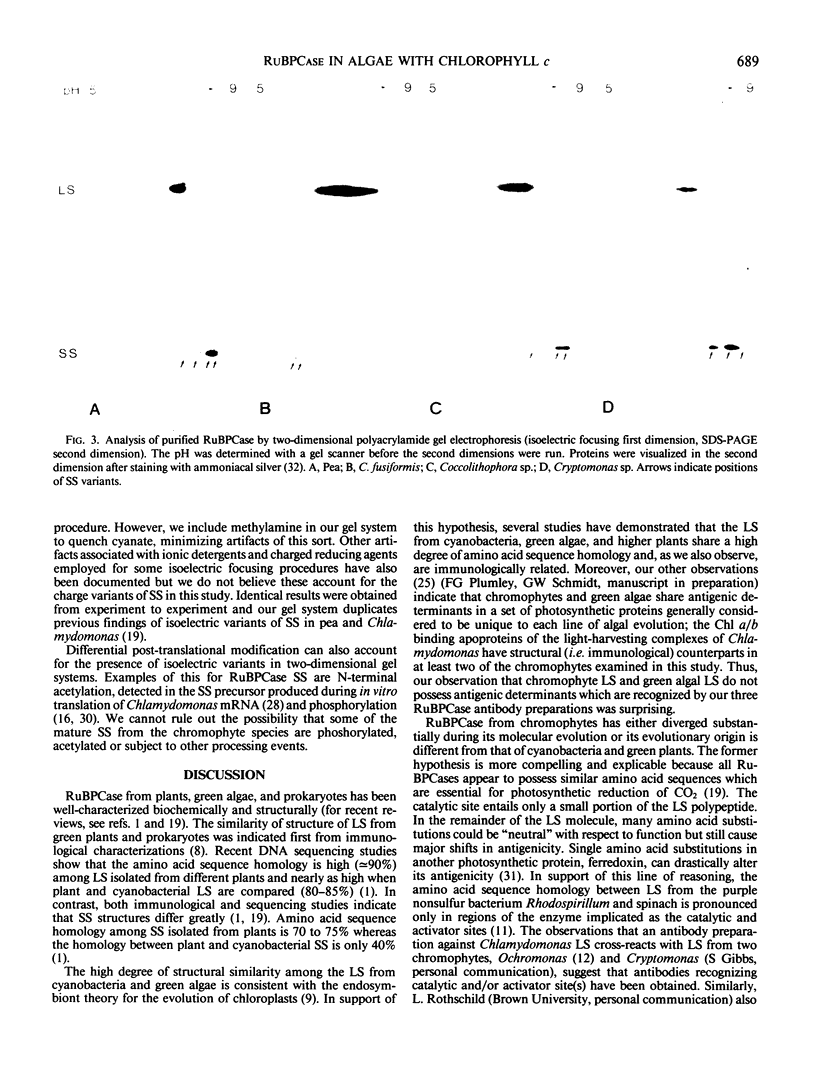
Distinctive properties are identified in the molecular structure of ribulose, 1,5-bisphosphate carboxylase/oxygenase (RuBPCase) in chlorophyll c-containing algae (i.e., chromophytes). Using purified enzyme from Cryptomonas sp., Coccolithophora sp., and Cylindrotheca fusiformis, we have determined that the RuBPCase holoenzyme of each species has a molecular weight, subunit composition, and isoelectric points of its subunits similar to the purified enzymes from pea and Chlamydomonas reinhardtii. The large subunits from chromophytes exhibit microheterogeneity in their isoelectric points, whereas two to four well-resolved isoelectric variants of the small subunit were observed in each RuBPCase preparation. In spite of the high degree of similarity in terms of physical properties, both the small and large RuBPCase subunits of the chromophytes are structurally different from those of chlorophytes; immunological studies demonstrate that RuBPCase subunits of these two groups have few antigenic determinants in common.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba M. L., Goodman M., Berger-Cohn J., Demaille J. G., Matsuda G. The early adaptive evolution of calmodulin. Mol Biol Evol. 1984 Nov;1(6):442–455. doi: 10.1093/oxfordjournals.molbev.a040330. [DOI] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Chory J., Muller E. D., Kaplan S. DNA-directed in vitro synthesis and assembly of the form II D-ribulose-1,5-bisphosphate carboxylase/oxygenase from Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jan;161(1):307–313. doi: 10.1128/jb.161.1.307-313.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Gray J. C., Kerwick R. G. An immunological investigation of the structure and function of ribulose 1,5-bisphosphate carboxylase. Eur J Biochem. 1974 May 15;44(2):481–489. doi: 10.1111/j.1432-1033.1974.tb03506.x. [DOI] [PubMed] [Google Scholar]
- Gray M. W., Doolittle W. F. Has the endosymbiont hypothesis been proven? Microbiol Rev. 1982 Mar;46(1):1–42. doi: 10.1128/mr.46.1.1-42.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman F. C., Stringer C. D., Lee E. H. Complete primary structure of ribulosebisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. Arch Biochem Biophys. 1984 Jul;232(1):280–295. doi: 10.1016/0003-9861(84)90544-7. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste-Royal G., Gibbs S. P. Ochromonas mitochondria contain a specific chloroplast protein. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1456–1459. doi: 10.1073/pnas.82.5.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero H. A., Lin Z. F., Racker E. Protein kinases from spinach chloroplasts. II. Protein substrate specificity and kinetic properties. J Biol Chem. 1982 Oct 25;257(20):12157–12160. [PubMed] [Google Scholar]
