Summary
Background
Infections are the main reason for mortality during acute leukaemia treatment and invasive aspergillosis (IA) is a major concern. Allogeneic stem cell transplantation (alloSCT) is a standard therapy and often is the only live-saving procedure in leukaemia patients. The profound immunodeficiency occurring after alloSCT led to high IA-associated mortality in the past. Therefore, patients with IA were historically considered transplant-ineligible. Recently, there has been improvement of anti-fungal management including novel anti-fungal agents. As a result, more leukaemia patients with IA are undergoing alloSCT. Outcome has not been prospectively assessed.
Methods
We performed a prospective study in acute leukaemia patients undergoing alloSCT to analyse the impact of a prior history of probable or proven IA (pre-SCT IA). The primary endpoint was 1-year non-relapse mortality (NRM). Relapse free survival and overall survival were analysed as secondary endpoints.
Findings
1439 patients were included between 2016 and 2021. The incidence of probable or proven pre-SCT IA was 6.0% (n = 87). The cumulative incidence of 1-year NRM was 17.3% (95% CI 10.2–26.0) and 11.2% (9.6–13.0) for patients with and without pre-SCT IA. In multivariate analyses the hazard ratio (HR) for 1-year NRM was 2.1 (1.2–3.6; p = 0.009) for patients with pre-SCT IA. One-year relapse-free survival was inferior in patients with pre-SCT IA (59.4% [48.3–68.9] vs. 70.4 [67.9–72.8]; multivariate HR 1.5 [1.1–2.1]; p = 0.02). Consequently, 1-year overall survival was lower in patients with pre-SCT IA: (68.8% [57.8–77.4] vs. 79.0% [76.7–81.1]; multivariate HR 1.7 [1.1–2.5]; p = 0.01).
Interpretation
Pre-SCT IA remains to be significantly associated with impaired alloSCT outcome. On the other hand, more than two thirds of patients with pre-SCT IA were alive at one year after alloSCT. IA is not anymore an absolute contraindication for alloSCT because the majority of patients with IA who undergo alloSCT benefit from this procedure.
Funding
There was no external funding source for this study.
Keywords: Invasive, Aspergillosis, Stem cell transplantation, Mortality
Research in context.
Evidence before this study
Invasive aspergillosis (IA) occurs frequently in patients with acute leukaemia undergoing intensive treatments. Allogeneic stem cell transplantation (alloSCT) is part of the treatment regimen in many acute leukaemia patients. IA could potentially influence outcome after alloSCT. To identify previous publications, we searched PubMed with the terms “aspergillosis”, “invasive”, “fungal infection”, “stem cell transplantation”, “leukaemia”, “mortality” and “outcome” in between Jan 1, 1980, and March 31st, 2023. This search retrieved a number of studies with variable outcomes. However, we could not identify a prospective study in a larger patient population in the recent era addressing the question on outcome of patients with IA undergoing alloSCT.
Added value of this study
This study provides the underlying clinical evidence for decision making on alloSCT indications in patients with acute leukaemia and IA. We found that 67% of patients with pre-SCT IA and 79% of control patients were alive one year after alloSCT. We show that IA remains to be a significant risk factor. More importantly, we demonstrate that patients with IA have a high chance of surviving alloSCT.
Implications of all the available evidence
The majority of leukaemia patients with IA who undergo alloSCT benefit from this procedure. Therefore, IA should not anymore be considered an absolute contraindication for alloSCT. Nonetheless, in spite of the improving outcome of IA, better prevention of pre-SCT IA is an important aspect of improving SCT results. Improved management of allogeneic SCT with pre-SCT IA is another area to investigate.
Introduction
Allogeneic stem cell transplantation (alloSCT) is a standard therapy for hematologic malignancies and it is also used for treatment of a variety of non-malignant diseases in children and adults. The number of performed alloSCTs is constantly increasing with nearly 20.000 transplantations reported to the European Society for Blood and Marrow Transplantation (EBMT) per year.1 The main clinical challenge of alloSCT is its high treatment-associated mortality (non-relapse mortality, NRM).2 Due to the profound secondary immunodeficiency that is associated with alloSCT, infectious complications are a major contributor to alloSCT-associated NRM.
Acute leukaemia is the main indication for alloSCT.3 Patients with acute leukaemia are initially treated with intensive chemotherapy to induce a remission (induction therapy). Patients with a high risk for relapse subsequently receive alloSCT as the only live saving procedure in many cases. Almost all acute leukaemia patients experience infectious complications during remission induction therapy.4 One frequent and severe infectious complication in this setting is invasive aspergillosis (IA), which most commonly affects exclusively the lungs, but can spread to the central nervous system, soft tissues, intestines and other parts of the body. Because of its high incidence during leukaemia induction therapy and its long persistence in immunocompromised hosts, a considerable number of alloSCT recipients have a history of IA.5
Historically, patients with IA were considered non-eligible to receive alloSCT because of the very high risk of IA progression and mortality. Over the years, there has been improvement of anti-fungal management including novel anti-fungal agents. As a result, more leukaemia patients with IA are undergoing alloSCT in modern times. Outcome has not been prospectively assessed impeding evidence-based transplant decisions. Previous studies have shown feasibility of alloSCT in patients with history of IA,6, 7, 8, 9 but had mixed results regarding the impact of pre-existing IA (pre-SCT IA) on clinical outcome of alloSCT,5,7,10, 11, 12 underlining the need for a prospective evaluation of a larger patient cohort.
