Abstract
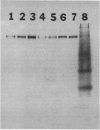
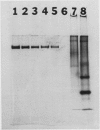
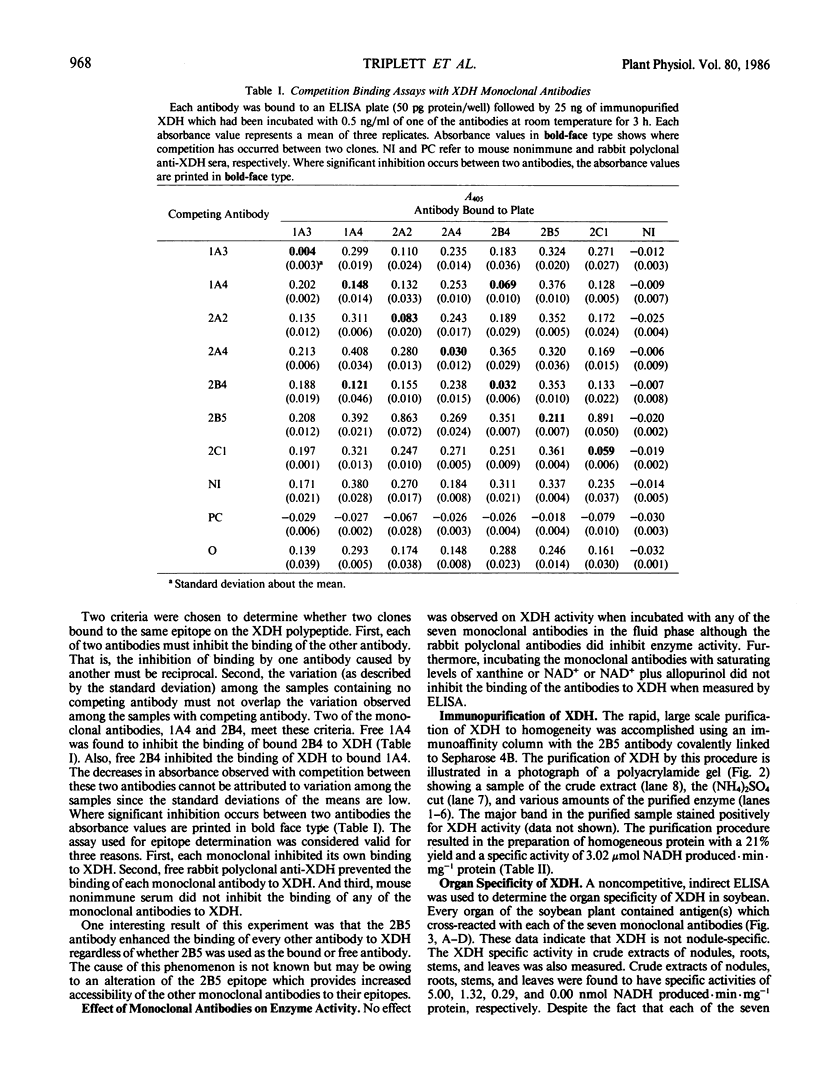
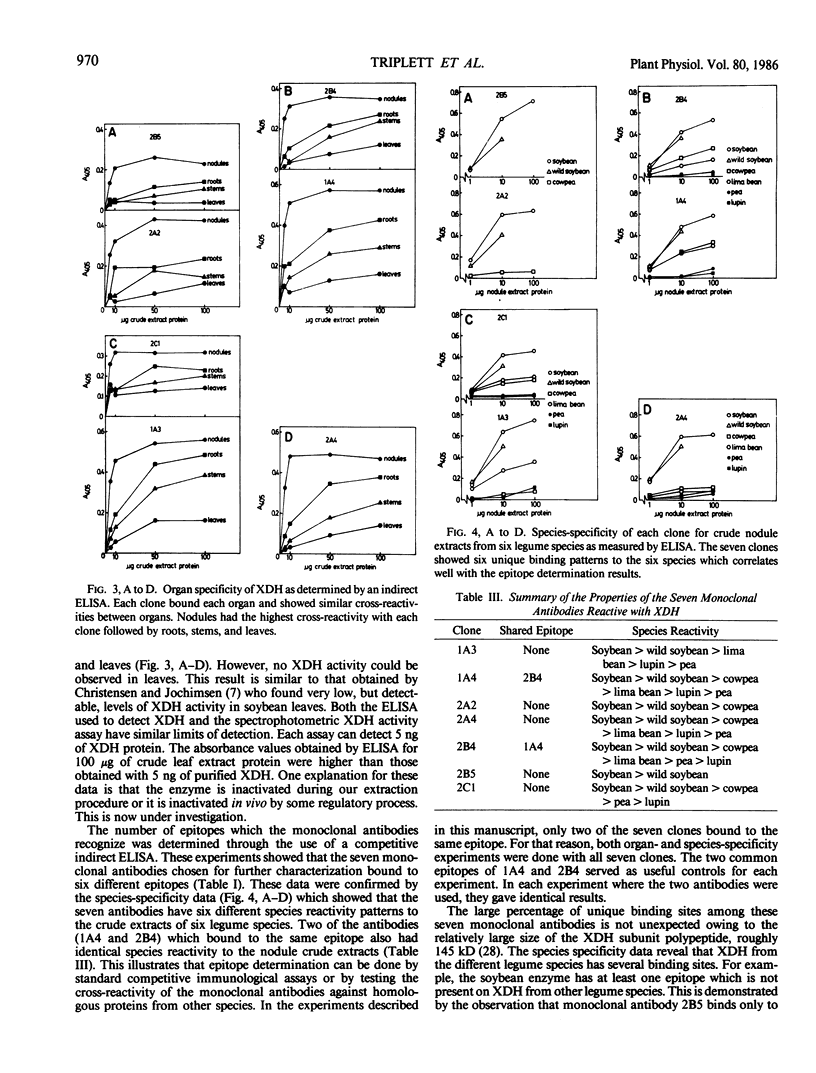
Seven monoclonal antibodies were produced against soybean nodule xanthine dehydrogenase, an enzyme involved in ureide synthesis. Specificity of the seven monoclonal antibodies for xanthine dehydrogenase was demonstrated by immunopurifying the enzyme to homogeneity from a crude nodule extract using antibodies immobilized to Sepharose 4B beads. Each monoclonal antibody was covalently bound to Sepharose 4B beads for the preparation of immunoaffinity columns for each antibody. All seven antibodies were found to be of the IgG1,K subclass. A competitive, indirect enzyme-linked immunosorbent assay demonstrated that two of the seven antibodies shared a common epitope while the remaining five antibodies defined unique determinants on the protein. Rapid, large scale purification of active xanthine dehydrogenase to homogeneity was performed by immunoaffinity chromatography. The presence of xanthine dehydrogenase activity and protein in every organ of the soybean plant was determined. Crude extracts of nodules, roots, stems, and leaves cross-reacted with all seven monoclonal antibodies in an indirect enzyme-linked immunosorbent assay. A positive correlation was observed between the degree of cross-reactivity of a given organ and the level of enzyme activity in that organ. These data demonstrate that xanthine dehydrogenase is not nodule specific. Antigenic variability of xanthine dehydrogenase present in crude extracts from nodules of soybean, wild soybean, cowpea, lima bean, pea, and lupin were detected in the indirect enzyme-linked immunosorbent assay which corresponded to six binding patterns for xanthine dehydrogenase from these plant species. These results correspond well with the epitope determination data which showed that the seven antibodies bind to six different binding determinants on the enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland M. J., Blevins D. G., Randall D. D. Soybean nodule xanthine dehydrogenase: a kinetic study. Arch Biochem Biophys. 1983 Apr 15;222(2):435–441. doi: 10.1016/0003-9861(83)90542-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brewin N. J., Robertson J. G., Wood E. A., Wells B., Larkins A. P., Galfre G., Butcher G. W. Monoclonal antibodies to antigens in the peribacteroid membrane from Rhizobium-induced root nodules of pea cross-react with plasma membranes and Golgi bodies. EMBO J. 1985 Mar;4(3):605–611. doi: 10.1002/j.1460-2075.1985.tb03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherel I., Grosclaude J., Rouze P. Monoclonal antibodies identify multiple epitopes on maize leaf nitrate reductase. Biochem Biophys Res Commun. 