- Mishkind M. L., Schmidt G. W. Posttranscriptional Regulation of Ribulose 1,5-bisphosphate Carboxylase Small Subunit Accumulation in Chlamydomonas reinhardtii. Plant Physiol. 1983 Jul;72(3):847–854. doi: 10.1104/pp.72.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Muller E. D., Chory J., Kaplan S. Cloning and characterization of the gene product of the form II ribulose-1,5-bisphosphate carboxylase gene of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jan;161(1):469–472. doi: 10.1128/jb.161.1.469-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumley F. G., Schmidt G. W. Rocket and crossed immunoelectrophoresis of proteins solubilized with sodium dodecyl sulfate. Anal Biochem. 1983 Oct 1;134(1):86–95. doi: 10.1016/0003-2697(83)90267-1. [DOI] [PubMed] [Google Scholar]
- Reith M. E., Cattolico R. A. In vivo chloroplast protein synthesis by the chromophytic alga Olisthodiscus luteus. Biochemistry. 1985 May 7;24(10):2556–2561. doi: 10.1021/bi00331a024. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Devillers-Thiery A., Desruisseaux H., Blobel G., Chua N. H. NH2-terminal amino acid sequences of precursor and mature forms of the ribulose-1,5-bisphosphate carboxylase small subunit from Chlamydomonas reinhardtii. J Cell Biol. 1979 Dec;83(3):615–622. doi: 10.1083/jcb.83.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Badger M. R., Berry J. A. Variations in the Specific Activity of Ribulose-1,5-bisphosphate Carboxylase between Species Utilizing Differing Photosynthetic Pathways. Plant Physiol. 1984 Apr;74(4):791–794. doi: 10.1104/pp.74.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J., Buchanan B. B. Phosphorylation of chloroplast ribulose bisphosphate carboxylase/oxygenase small subunit by an envelope-bound protein kinase in situ. J Biol Chem. 1983 Jun 10;258(11):6686–6689. [PubMed] [Google Scholar]
- Tel-Or E., Cammack R., Rao K. K., Rogers L. J., Stewart W. D., Hall D. O. Comparative immunochemistry of bacterial, algal and plant ferredoxins. Biochim Biophys Acta. 1977 Jan 25;490(1):120–131. doi: 10.1016/0005-2795(77)90112-x. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Marrs B. L. Molecular genetics and the light reactions of photosynthesis. Cell. 1984 Nov;39(1):1–3. doi: 10.1016/0092-8674(84)90185-5. [DOI] [PubMed] [Google Scholar]