In the present study, the Infectious Diseases and Acute Leukaemia Working Parties of The European Society for Blood and Marrow Transplantation (EBMT) prospectively analysed the long-term outcome of patients with acute leukaemia undergoing alloSCT according to the presence- or absence of pre-SCT IA.
Methods
This is a prospective multicentre analysis by the EBMT, which is a voluntary working group of transplant centres that are required to report regular follow up on all consecutive stem cell transplantations. Audits are routinely performed to determine the accuracy of the data. The study was planned and approved by the Infectious Diseases Working Party of the EBMT. Centres confirmed that all patients gave their written informed consent to use their personal information for research purposes. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
We asked EBMT centres performing alloSCTs if they were willing to participate in this study by completing data forms (Med-C, Supplementary data) with detailed information on IA, therapy, response, and complications for each included alloSCT recipient. From the centres who responded positively we asked data about all patients receiving first alloSCT for acute leukaemia between 5/2016-3/2021.
Sample size and definition
Assuming a 12% incidence of non-relapse mortality (NRM) in patients without proven or probable history of IA,2 a total of 1380 fully documented cases of alloSCT were sufficient in order to test the hypothesis that the incidence of NRM in cohort 1 (alloSCTs with previous history of proven or probable IA) is not higher than cohort 2 (alloSCT without history of IA or with previous history of possible IA) by more than a specified margin of 10%. The estimated proportion of alloSCTs in cohort 1 is 5%, thus 69 and 1311 alloSCTs were needed in cohort 1 and cohort 2, respectively, considering an alpha = 0.05, a beta = 0.2. On top of this number, we planned a drop out of 20% and we stopped the enrolment once the planned number of cases was reached. The final sample size of cases with a complete reported data set included a total of 1439 patients: 87 with and 1352 without a previous history of IA. Definition of IA into possible, probable or proven were used as proposed by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group in 2008.
Statistical analysis
The primary endpoint was 1-year NRM, estimated using the cumulative incidence method. The cause-specific Cox regression model was used to adjust the results for the main confounders: age, gender, underlying disease, status at SCT, time from diagnosis to SCT, time from IA to alloSCT, donor type, source of SCT, donor age, donor/recipient (D/R) gender match, D/R CMV status, conditioning regimens, type of immunosuppression. The relapse free survival (RFS) and the overall survival (OS) were analysed as secondary endpoints, using Kaplan–Meier method and Cox regression model for univariate and multivariate analysis using the main confounders, respectively. The relapse incidence and the incidence of acute and chronic graft-vs.-host disease (GVHD) were also estimated by the cumulative incidence methods, with the risk factor analysis performed by the cause-specific Cox regression model.
Non-relapse cause of death was considered as event of interest for the NRM, whilst the relapse was considered as competing events. Death due to any cause was considered as an event for the OS; death due to any cause and relapse were considered as events for the RFS. Relapse, acute and chronic GVHD were considered as event of interest for the cumulative incidence of relapse, acute and chronic GVHD, respectively; whilst the death was considered as a competing event. The proportional hazards assumption of the Cox models was verified.
The analyses were performed by using the software SAS v 9.4 and R. A p-value <0.05 was considered as statistically significant.
Role of funding
No external support was received for this project.
Results
Patient characteristics
We sent a feasibility questionnaire to 366 EBMT centers in July 2015 for participation in the study. A total of 70 centers were initially willing to participate and received the start announcement in April 2016 including the MEDC forms. Finally, thirty-six of 366 (9.8%) EBMT centres from 17 countries actively participated by providing detailed data in MED-C forms. We started enrolment in 2016 and stopped in 2021 after the planned sample size was reached. We asked centers to include all consecutive patients meeting the inclusion criteria during the study period. However, due to the extensive workload some of the centers terminated inclusion of patients prior to the end of study. The final analysis was based on 1439 patients including 87 patients with pre-SCT probable/proven IA who underwent first alloSCT. Patient characteristics are given in Table 1. The majority were adult patients (69%) with acute myeloid leukaemia (68%) in complete remission at time of transplant (78%). Donors were mostly unrelated (57%) and the stem cell source was peripheral blood in the majority of cases (72%). The conditioning regimen was myeloablative in 76% and did not contain total body irradiation (69%) in most cases. In vivo T cell depletion with anti-T—cell globulin (ATG, also termed anti-thymocyte globulin) was performed in 49% of patients. Most patient characteristics were balanced in between the groups of patients with pre-SCT IA vs. no pre-existing-IA. However, the pre-SCT IA group contained significantly more HLA-identical sibling donors (41% vs. 26%, p = 0.004).
Table 1.
