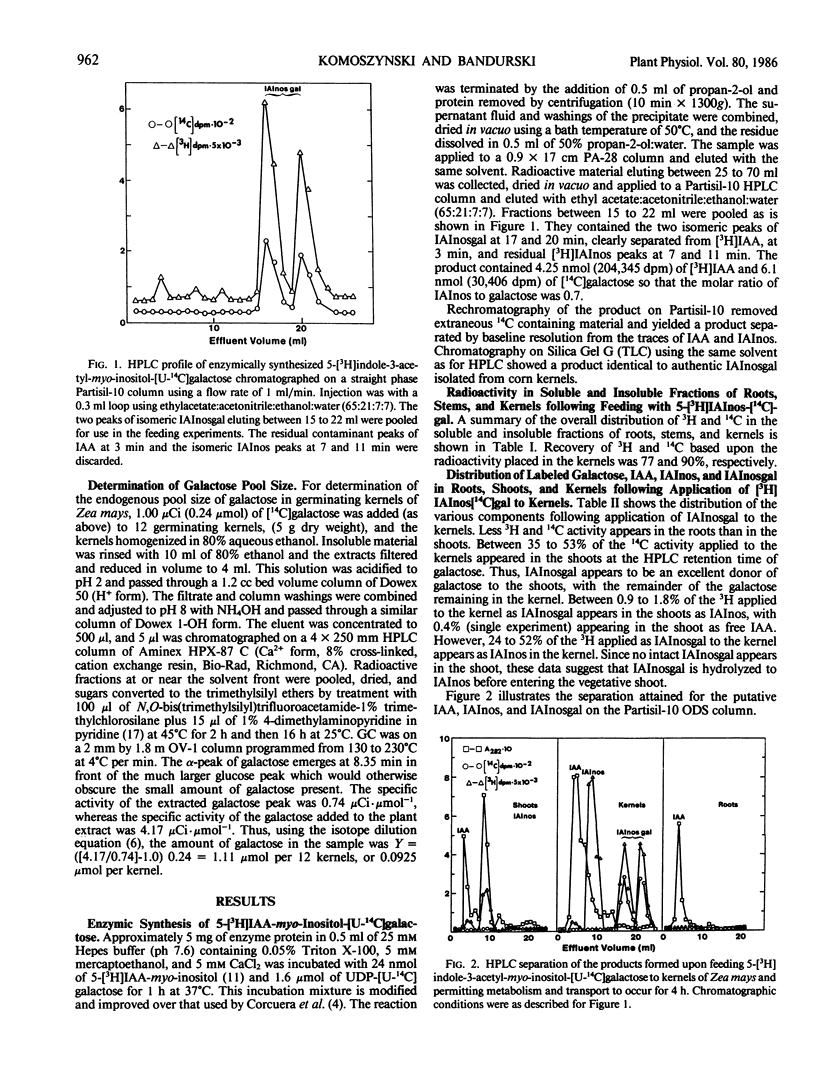
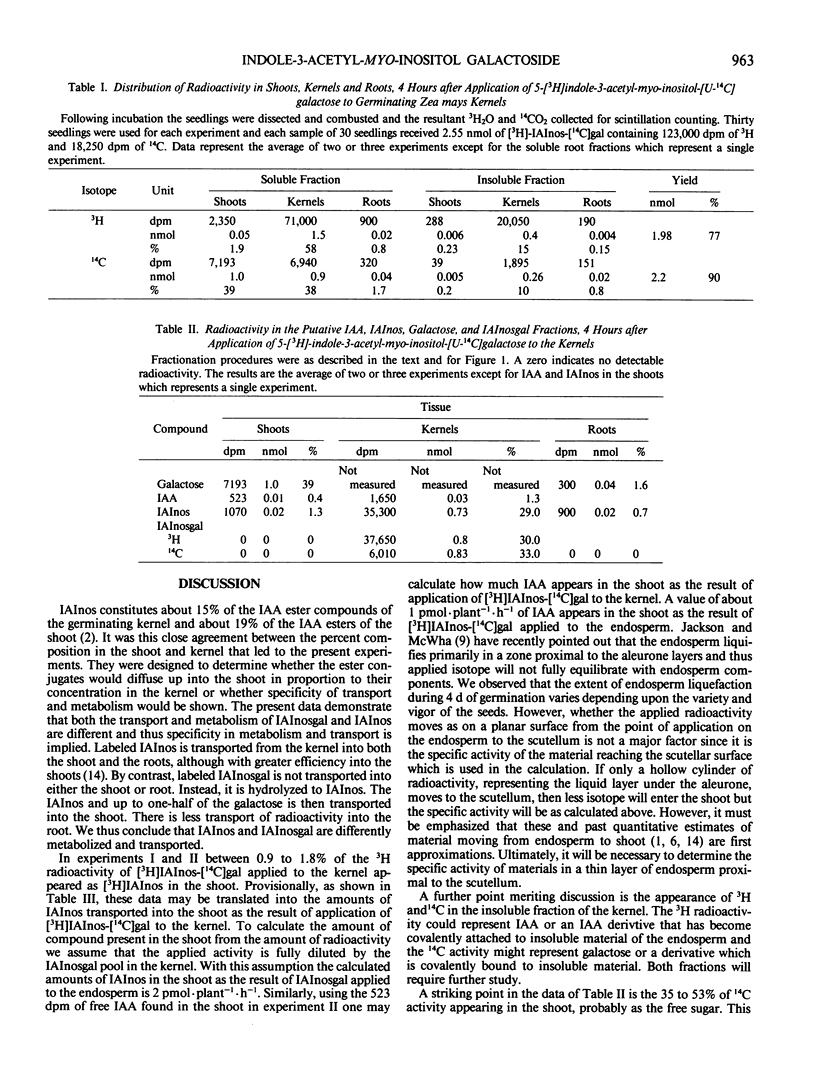
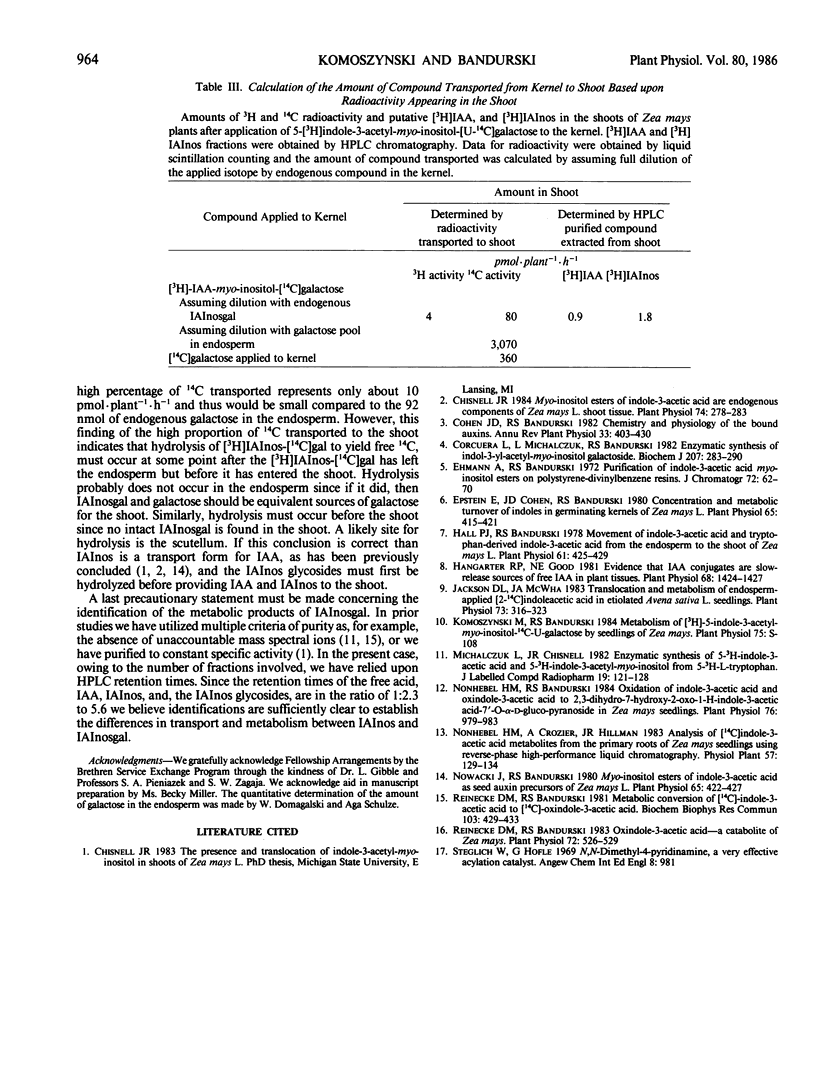
Abstract
Indole-3-acetyl-myo-inositol galactoside labeled with 3H in the indole and 14C in the galactose moieties was applied to kernels of 5 day old germinating seedlings of Zea mays. Indole-3-acetyl-myo-inositol galactoside was not transported into either the shoot or root tissue as the intact molecule but was instead hydrolyzed to yield [3H]indole-3-acetyl-myo-inositol and [3H]indole-3-acetic acid which were then transported to the shoot with little radioactivity going to the root. With certain assumptions concerning the equilibration of applied [3H]indole-3-acetyl-myo-inositol-[U-14C]galactose with the endogenous pool, it may be concluded that indole-3-acetyl-myo-inositol galactoside in the endosperm supplies about 2 picomoles per plant per hour of indole-3-acetyl-myo-inositol and 1 picomole per plant per hour of indole-3-acetic acid to the shoot and thus is comparable to indole-3-acetyl-myo-inositol as a source of indole-acetic acid for the shoot. Quantitative estimates of the amount of galactose in the kernels suggest that [3H]indole-3-acetyl-myo-inositol-[14C] galactose is hydrolyzed after the compound leaves the endosperm but before it reaches the shoot. In addition, [3H]indole-3-acetyl-myo-inositol-[14C]galactose supplies appreciable amounts of 14C to the shoot and both 14C and 3H to an uncharacterized insoluble fraction of the endosperm.