1985 Jun 28;129(3):686–693. doi: 10.1016/0006-291X(85)91946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T. M., Jochimsen B. U. Enzymes of ureide synthesis in pea and soybean. Plant Physiol. 1983 May;72(1):56–59. doi: 10.1104/pp.72.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Andrews J., Mersey B., Newcomb E. H., Keegstra K. Separation and characterization of inner and outer envelope membranes of pea chloroplasts. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3595–3599. doi: 10.1073/pnas.78.6.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier M. M., Greppin H., Pratt L. H. Characterization by enzyme-linked immunosorbent assay of monoclonal antibodies to pisum and Avena phytochrome. Plant Physiol. 1984 Jan;74(1):123–127. doi: 10.1104/pp.74.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels S. M., Quail P. H. Monoclonal antibodies to three separate domains on 124 kilodalton phytochrome from Avena. Plant Physiol. 1984 Nov;76(3):622–626. doi: 10.1104/pp.76.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Schubert K., Tolbert N. E. Isolation and characterization of infected and uninfected cells from soybean nodules : role of uninfected cells in ureide synthesis. Plant Physiol. 1983 Apr;71(4):869–873. doi: 10.1104/pp.71.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie S. Subunit constitution of electrophoretically purified xanthine dehydrogenase of avian liver. J Biochem. 1984 Feb;95(2):405–412. doi: 10.1093/oxfordjournals.jbchem.a134621. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Gilbert S. F. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science. 1983 Jun 17;220(4603):1297–1300. doi: 10.1126/science.220.4603.1297. [DOI] [PubMed] [Google Scholar]
- Kaetzel C. S., Mather I. H., Bruder G., Madara P. J. Characterization of a monoclonal antibody to bovine xanthine oxidase. Biochem J. 1984 May 1;219(3):917–925. doi: 10.1042/bj2190917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Kolb L. M., Podack E. R. C1q: isolation from human serum in high yield by affinity chromatography and development of a highly sensitive hemolytic assay. J Immunol. 1979 May;122(5):2103–2111. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liedgens W., Grützmann R., Schneider H. A. Highly efficient purificaton of the labile plant enzyme 5-aminolevulinate dehydratase (EC 4.2.1.24) by means of monoclonal antibodies. Z Naturforsch C. 1980 Nov-Dec;35(11-12):958–962. doi: 10.1515/znc-1980-11-1215. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Mather I. H., Nace C. S., Johnson V. G., Goldsby R. A. Preparation of monoclonal antibodies to xanthine oxidase and other proteins of bovine milk-fat-globule membrane. Biochem J. 1980 Jun 15;188(3):925–928. doi: 10.1042/bj1880925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb E. H., Tandon S. R. Uninfected cells of soybean root nodules: ultrastructure suggests key role in ureide production. Science. 1981 Jun 19;212(4501):1394–1396. doi: 10.1126/science.212.4501.1394. [DOI] [PubMed] [Google Scholar]
- Silberman L. G., Datta N., Hoops P., Roux S. J. Characterization of monoclonal antibodies to oat phytochrome by competitive radioimmunoassays and comparative immunoblots of phytochrome peptides. Arch Biochem Biophys. 1985 Jan;236(1):150–158. doi: 10.1016/0003-9861(85)90614-9. [DOI] [PubMed] [Google Scholar]
- Streeter J. G. Allantoin and Allantoic Acid in Tissues and Stem Exudate from Field-grown Soybean Plants. Plant Physiol. 1979 Mar;63(3):478–480. doi: 10.1104/pp.63.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Blevins D. G., Randall D. D. Allantoic Acid Synthesis in Soybean Root Nodule Cytosol via Xanthine Dehydrogenase. Plant Physiol. 1980 Jun;65(6):1203–1206. doi: 10.1104/pp.65.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Blevins D. G., Randall D. D. Purification and properties of soybean nodule xanthine dehydrogenase. Arch Biochem Biophys. 1982 Nov;219(1):39–46. doi: 10.1016/0003-9861(82)90131-x. [DOI] [PubMed] [Google Scholar]
- Triplett E. W., Heitholt J. J., Evensen K. B., Blevins D. G. Increase in Internode Length of Phaseolus lunatus L. Caused by Inoculation with a Nitrate Reductase-deficient Strain of Rhizobium sp. Plant Physiol. 1981 Jan;67(1):1–4. doi: 10.1104/pp.67.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W. Intercellular nodule localization and nodule specificity of xanthine dehydrogenase in soybean. Plant Physiol. 1985 Apr;77(4):1004–1009. doi: 10.1104/pp.77.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora S. Monoclonal antibodies in enzyme research: present and potential applications. Anal Biochem. 1985 Feb 1;144(2):307–318. doi: 10.1016/0003-2697(85)90123-x. [DOI] [PubMed] [Google Scholar]
- Ware C. F., Reade J. L., Der L. C. A rat anti-mouse kappa chain specific monoclonal antibody, 187.1.10: purification, immunochemical properties and its utility as a general second-antibody reagent. J Immunol Methods. 1984 Nov 16;74(1):93–104. doi: 10.1016/0022-1759(84)90371-5. [DOI] [PubMed] [Google Scholar]
- Weeden N. F., Higgins R. C., Gottlieb L. D. Immunological similarity between a cyanobacterial enzyme and a nuclear DNA-encoded plastid-specific isozyme from spinach. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5953–5955. doi: 10.1073/pnas.79.19.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]