Patient characteristics.
| IA before alloSCT |
Total (N = 1439) | p Value | ||
|---|---|---|---|---|
| No (N = 1352) | Yes (N = 87) | |||
| Age at alloSCT Median (min—max) | 41.0 (0.4–75.8) | 45.4 (1.7–68.0) | 41.0 (0.4–75.8) | 0.9 |
| Children <18 years old | 420 (31.1) | 29 (33.3) | 449 (31.2) | |
| Adults | 932 (68.9) | 58 (66.7) | 990 (68.8) | |
| Underlying malignancy | 0.8 | |||
| ALL | 434 (32.1) | 29 (33.3) | 463 (32.2) | |
| AML | 918 (67.9) | 58 (66.7) | 976 (67.8) | |
| Interval from leukaemia diagnosis to alloSCT (months) Median (min—max) | 6.9 (0.3–267.8) | 6.3 (2.2–165.3) | 6.9 (0.3–267.8) | 0.5 |
| Acute leukaemia status before alloSCT | 0.1 | |||
| CR | 1052 (77.8) | 74 (85.1) | 1126 (78.2) | |
| Other | 300 (22.2) | 13 (14.9) | 313 (21.8) | |
| Donor type | 0.004 | |||
| HLA-identical sibling | 347 (25.7) | 36 (41.4) | 383 (26.6) | |
| Haplo | 223 (16.5) | 14 (16.1) | 237 (16.5) | |
| Unrelated donor | 782 (57.8) | 37 (42.5) | 819 (56.9) | |
| Stem cell source | 0.5 | |||
| Bone marrow | 351 (26.0) | 18 (20.7) | 369 (25.6) | |
| Peripheral blood | 964 (71.3) | 67 (77.0) | 1031 (71.6) | |
| Cord blood | 37 (2.7) | 2 (2.3) | 39 (2.7) | |
| Conditioning regimen | 0.051 | |||
| Myeloablative | 1026 (75.9) | 74 (85.1) | 1100 (76.4) | |
| Reduced | 326 (24.1) | 13 (14.9) | 339 (23.6) | |
| Total body irradiation | 0.6 | |||
| No | 935 (69.2) | 58 (66.7) | 993 (69.0) | |
| Yes | 417 (30.8) | 29 (33.3) | 446 (31.0) | |
| In-vivo T-cell depletion (ATG or Campath) | 0.5 | |||
| No | 682 (50.4) | 47 (54.0) | 729 (50.7) | |
| Yes | 670 (49.6) | 40 (46.0) | 710 (49.3) | |
Description of pre-SCT IA
The incidence of probable or proven IA prior to alloSCT in our study population of acute leukaemia patients was 6.0% (87 cases of IA in 1439 alloSCT patients). A description of IA characteristics is given in Table 2. There were 11 proven (6 aspergillus fumigatus, 1 aspergillus flavus, 4 other aspergillus) and 76 probable IA infections. In 47% the interval between diagnosis of IA to alloSCT was <3 months and in 35% the interval was ≥3 months to <6 months. Only 6% of patients had a long interval between IA diagnosis and alloSCT of more than one year.
Table 2.
Characteristics of pre-existing IA.
| Characteristics of IA | N = 87 |
|---|---|
| Interval between diagnosis of IA and alloSCT | |
| Median: 99 days (9 days–4 years) | |
| 0–2.9 months | 41 (47.1) |
| 3–5.9 months | 30 (34.5) |
| 6–11.9 months | 11 (12.6) |
| >1 year | 5 (5.7) |
| Organ Involvement | |
| Lung only | 75 (86.2%) |
| Involvement of other sites ± lung involvement | 12 (13.8%) |
| Intraabdominal + lung | 4 |
| Paranasal sinuses only | 1 |
| Paranasal sinuses + lung | 2 |
| CNS + lung | 2 |
| Skin and soft tissue + lung | 2 |
| Skin and soft tissue only | 1 |
| Remission status of the IA at time of alloSCT | |
| Complete remission | 41/62 (66.1%) |
| Partial remission | 11/62 (17.7%) |
| Stable disease | 5/62 (8.1%) |
| Progression | 5/62 (8.1%) |
| Missing | 25 |
Most patients had exclusively lung involvement of the IA (86%) and 14% had other organs involved either in addition to lung involvement (12%) or without diagnosed lung involvement (2%). In patients with a known IA response to therapy before alloSCT, the response status was complete (66%), partial (18%), stable (8%) and progressive disease (8%).
The use of mould-active anti-fungal drugs after HSCT is described in Table 3. Many patients after alloSCT received Aspergillus active anti-fungal drugs between the day of alloSCT and day+99 (65%) as well as between day+100 and day +365 (45%). As expected, more patients in the IA arm received anti-fungal drugs vs. in the no-IA arm (until day +99 (88% vs. 63%); between day +100 and + 365 (66% vs. 44%). Posaconazole (28% until day +99 and 22% between +100 and +365), Voriconazole (23% until day +99 and 18% between +100 and +365) and liposomal Amphotericin B (20% until day +99 and 11% between +100 and +365) were the drugs most frequently administered.
Table 3.
Use of mould-active anti-fungal drugs after alloSCT in the subgroup of patients with available comprehensive information on drug use.
