Abstract
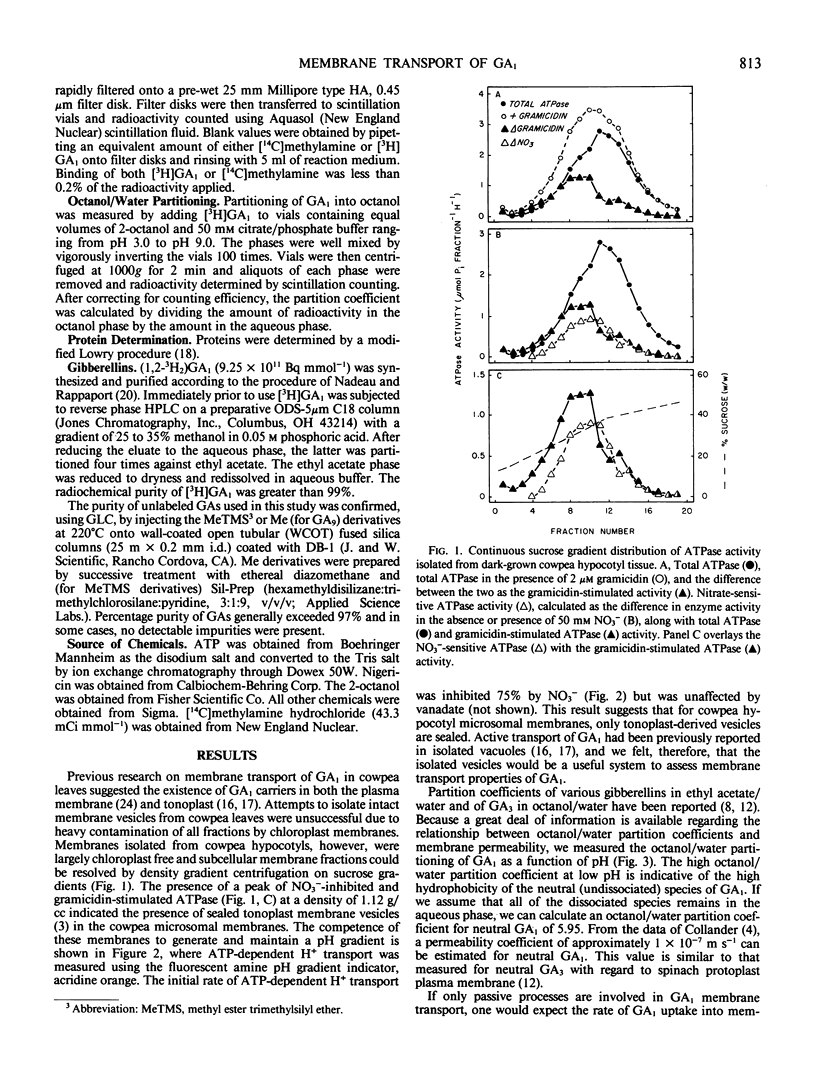
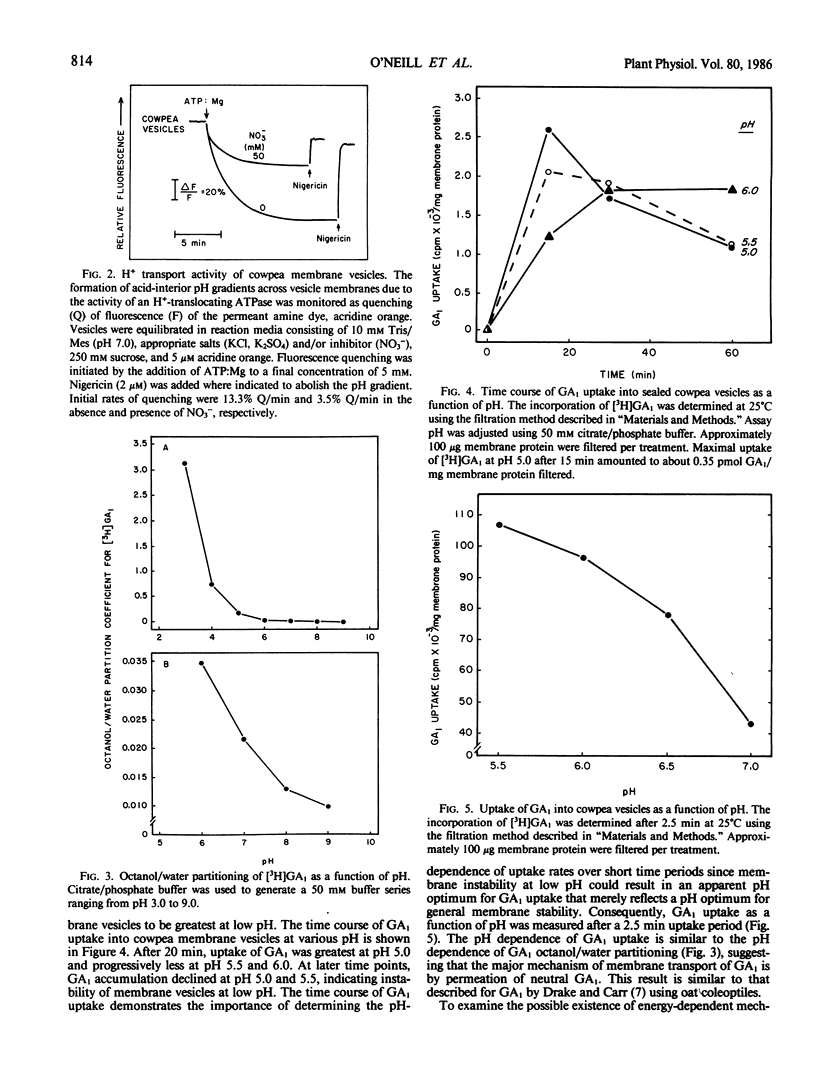
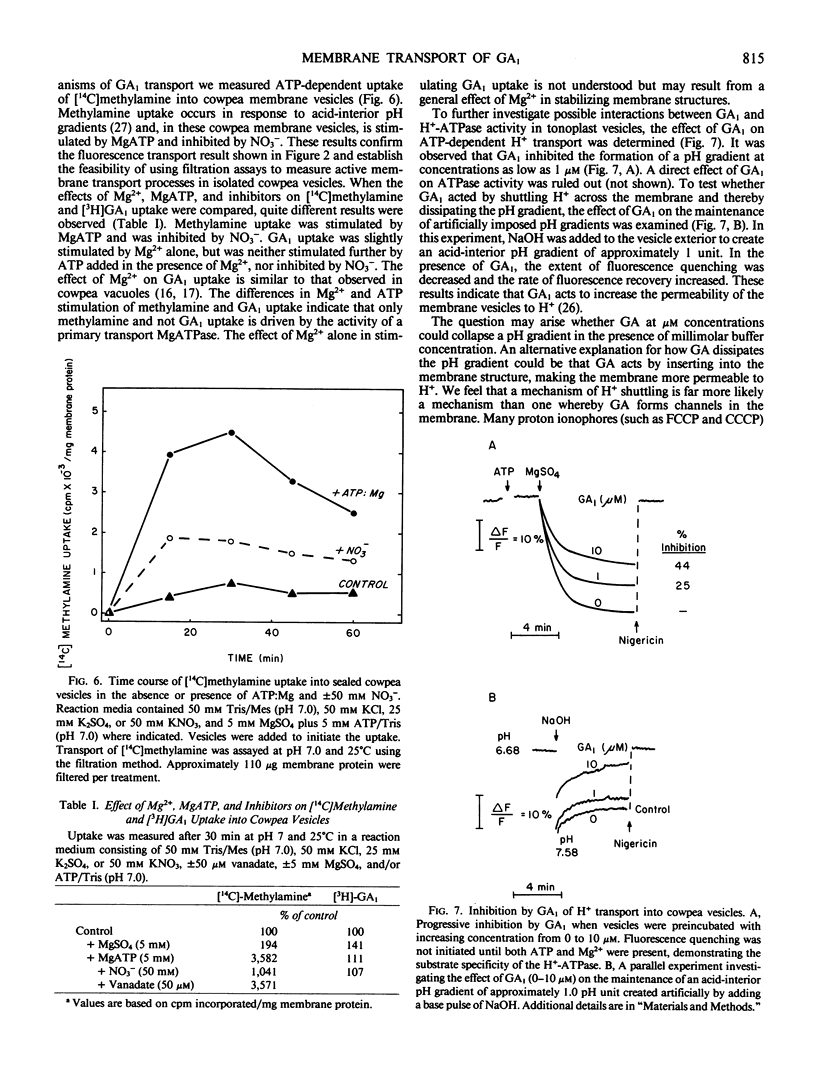
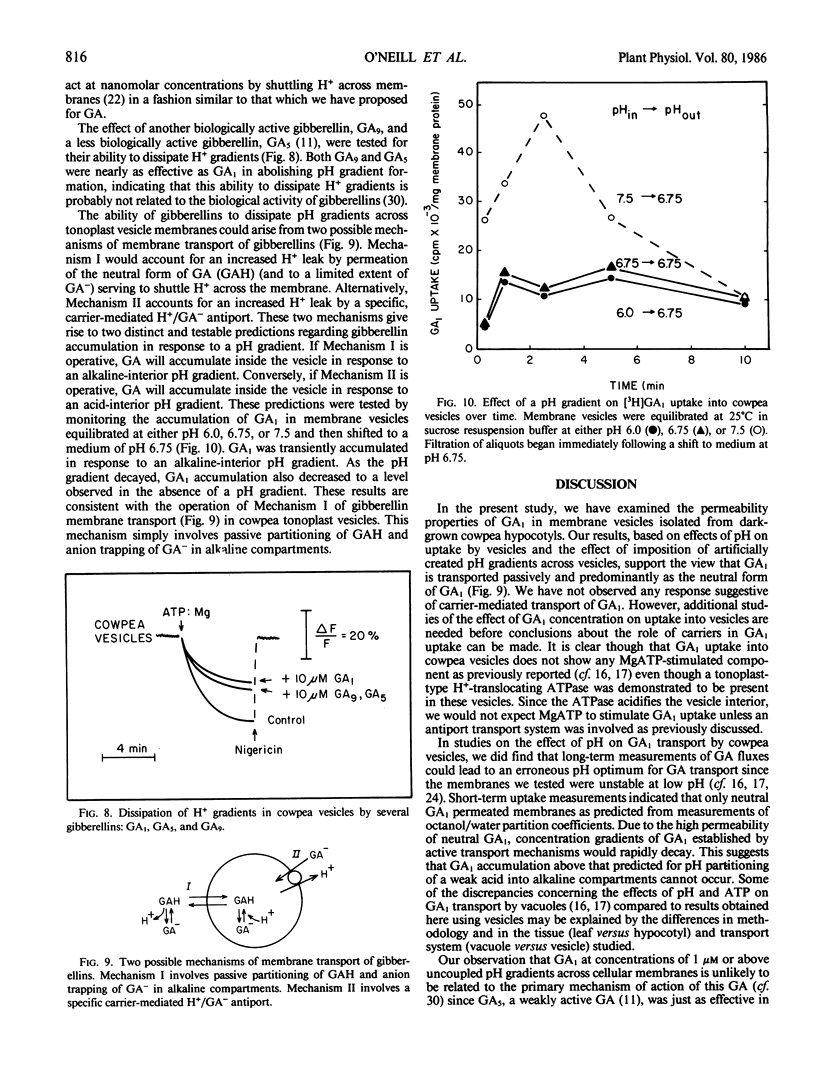
The permeability properties of gibberellin A1 (GA1) were examined in membrane vesicles isolated from cowpea hypocotyls. The rate of GA1 uptake was progressively greater as pH decreased, indicating that the neutral molecule is more permeable than anionic GA1. Membrane vesicles used in this study possessed a tonoplast-type H+-translocating ATPase as assayed by MgATP-dependent quenching of acridine orange fluorescence and methylamine uptake. However, GA1 uptake was not stimulated by MgATP. At concentrations in excess of 1 micromolar, GA1, GA5, and GA, collapsed both MgATP-generated and artifically imposed pH gradients, apparently by shuttling H+ across the membrane as neutral GA. The relatively high permeability of