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chisnell J. R. Myo-inositol esters of indole-3-acetic acid are endogenous components of Zea mays L. shoot tissue. Plant Physiol. 1984;74:278–283. doi: 10.1104/pp.74.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcuera L. J., Michalczuk L., Bandurski R. S. Enzymic synthesis of indol-3-ylacetyl-myo-inositol galactoside. Biochem J. 1982 Nov 1;207(2):283–290. doi: 10.1042/bj2070283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Cohen J. D., Bandurski R. S. Concentration and Metabolic Turnover of Indoles in Germinating Kernels of Zea mays L. Plant Physiol. 1980 Mar;65(3):415–421. doi: 10.1104/pp.65.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. L., Bandurski R. S. Movement of Indole-3-acetic Acid and Tryptophan-derived Indole-3-acetic Acid from the Endosperm to the Shoot of Zea mays L. Plant Physiol. 1978 Mar;61(3):425–429. doi: 10.1104/pp.61.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter R. P., Good N. E. Evidence That IAA Conjugates Are Slow-Release Sources of Free IAA in Plant Tissues. Plant Physiol. 1981 Dec;68(6):1424–1427. doi: 10.1104/pp.68.6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. L., McWha J. A. Translocation and Metabolism of Endosperm-Applied [2-C] Indoleacetic Acid in Etiolated Avena sativa L. Seedlings. Plant Physiol. 1983 Oct;73(2):316–323. doi: 10.1104/pp.73.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momonoki Y. S., Schulze A., Bandurski R. S. Effect of Deseeding on the Indole-3-acetic Acid Content of Shoots and Roots of Zea mays Seedlings. Plant Physiol. 1983 Jun;72(2):526–529. doi: 10.1104/pp.72.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonhebel H. M., Bandurski R. S. Oxidation of indole-3-acetic acid and oxindole-3-acetic acid to 2,3-dihydro-7-hydroxy-2-oxo-1H indole-3-acetic acid-7'-O-beta-D-glucopyranoside in Zea mays seedlings. Plant Physiol. 1984;76:979–983. doi: 10.1104/pp.76.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacki J., Bandurski R. S. Myo-Inositol Esters of Indole-3-acetic Acid as Seed Auxin Precursors of Zea mays L. Plant Physiol. 1980 Mar;65(3):422–427. doi: 10.1104/pp.65.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke D. M., Bandurski R. S. Metabolic conversion of 14C-indole-3-acetic acid to 14C-oxindole-3-acetic acid. Biochem Biophys Res Commun. 1981 Nov 30;103(2):429–433. doi: 10.1016/0006-291x(81)90470-8. [DOI] [PubMed] [Google Scholar]