| IA pre alloSCT |
Total (N = 1152) |
||
|---|---|---|---|
| No (N = 1083) |
Yes (N = 69) |
||
| N (%) | N (%) | N (%) | |
| Any anti-mould drug given day 0- day +99 | |||
| No | 398 (36.7) | 8 (11.6) | 406 (35.2) |
| Yes | 685 (63.3) | 61 (88.4) | 746 (64.8) |
| Posaconazole given day 0 to +99 | |||
| No | 785 (72.5) | 45 (65.2) | 830 (72.0) |
| Yes | 298 (27.5) | 24 (34.8) | 322 (28.0) |
| Voriconazole given day 0–day +99 | |||
| No | 841 (77.7) | 42 (60.9) | 883 (76.6) |
| Yes | 242 (22.3) | 27 (39.1) | 269 (23.4) |
| Ambisome given day 0–day +99 | |||
| No | 877 (81.0) | 46 (66.7) | 923 (80.1) |
| Yes | 206 (19.0) | 23 (33.3) | 229 (19.9) |
| Echinocandin given day 0–day +99 | |||
| No | 937 (86.5) | 61 (88.4) | 998 (86.6) |
| Yes | 146 (13.5) | 8 (11.6) | 154 (13.4) |
| Amphotericin given day 0–day +99 | |||
| No | 1027 (94.8) | 69 (100.0) | 1096 (95.1) |
| Yes | 56 (5.2) | 0 (0.0) | 56 (4.9) |
| Isavuconazole given day 0–day +99 | |||
| No | 1054 (97.3) | 65 (94.2) | 1119 (97.1) |
| Yes | 29 (2.7) | 4 (5.8) | 33 (2.9) |
|
N = 924 |
N = 56 |
N = 980 |
|
| Any anti-mould drug given day +100–day +365 | |||
| No | 520 (56.3) | 19 (33.9) | 539 (55.0) |
| Yes | 404 (43.7) | 37 (66.1) | 441 (45.0) |
| Posaconazole given day +100–day +365 | |||
| No | 720 (77.9) | 46 (82.1) | 766 (78.2) |
| Yes | 204 (22.1) | 10 (17.9) | 214 (21.8) |
| Voriconazole given day +100–day +365 | |||
| No | 765 (82.8) | 37 (66.1) | 802 (81.8) |
| Yes | 159 (17.2) | 19 (33.9) | 178 (18.2) |
| Ambisome given day +100–day +365 | |||
| No | 833 (90.2) | 42 (75.0) | 875 (89.3) |
| Yes | 91 (9.8) | 14 (25.0) | 105 (10.7) |
| Echinocandin given day +100–day +365 | |||
| No | 863 (93.4) | 51 (91.1) | 914 (93.3) |
| Yes | 61 (6.6) | 5 (8.9) | 66 (6.7) |
| Amphotericin given day +100–day +365 | |||
| No | 902 (97.6) | 56 (100.0) | 958 (97.8) |
| Yes | 22 (2.4) | 0 (0.0) | 22 (2.2) |
| Isavuconazole given day +100–day +365 | |||
| No | 897 (97.1) | 52 (92.9) | 949 (96.8) |
| Yes | 27 (2.9) | 4 (7.1) | 31 (3.2) |
Influence of IA on survival and relapse
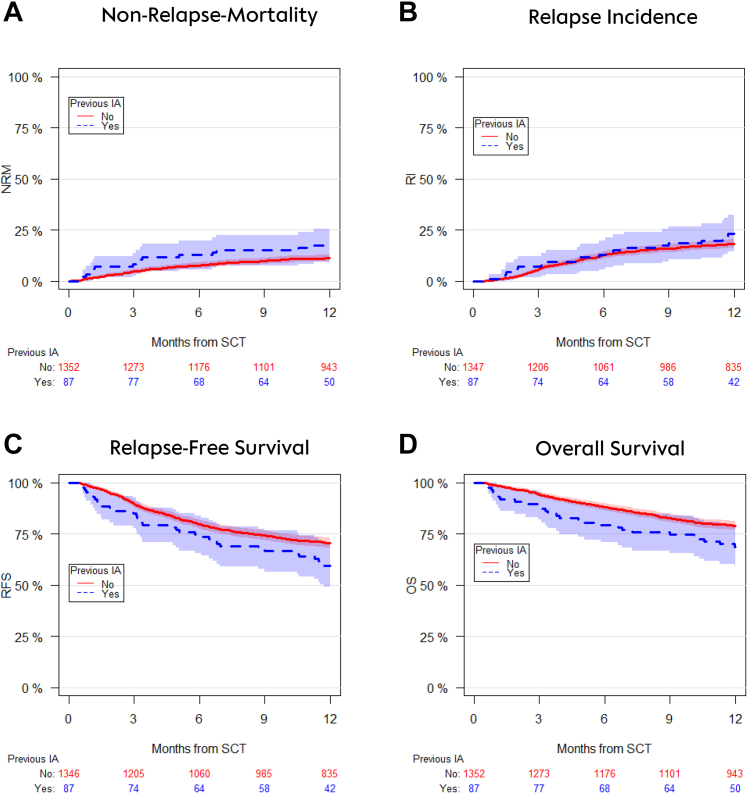
The cumulative incidence of NRM at one year post alloSCT was 17.3% (95% CI 10.2–26.0) in patients with pre-existing probable/proven IA vs. 11.2% (95% CI 9.6–13.0) in patients without IA (Fig. 1A). In multivariate analyses the hazard ratio for experiencing NRM was 2.1 (95% CI 1.2–3.6) (p = 0.009).
Fig. 1.
Univariate outcome graphs showing non-relapse-mortality (panel A), relapse incidence (panel B), relapse-free survival (panel C) and overall survival (panel D) after alloSCT in patients with pre-existing IA (blue dotted lines) vs. patients who did not have IA prior to alloSCT (red lines).