neutral GA and the potentially detrimental effects of GA in uncoupling pH gradients across intracellular membranes supports the view that GA1 accumulation and compartmentation must occur by conversion of GA1 to more polar metabolites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daie J., Wyse R. ABA Uptake in Source and Sink Tissues of Sugar Beet. Plant Physiol. 1983 Jun;72(2):430–433. doi: 10.1104/pp.72.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martinez J. L. Differential compartmentation of gibberellin a(1) and its metabolites in vacuoles of cowpea and barley leaves. Plant Physiol. 1981 Oct;68(4):865–867. doi: 10.1104/pp.68.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- O'neill S. D., Bennett A. B., Spanswick R. M. Characterization of a NO(3)-Sensitive H-ATPase from Corn Roots. Plant Physiol. 1983 Jul;72(3):837–846. doi: 10.1104/pp.72.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., García-Martínez J. L., Adams D., Rappaport L. Uptake and subcellular compartmentation of gibberellin a(1) applied to leaves of barley and cowpea. Plant Physiol. 1980 Sep;66(3):422–427. doi: 10.1104/pp.66.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Determination of pH in chloroplasts. I. Distribution of ( 14 C) methylamine. Eur J Biochem. 1972 Jan 31;25(1):54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Tyree M. T. A simple theory regarding ambimobility of xenobiotics with special reference to the nematicide, oxamyl. Plant Physiol. 1979 Feb;63(2):367–374. doi: 10.1104/pp.63.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A., Paleg L. G. The influence of gibberellic Acid on the permeability of model membrane systems. Plant Physiol. 1972 Jul;50(1):103–108. doi: 10.1104/pp.50.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]


