Abstract
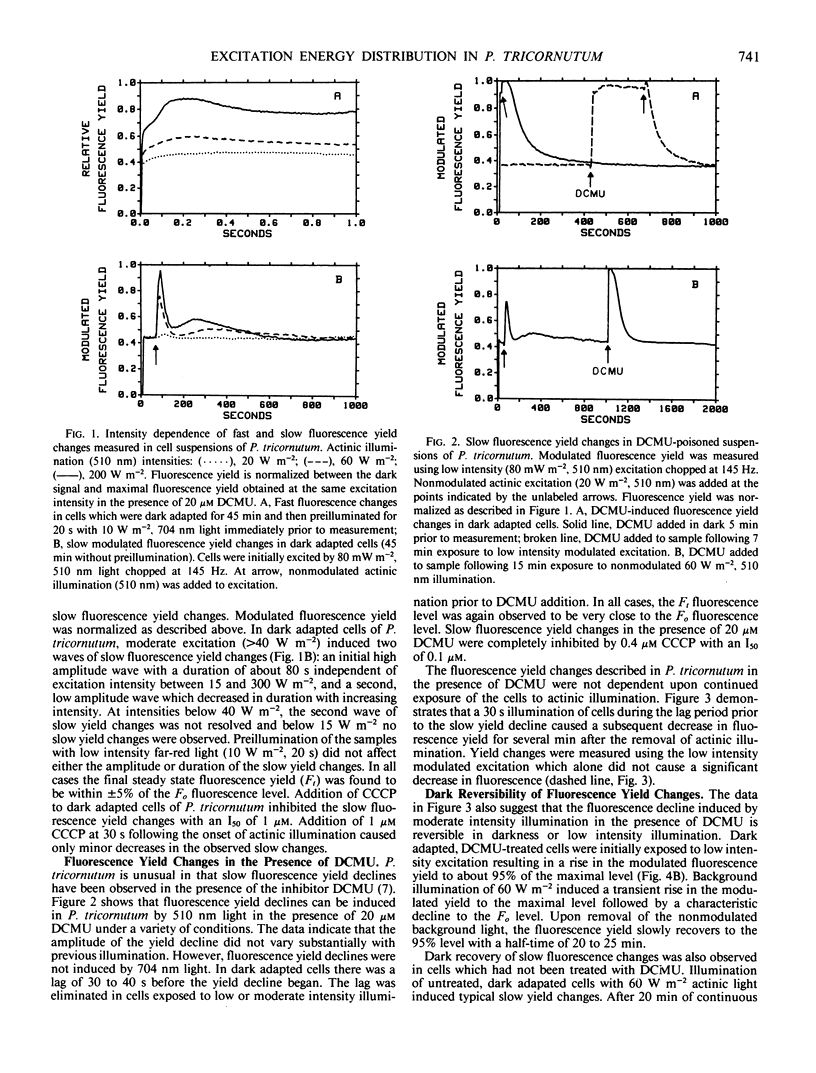
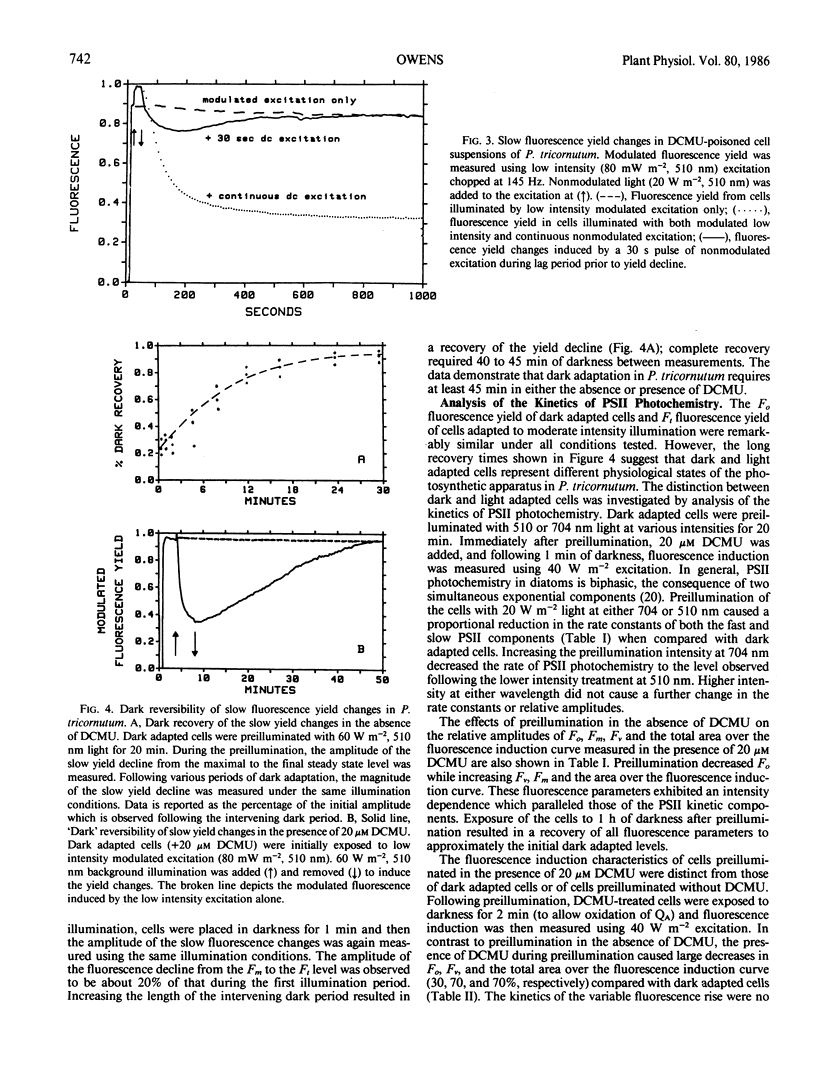
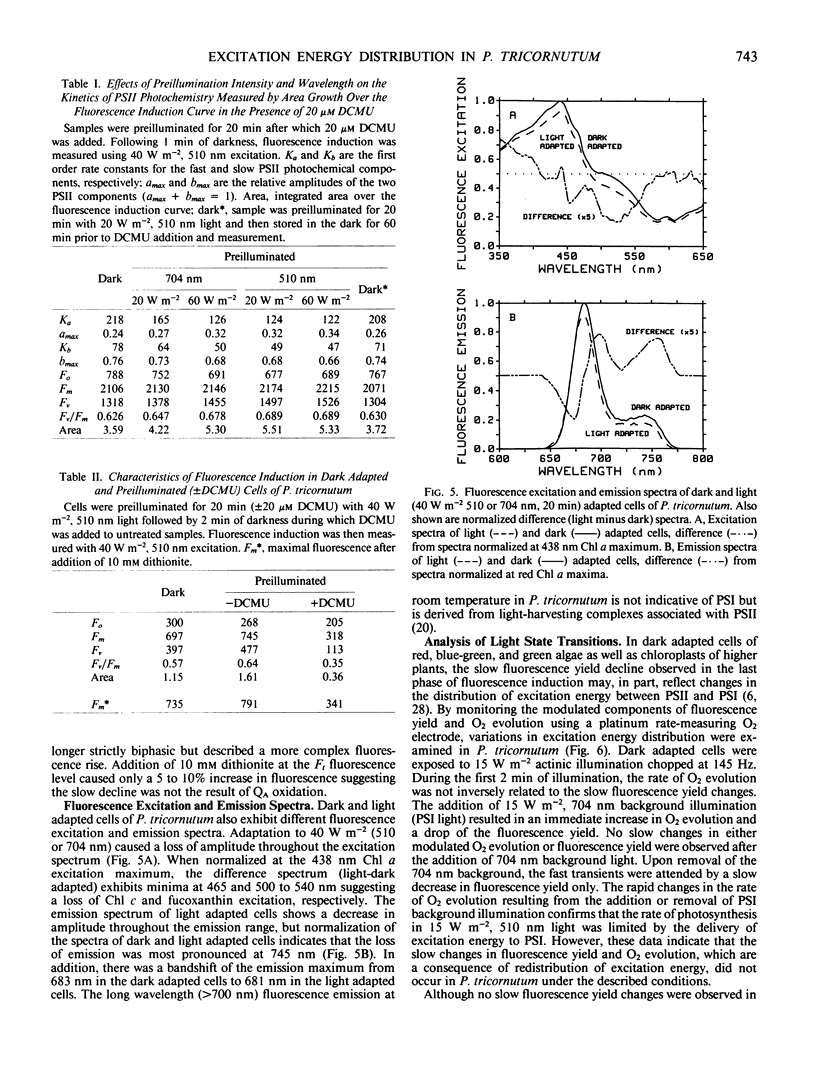
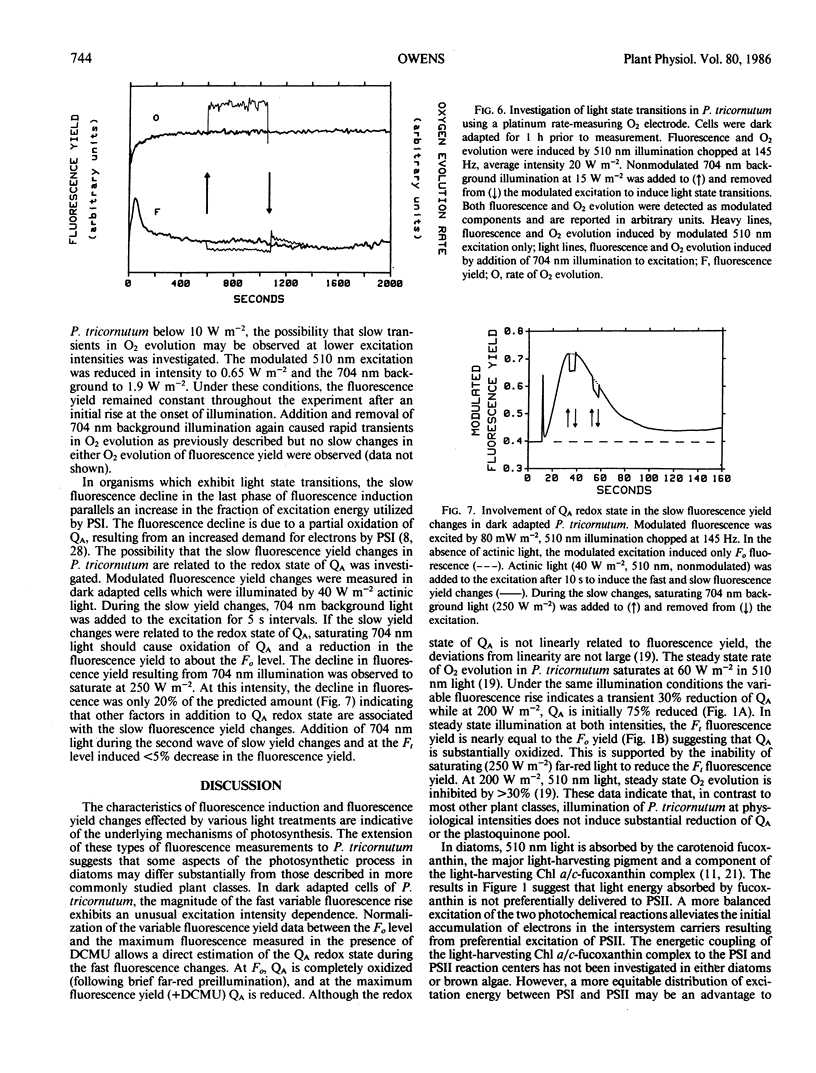
The distribution of excitation energy between photosystems I and II (PSI and PSII) was investigated in the marine diatom Phaeodactylum tricornutum (Bohlin) using light-induced changes in fluorescence yield and rate of modulated O2 evolution. The intensity dependence of the fast fluorescence rise in dark adapted cells (±DCMU) suggests that light absorbed by the major antenna complex was not delivered preferentially to PSII but is more equally distributed between the photosystems. Reversible, slow fluorescence yield changes measured in the absence of DCMU were correlated with decreased initial fluorescence and rate constants for PSII photochemistry, increased variable fluorescence, alteration of the fluorescence excitation and emission spectra, and could be effected by either 510 nm (PSII) or 704 nm (PSI) light. Slow, reversible