The incidence of relapse at one year was 23.2% (14.9–32.7) in patients with pre-SCT IA vs. 18.3 (16.3–20.5) in patients without IA prior to alloSCT (Fig. 1B). This difference was not statistically significant after adjusting for confounders (HR 1.4 [0.9–2.1]), (p = 0.19). Relapse free survival at one year was significantly inferior in alloSCT recipients with pre-SCT IA as compared to alloSCT recipients without pre-SCT IA (59.4% [95% CI 48.3–68.9] vs. 70.4% [95% CI 67.9–72.8] (Fig. 1C); HR in multivariate analyses 1.5 [95% CI 1.1–2.1], p = 0.02). Consequently, 1-year overall survival was lower in patients with pre-SCT IA: (68.8 [57.8–77.4] vs. 79.0 [76.7–81.1]; Fig. 1D; multivariate HR 1.7 [1.1–2.5]; p = 0.01). Reasons of death are given in Table 4. Of note, relapse of acute leukaemia was the most frequent reason of death in both groups. Death was infection related in 29.1% and 29.3% of patients with and without pre-SCT IA, respectively.
Table 4.
Reasons of death after alloSCT in both cohorts of patients with pre-existing IA vs. no pre-existing IA.
| Total patients | IA pre alloSCT |
|
|---|---|---|
| No 1352 | Yes 87 | |
| Number of overall deaths | (N = 444) | (N = 41) |
| Cause of death | N (%) | N (%) |
| Relapse of primary disease | 145 (10.7) | 16 (18.4) |
| Infection related | 129 (9.5) | 12 (13.8) |
| Bacterial | 42 | 4 |
| Viral | 20 | 1 |
| Fungal | 14 | 1 |
| Bacterial + fungal | 8 | 1 |
| Bacterial + viral | 7 | 0 |
| Viral + fungal | 4 | 0 |
| Bacterial + viral + fungal | 4 | 0 |
| Parasitic | 1 | 0 |
| Fungal + parasitic | 1 | 0 |
| Viral + parasitic | 1 | 0 |
| Viral + fungal + parasitic | 1 | 0 |
| Type of infection not specified | 26 | 5 |
| Graft-vs.-host disease related | 85 (6.3) | 7 (8.0) |
| Secondary malignancy | 9 (0.7) | 1 (1.1) |
| Multi-organ failure related | 35 (2.6) | 0 (0.0) |
| Other | 34 (2.5) | 5 (5.7) |
| Missing | 7 (0.5) | 0 (0.0) |
Next, we investigated if the time interval between diagnosis of IA and alloSCT influences alloSCT outcome. The main clinical outcome parameters in relation to the time intervals of IA are described in Table 5. We found no significant differences in one-year cumulative incidence on NRM between patients who were diagnosed with IA shortly before alloSCT (0 to <3 months) as compared to ≥ 3 months. In addition, the IA time interval was not associated to significant differences in the cumulative incidence of relapse. Interestingly, we found that patients with IA shortly before alloSCT (0–2.9 months) had a not statistically significant tendency towards a lower progression-free survival of 58.4% [95% CI 41.9–71.8]) as compared to patients with a longer time interval between IA and alloSCT of between 3 and 6 months with 59.1% [95% CI 39.3–74.4]) or to patients with IA longer than 6 months/no IA of 70.3% [95% CI 67.8–72.7]) (p = 0.08). This resulted in similar non-significant differences in overall survival: patients with IA shortly before alloSCT had overall survival of 70.7% [95% CI 54.1–82.1]) and patients with a longer time interval between IA and alloSCT of between 3 and 6 months had 66.0% [95% CI 46.0–80.1]). Patients with IA longer than 6 months or no IA had overall survival 78.9% [95% CI 76.6–81.0]) (p = 0.077). We also asked the question if the timing of IA influences the remission status of IA at time of alloSCT. These data were available only in a subgroup of 62 patients. As expected, more patients with IA episodes ≥3 months prior to alloSCT had complete remission of IA at time of transplant as compared to patients with IA episodes <3 months prior to alloSCT (82.4% CR vs. 46.4%; p = 0.003). We found no statistical significant association of IA remission status at time of transplant to one year NRM (CR = 14.6% (5.9–27.2) vs. no CR 14.3% (3.4–32.7)). There was a tendency towards a higher relapse incidence in the no CR group, which was not statistically significant (CR = 17.1% (7.4–30.1) vs. no CR 28.6% (11.2–48.8), p = 0.2).
Table 5.
Major clinical outcome parameters in alloSCT patients according to time intervals between diagnosis of IA to alloSCT.
| Non-Relapse-Mortality | Total | Events | 1-year NRM (95% C.I.) | p |
|---|---|---|---|---|
| IA No or more than 6 months before | 1368 | 155 | 11.40 (9.78–13.16) | 0.5 |
| IA 3–5.9 months before | 30 | 5 | 16.94 (6.02–32.58) | |
| IA 0–2.9 months before | 41 | 6 | 14.63 (5.85–27.23) | |
|
Relapse incidence |
Total |
Events |
1-year RI (95% C.I.) |
p |
| IA No or more than 6 months before | 1368 | 249 | 18.26 (16.25–20.37) | 0.28 |
| IA 3–5.9 months before | 41 | 11 | 26.93 (14.35–41.21) | |
| IA 0–2.9 months before | 30 | 7 | 23.91 (10.30–40.64) | |
|
Progression-free survival |
Total |
Events |
1-year RFS (95% C.I.) |
p |
| IA No or more than 6 months before | 1368 | 404 | 70.34 (67.83–72.70) | 0.08 |
| IA 3–5.9 months before | 30 | 12 | 59.13 (39.34–74.38) | |
| IA 0–2.9 months before | 41 | 17 | 58.43 (41.91–71.75) | |
|
Overall survival |
Total |
Events |
1-year OS (95% C.I.) |
p |
| IA No or more than 6 months before | 1368 | 287 | 78.88 (76.61–80.96) | 0.077 |
| IA 3–5.9 months before | 30 | 10 | 65.97 (45.95–80.05) | |
| IA 0–2.9 months before | 41 | 12 | 70.65 (54.13–82.14) |
Roughly, one third of patients in our study were below 18 years (Table 1) and we were interested if the pre-SCT IA-related risk for NRM depends on the age of the alloSCT patients. By testing the interaction between age (adults vs. children) and pre-SCT IA, we obtained no significant association in the multivariate model. We also found no significant association between the frequency of alloSCTs a center performs and NRM or overall survival.
