Abstract
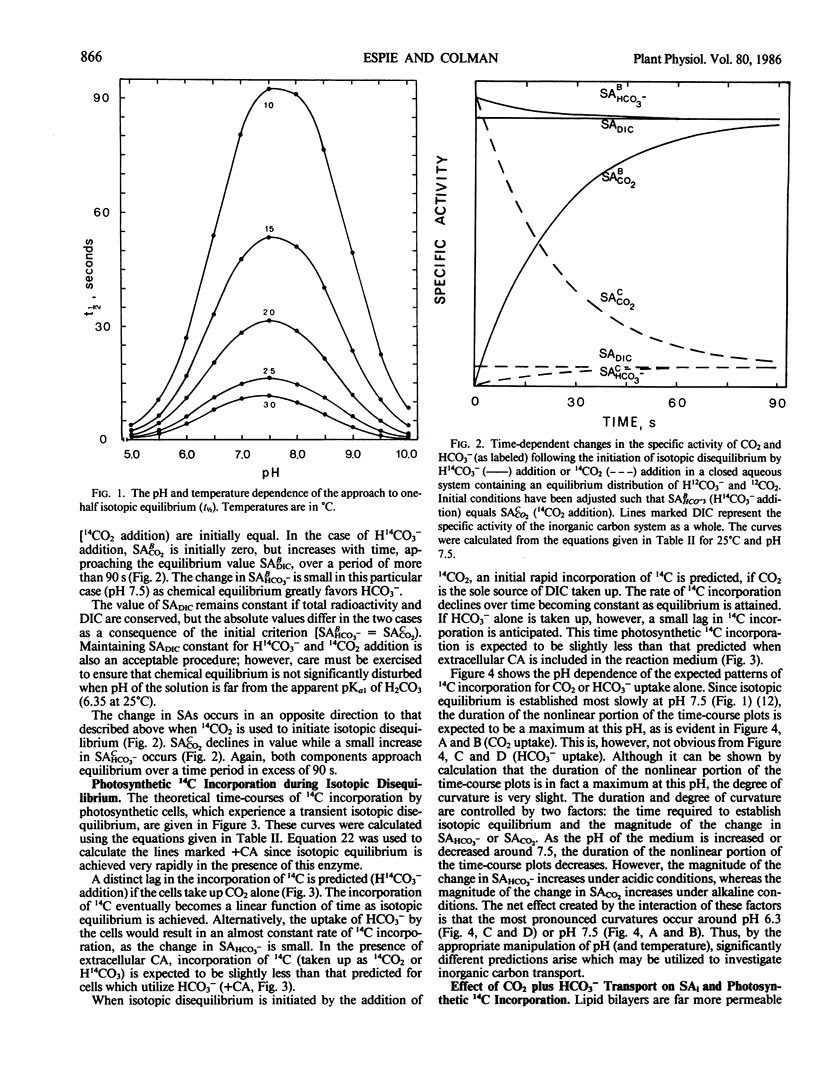
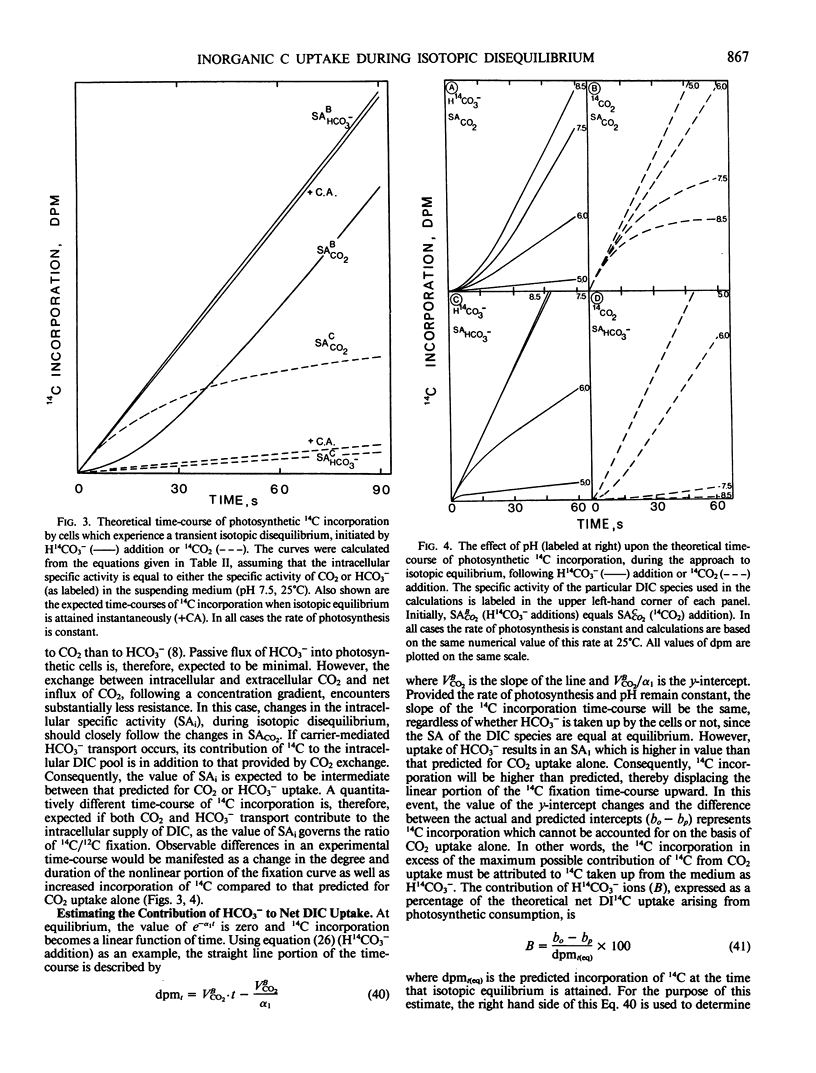
Equations have been developed which quantitatively predict the theoretical time-course of photosynthetic 14C incorporation when CO2 or HCO3− serves as the sole source of exogenous inorganic carbon taken up for fixation by cells during steady state photosynthesis. Comparison between the shape of theoretical (CO2 or HCO3−) and experimentally derived time-courses of 14C incorporation permits the identification of the major species of inorganic carbon which crosses the plasmalemma of photosynthetic cells and facilitates the detection of any combined contribution of CO2 and HCO3− transport to the supply of intracellular inorganic carbon. The ability to discriminate between CO2 or HCO3− uptake relies upon monitoring changes in the intracellular specific activity (by 14C fixation) which occur when the inorganic carbon, present in the suspending medium, is in a state of isotopic disequilibrium (JT Lehman 1978 J Phycol 14: 33-42). The presence of intracellular carbonic anhydrase or some other catalyst of the CO2-HCO3− interconversion reaction is required for quantitatively accurate predictions. Analysis of equations describing the rate of 14C incorporation provides two methods by which any contribution of HCO3− ions to net photosynthetic carbon uptake can be estimated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Colman B. Photosynthesis and inorganic carbon transport in isolated asparagus mesophyll cells. Plant Physiol. 1982 Sep;70(3):649–654. doi: 10.1104/pp.70.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmer D. L., Ooper T. G. Effect of varying temperature and pH upon the predicted rate of "CO2" utilization by carboxylases. J Theor Biol. 1970 Oct;29(1):131–145. doi: 10.1016/0022-5193(70)90122-0. [DOI] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Evidence for HCO(3) Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980 Feb;65(2):397–402. doi: 10.1104/pp.65.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Kaplan A., Reinhold L. Nature of the rate-limiting step in the supply of inorganic carbon for photosynthesis in isolated asparagus mesophyll cells. Plant Physiol. 1983 Jul;72(3):886–890. doi: 10.1104/pp.72.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]