fluorescence yield changes were also observed in the presence of DCMU, but were characterized by a loss of both initial and variable fluorescence and could not be induced by PSI light. The absence of slow changes in the yield of fluorescence and rate of modulated O2 evolution, following addition or removal of PSI background light to modulated PSII excitation, does not support regulation of excitation energy density in PSI at the expense of PSII. The results suggest that adjustments are made at the level of excitation energy transfer to the PSII reaction center which prevent prolonged loss of photosynthetic capacity. Energy distribution is regulated by ionic distributions independently of the plastoquinone pool redox state. These differences in light-harvesting function are probably a response to the aquatic light field and may account for the success of diatoms in low and variable light environments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Brown J. S. Fluorometric evidence for the participation of chlorophyll a-695 in system 2 of photosynthesis. Biochim Biophys Acta. 1967 Sep 6;143(2):391–398. doi: 10.1016/0005-2728(67)90092-8. [DOI] [PubMed] [Google Scholar]
- Friedman A. L., Alberte R. S. A diatom light-harvesting pigment-protein complex : purification and characterization. Plant Physiol. 1984 Oct;76(2):483–489. doi: 10.1104/pp.76.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. G., Wold E. R. Light-Harvesting Function in the Diatom Phaeodactylum tricornutum: I. Isolation and Characterization of Pigment-Protein Complexes. Plant Physiol. 1986 Mar;80(3):732–738. doi: 10.1104/pp.80.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Fork D. C. A New Mechanism for Adaptation to Changes in Light Intensity and Quality in the Red Alga Porphyra perforata: III. Fluorescence Transients in the Presence of 3-(3,4-Dichlorophenyl)-1,1-dimethylurea. Plant Physiol. 1983 Mar;71(3):673–676. doi: 10.1104/pp.71.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]