Influence of pre-SCT IA on graft-vs.-host disease (aGVHD)
The cumulative incidence of aGVHD all grades did not significantly differ between patients with pre-SCT IA (39.1% [95% CI 28.5–49.5]) vs. patients without IA (45.5% [95% CI 42.8–48.2]) (p = 0.2). In addition, the incidence of severe aGVHD grades III-IV was not significantly different in both groups (IA group 8.6% [95% CI 3.7–15.9] vs. without IA group 12.4% [95% CI 10.7–14.3]) (p = 0.3).
The same was true for chronic GVHD (cGVHD): The cumulative incidence of cGVHD all grades did not significantly differ between patients with pre-SCT IA (28.1% [95% CI 18.2–38.9]) vs. patients without IA (25.1% [95% CI 22.7–27.5]) (p = 0.5). In line with these results, also the incidence of extensive cGVHD was not significantly different in both groups (IA group 18.3% [95% CI 10.3–28.1] vs. without IA group 15.0% [95% CI 13.0–17.0]) (p = 0.4).
Incidence and risk factors of post alloSCT IA
In addition to the main objective of our study on pre-SCT IA before alloSCT, we were able to collect prospective data on the occurrence of post alloSCT IA. In the entire cohort (n = 1439) the cumulative incidence of probable or proven IA at day +100 post alloSCT was 1.9% (95% CI 1.3–2.7) and at one year post alloSCT was 3.5% (95% CI 2.6–4.5). Four out of 87 patients with pre-SCT IA developed a post alloSCT IA.
In multivariate analyses the donor type was the strongest risk factor for post alloSCT IA: as compared to HLA-identical siblings the use of haplo-identical donors was associated to significantly increased risk for IA with a hazard ratio of 5.0 (1.8–14.0) (p = 0.003). The other significant risk factor for post alloSCT development of IA was the use of in vivo T-cell depletion with ATG or Campath (HR 2.0 [1.0–4.0], p = 0.04). The use of unrelated donors was non-significantly associated to a higher hazard ratio for post IA development of 2.3 (0.9–6.1) (p = 0.1) as compared to HLA-identical sibling donors.
Discussion
In this prospective EBMT study, we were able to estimate the mortality risk after alloSCT in patients with- and without pre-SCT IA. Our most important findings is that more than two thirds of patients with pre-SCT IA were alive at one year after alloSCT. Our second most important finding is that pre-SCT IA remains a significant risk factor for impaired alloSCT outcome in this recent cohort. Also importantly, we found that in patients with pre-SCT IA as well as in control patients without IA the main reasons of death after alloSCT were non-infection related. Namely, leukaemia relapse was the most prominent reason for deaths in patients with pre-SCT IA. The fact that patients with the previous IA group have lost the dose-intensity of the treatment may have played role. This is because the treatment of Aspergillosis is usually associated to temporary suspension of chemotherapy (or dose reduction) or at least postponement of the treatment schedule to give time to achieve an acceptable haematological recovery (if neutropenic) and an adequate control of organ infection. The lowering of dose-intensity may affect the efficacy of the treatment and make these patients more prone to relapse. Taken together these data demonstrate that IA is not anymore an absolute contraindication for alloSCT because the majority of patients with IA who undergo alloSCT benefit from this procedure.
