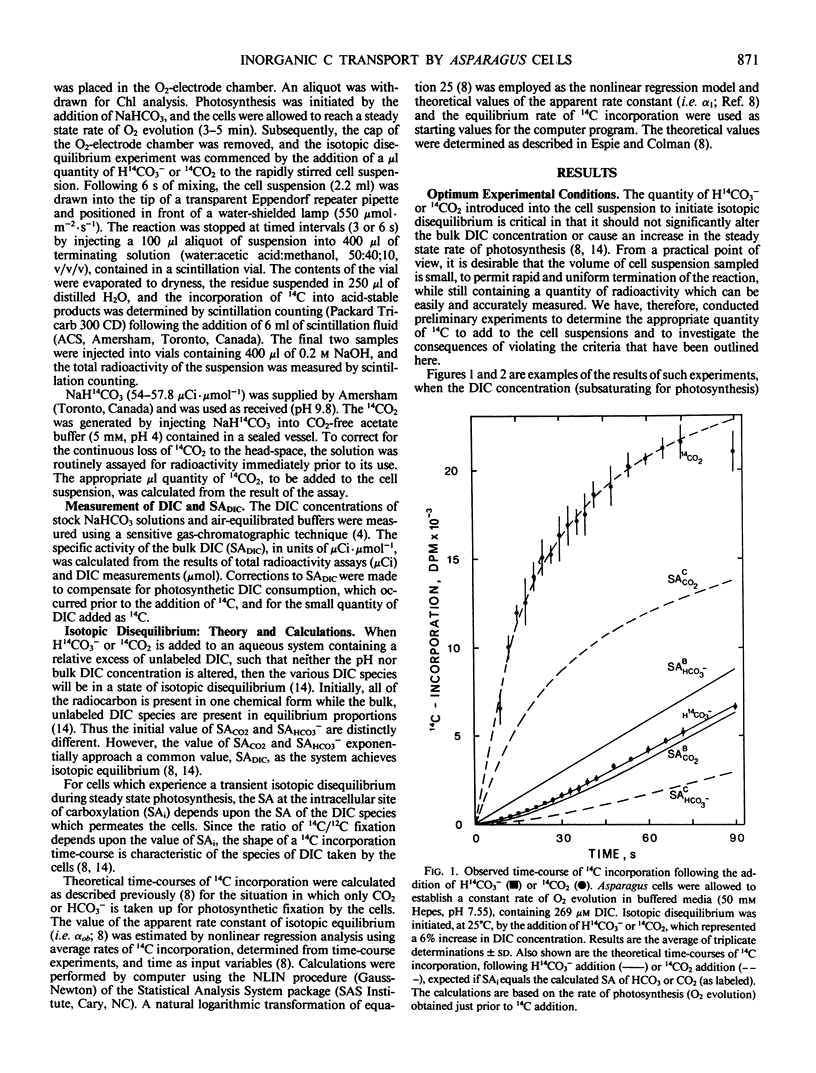
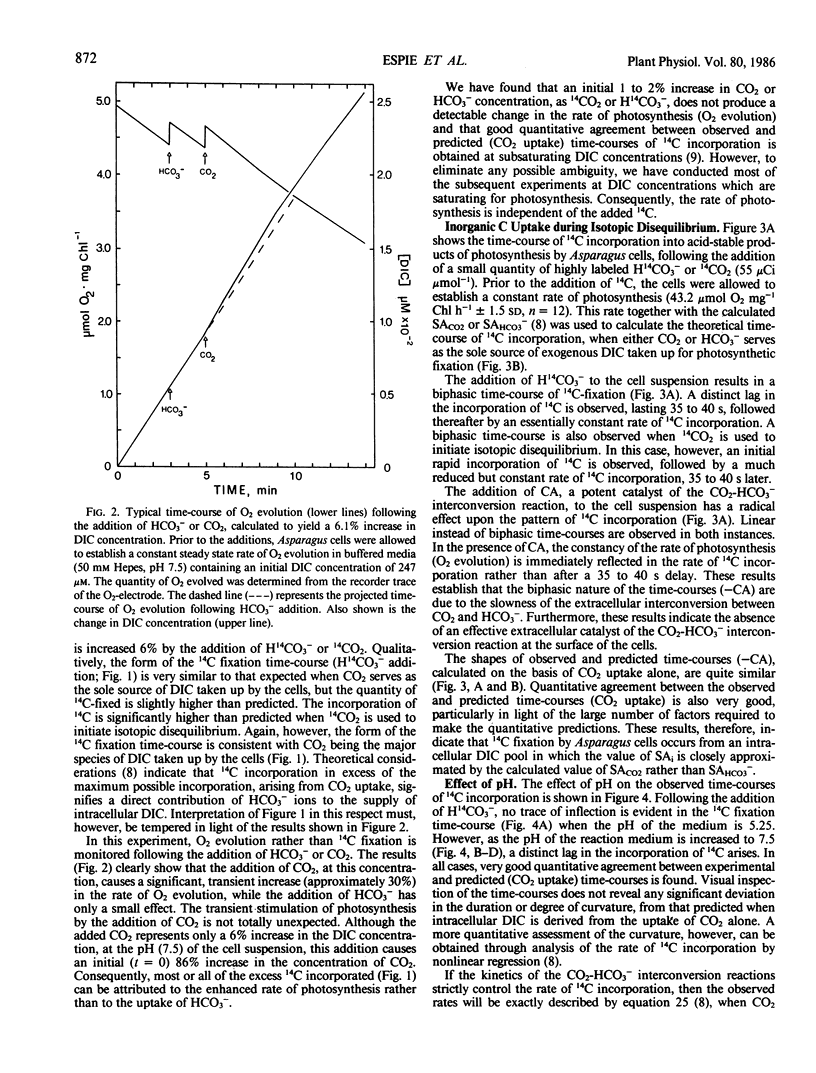
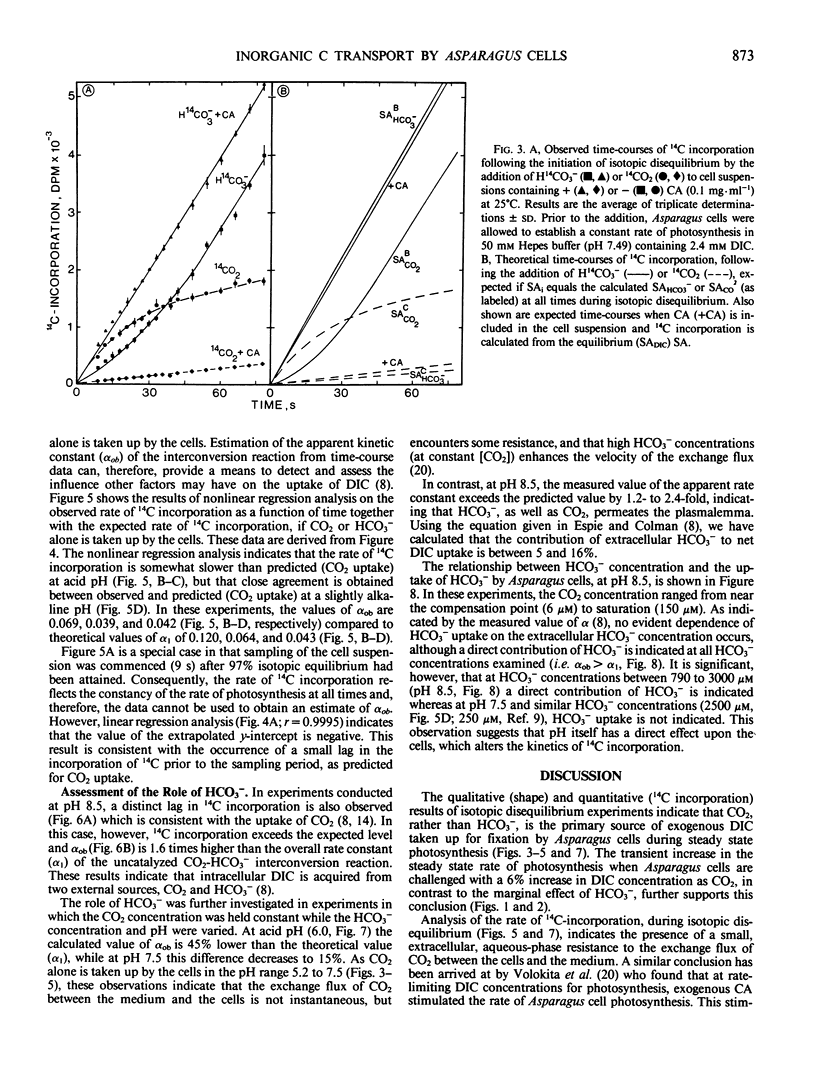
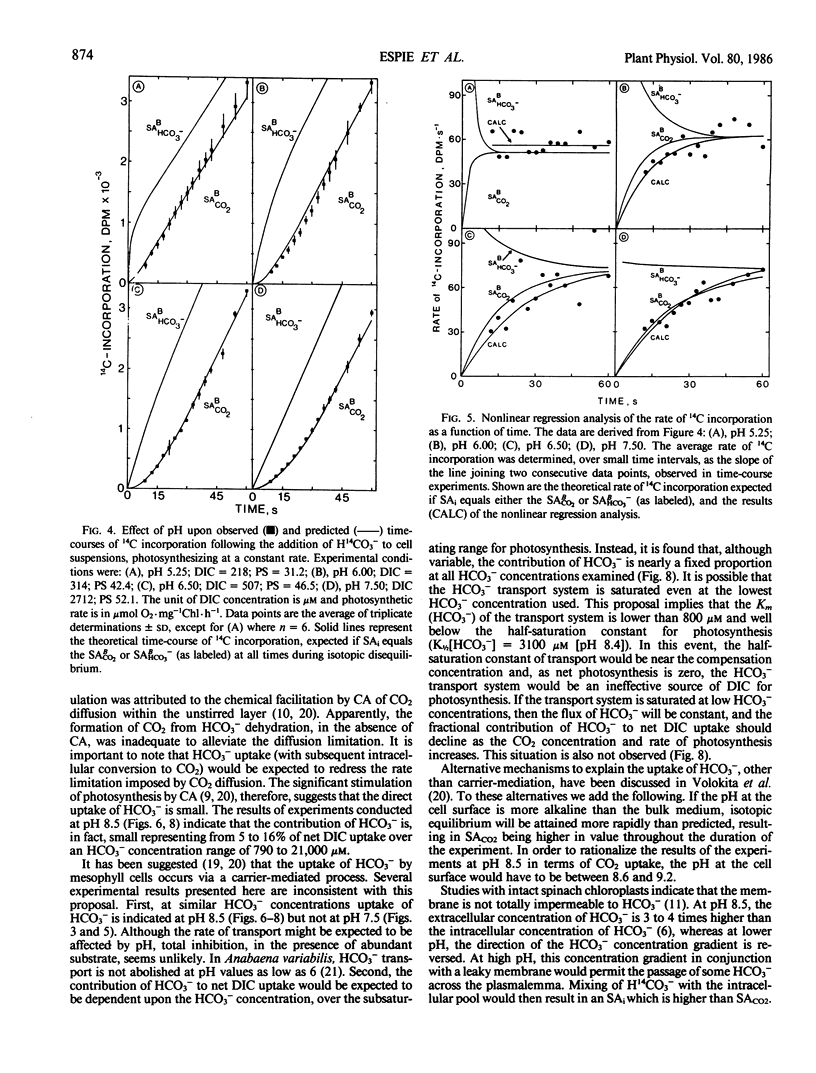
Abstract
The species of inorganic carbon (CO2 or HCO3−) taken up a source of substrate for photosynthetic fixation by isolated Asparagus sprengeri mesophyll cells is investigated. Discrimination between CO2 or HCO3− transport, during steady state photosynthesis, is achieved by monitoring the changes (by 14C fixation) which occur in the specific activity of the intracellular pool of inorganic carbon when the inorganic carbon present in the suspending medium is in a state of isotopic disequilibrium. Quantitative comparisons between theoretical (CO2 or HCO3− transport) and experimental time-courses of 14C incorporation, over the pH range of 5.2 to 7.5, indicate that the specific activity of extracellular CO2, rather than HCO3−, is the appropriate predictor of the intracellular specific activity. It is concluded, therefore, that CO2 is the major source of exogenous inorganic carbon taken up by Asparagus