Our findings differ from historic reports describing a major adverse impact of IA on alloSCT outcome.11, 12, 13, 14 While this study was not designed to investigate the reasons for better outcome as compared to previous studies, we speculate that the widespread use of more active anti-mould drugs as well as improved supportive care management may have contributed to more favourable results in the present study. We found that a high proportion of alloSCT recipients were treated with mould-active drugs as secondary antifungal prophylaxis. This may have led to lower rates of IA-related deaths and improved outcome.6,13,15,16 An overall better supportive care management under current conditions may have improved specifically the outcome of alloSCT in patients with IA. This hypothesis is supported by publications demonstrating that the outcome of severe infectious complications in patients with malignancies has considerably improved during the last decade and is mostly determined by the status of the underlying malignancy (not by the infection).17,18
A specific difficulty in our study was to assess the remission status of invasive pulmonary aspergillosis at the time of alloSCT in these heavily pre-treated haematology patients. This is reflected by the fact that in 25 cases with pre-SCT IA it was not possible to determine the remission status at time of SCT. The standard procedure is to perform chest CT scan prior to SCT in patients who had previous evidence of invasive aspergillosis. However, in acute leukaemia patients, the chest CT scans often is abnormal and small nodules or infiltrates of unknown origin are frequently seen.19
Our current results add prospective evidence on results from two previous retrospective registry studies in North America of the Centre for International Blood and Marrow Transplant Research (CIBMTR)20 and in Europe of the EBMT.5 In 2017, the CIBMTR published their experience with pre-existing invasive fungal infections including older cases before the year 2000 and found that Aspergillus spp. and Candida spp. were the most commonly identified pathogens. AlloSCT recipients with invasive fungal infections had an inferior progression-free survival, a shorter overall survival, increased NRM as well as higher infection-related mortality. Similar to our current results, significant survivorship was observed and the predominant cause of death in alloSCT recipients with pre-existing invasive fungal disease was not infection but the underlying malignancy.20 The former EBMT study focused on patients with pre-SCT IA (excluding other types of fungi) and exclusively included a more recent patient population between 2005 and 2010.5 In this retrospective dataset, overall survival and NRM were not statistically different after alloSCT in patients with pre-SCT IA vs. controls but there was a trend towards impaired outcome. Of note, the registries contain less detailed and reliable data on invasive fungal infections and there is a risk of underreporting as well as difficulties with loss of follow up. The registry analyses may have underestimated the effect of IA on alloSCT outcome due to difficulties in diagnosing IA under ‘real life conditions’. In the previous retrospective study, we were unable to assess the impact of the type of IA (proven/probable vs. possible) and the status of IA before alloSCT. Therefore, we decided that a prospective trial is needed to draw definite conclusions on the impact of pre-SCT IA on alloSCT outcome.
One further interesting aspect of our study is that the use of in vivo T-cell depletion with ATG is a significant risk factor for development of post alloSCT IA. Based on our results it may be tempting to omit ATG in alloSCTs with a high risk of IA. This however would increase the risk of acute and chronic GVHD rates. We therefore recommend using ATG in alloSCTs at high risk of GVHD despite the somewhat higher risk of IA.21 An unanswered question is how ATG compares to post transplantation cyclophosphamide (PTCy), the other popular GVHD prophylaxis strategy, regarding the infectious disease risk. The EBMT IDWP is currently conducting a retrospective study to collect evidence.
In summary, our current results provide the underlying clinical evidence for decision making on alloSCT indications in patients with acute leukaemia and IA. The main message is that the majority of leukaemia patients with IA who undergo alloSCT will benefit from this procedure. Therefore, patients with pre-SCT IA should not anymore be excluded from alloSCT, which is the only lifesaving treatment for many patients with acute leukaemia.
Contributors
All authors contributed substantially to the manuscript. OP, GT and SC contributed to the study conception and design. GT, NK and LW accessed and verified the raw data. All authors contributed to data acquisition, analysis, or interpretation. OP drafted the article and all authors critically revised and approved the manuscript. All authors agree that they are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved. No writing assistance other than copy editing was provided.
Data sharing statement
Data is available upon request to the EBMT Leiden office, Rijnsburgerweg 10, 2333 Leiden, The Netherlands, +31715261444, info@ebmt.org.
Declaration of interests
R.dlC. Has received honoraria from Pfizer and MSD; J.S. reports personal fees from Gilead, outside the submitted work. EDK reports grants from Gilead sciences, personal fees from Pfizer, outside the submitted work. L.Y. reports grants and personal fees from Janssen, personal fees from Abbvie, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Beigene, personal fees and non-financial support from Kite/Gilead, personal fees and non-financial support from Pfizer, outside the submitted work; S.M reports other from Celgene/BMS, other from Novartis, other from Kite/Gilead, other from Pfizer, other from Miltenyi, other from Mendes, other from SWECARNET, other from Scientify Research, outside the submitted work; J.M. reports personal fees and non-financial support from Gilead Sciences, personal fees and non-financial support from Mundipharma, personal fees and non-financial support from F2G, personal fees and non-financial support from Takeda, personal fees and non-financial support from Basilea, outside the submitted work; M.M. reports grants and personal fees from Gilead, personal fees from Mundipharma, personal fees from Pfizer, from null, outside the submitted work; H.P. reports non-financial support from Neovii, during the conduct of the study; OP has no COIs directly related to this work. HP reports non-financial support from Neovii during the conduct of the study; OP has received honoraria or travel support from Gilead, Jazz, MSD, Neovii, Novartis, Pfizer and Therakos. He has received research support from Incyte and Priothera. He is member of advisory boards to Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Orca Bio, Priothera, Sanofi, Shionogi and SOBI. T.Z. reports personal fees from AbbVie, personal fees from Orgenesis Inc, personal fees from BioSight Ltd, personal fees from Cellect Biotechnology, personal fees from Janssen, personal fees from Novartis, personal fees from Gilead Sciences, outside the submitted work.
The remaining authors declare no conflict of interests.
Acknowledgements
The authors thank the following funding agencies for supporting their work.
OP acknowledges the support of José Carreras Leukämie-Stiftung (3R/2019, 23R/2021), Deutsche Krebshilfe (70113519), Deutsche Forschungsgemeinschaft (PE 1450/7-1, PE 1450/9-1) and Stiftung Charité BIH (BIH_PRO_549, Focus Group Vascular Biomedicine).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102393.
Appendix A. Supplementary data
References
- 1.Passweg J.R., Baldomero H., Chabannon C., et al. The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T's come into focus. Bone Marrow Transplant. 2020;55(8):1604–1613. doi: 10.1038/s41409-020-0826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penack O., Peczynski C., Mohty M., et al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020;4(24):6283–6290. doi: 10.1182/bloodadvances.2020003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snowden J.A., Sanchez-Ortega I., Corbacioglu S., et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022;57(8):1217–1239. doi: 10.1038/s41409-022-01691-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinz W.J., Buchheidt D., Christopeit M., et al. Diagnosis and empirical treatment of fever of unknown origin (FUO) in adult neutropenic patients: guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO) Ann Hematol. 2017;96(11):1775–1792. doi: 10.1007/s00277-017-3098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penack O., Tridello G., Hoek J., et al. Influence of pre-existing invasive aspergillosis on allo-HSCT outcome: a retrospective EBMT analysis by the Infectious Diseases and Acute Leukemia Working Parties. Bone Marrow Transplant. 2016;51(3):418–423. doi: 10.1038/bmt.2015.237. [DOI] [PubMed] [Google Scholar]
- 6.Cordonnier C., Rovira M., Maertens J., et al. Voriconazole as secondary antifungal prophylaxis in stem cell transplant recipients. Haematologica. 2011;96(2):e9–e10. doi: 10.3324/haematol.2010.038463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda T., Boeckh M., Guthrie K.A., et al. Invasive aspergillosis before allogeneic hematopoietic stem cell transplantation: 10-year experience at a single transplant center. Biol Blood Marrow Transplant. 2004;10(7):494–503. doi: 10.1016/j.bbmt.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Girmenia C., Raiola A.M., Piciocchi A., et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO) Biol Blood Marrow Transplant. 2014;20(6):872–880. doi: 10.1016/j.bbmt.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Martino R., Parody R., Fukuda T., et al. Impact of the intensity of the pretransplantation conditioning regimen in patients with prior invasive aspergillosis undergoing allogeneic hematopoietic stem cell transplantation: a retrospective survey of the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2006;108(9):2928–2936. doi: 10.1182/blood-2006-03-008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marr K.A., Carter R.A., Crippa F., Wald A., Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 11.Martino R., Lopez R., Sureda A., Brunet S., Domingo-Albos A. Risk of reactivation of a recent invasive fungal infection in patients with hematological malignancies undergoing further intensive chemo-radiotherapy. A single-center experience and review of the literature. Haematologica. 1997;82(3):297–304. [PubMed] [Google Scholar]
- 12.Offner F., Cordonnier C., Ljungman P., et al. Impact of previous aspergillosis on the outcome of bone marrow transplantation. Clin Infect Dis. 1998;26(5):1098–1103. doi: 10.1086/520274. [DOI] [PubMed] [Google Scholar]
- 13.Hill B.T., Kondapalli L., Artz A., et al. Successful allogeneic transplantation of patients with suspected prior invasive mold infection. Leuk Lymphoma. 2007;48(9):1799–1805. doi: 10.1080/10428190701534390. [DOI] [PubMed] [Google Scholar]
- 14.Martino R., Nomdedeu J., Altes A., et al. Successful bone marrow transplantation in patients with previous invasive fungal infections: report of four cases. Bone Marrow Transplant. 1994;13(3):265–269. [PubMed] [Google Scholar]
- 15.Allinson K., Kolve H., Gumbinger H.G., Vormoor H.J., Ehlert K., Groll A.H. Secondary antifungal prophylaxis in paediatric allogeneic haematopoietic stem cell recipients. J Antimicrob Chemother. 2008;61(3):734–742. doi: 10.1093/jac/dkm521. [DOI] [PubMed] [Google Scholar]
- 16.Cordonnier C., Maury S., Pautas C., et al. Secondary antifungal prophylaxis with voriconazole to adhere to scheduled treatment in leukemic patients and stem cell transplant recipients. Bone Marrow Transplant. 2004;33(9):943–948. doi: 10.1038/sj.bmt.1704469. [DOI] [PubMed] [Google Scholar]
- 17.Azoulay E., Mokart D., Pene F., et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium--a groupe de recherche respiratoire en reanimation onco-hematologique study. J Clin Oncol. 2013;31(22):2810–2818. doi: 10.1200/JCO.2012.47.2365. [DOI] [PubMed] [Google Scholar]
- 18.Legrand M., Max A., Peigne V., et al. Survival in neutropenic patients with severe sepsis or septic shock. Crit Care Med. 2012;40(1):43–49. doi: 10.1097/CCM.0b013e31822b50c2. [DOI] [PubMed] [Google Scholar]
- 19.Muslimani A., Chisti M.M., Margolis J., et al. Pulmonary infiltrates in acute myeloid leukemia during induction treatment: how much do we know? Am J Clin Oncol. 2014;37(4):377–383. doi: 10.1097/COC.0b013e31827b4702. [DOI] [PubMed] [Google Scholar]
- 20.Maziarz R.T., Brazauskas R., Chen M., et al. Pre-existing invasive fungal infection is not a contraindication for allogeneic HSCT for patients with hematologic malignancies: a CIBMTR study. Bone Marrow Transplant. 2017;52(2):270–278. doi: 10.1038/bmt.2016.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penack O., Marchetti M., Ruutu T., et al. Prophylaxis and management of graft vs. host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7(2):e157–e167. doi: 10.1016/S2352-3026(19)30256-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.