cells. However, at high pH (8.5), a component of net DIC uptake may be attributable to HCO3− transport, as the incorporation of 14C during isotopic disequilibrium exceeds the maximum possible incorporation predicted on the basis of CO2 uptake alone. The contribution of HCO3− to net inorganic carbon uptake (pH 8.5) is variable, ranging from 5 to 16%, but is independent of the extracellular HCO3− concentration. The evidence for direct HCO3− transport is subject to alternative explanations and must, therefore, be regarded as equivocal. Nonlinear regression analysis of the rate of 14C incorporation as a function of time indicates the presence of a small extracellular resistance to the diffusion of CO2, which is partially alleviated by a high extracellular concentration of HCO3−.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham B. C., Colman B. Measurement of carbon dioxide compensation points of freshwater algae. Plant Physiol. 1979 Nov;64(5):892–895. doi: 10.1104/pp.64.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Colman B. Photosynthesis and inorganic carbon transport in isolated asparagus mesophyll cells. Plant Physiol. 1982 Sep;70(3):649–654. doi: 10.1104/pp.70.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J., Bisson M. A., Tosteson F. C. Diffusion of carbon dioxide through lipid bilayer membranes: effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol. 1977 Jun;69(6):779–794. doi: 10.1085/jgp.69.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Evidence for HCO(3) Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980 Feb;65(2):397–402. doi: 10.1104/pp.65.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Kaplan A., Reinhold L. Evidence for Mediated HCO(3) Transport in Isolated Pea Mesophyll Protoplasts. Plant Physiol. 1981 Jun;67(6):1119–1123. doi: 10.1104/pp.67.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Kaplan A., Reinhold L. Nature of the rate-limiting step in the supply of inorganic carbon for photosynthesis in isolated asparagus mesophyll cells. Plant Physiol. 1983 Jul;72(3):886–890. doi: 10.1104/pp.72.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]


