Abstract
The enzyme aldose (aldehyde) reductase was partially purified (142-fold) and characterized from Euonymus japonica leaves. The reductase, a dimer, had an average molecular weight of 67,000 as determined by gel filtration on Sephadex G-100. The enzyme was NADPH specific and reduced a broad range of substrates including aldoses, aliphatic aldehydes, and aromatic aldehydes. Maximum activity was observed at pH 8 in phosphate and Tris-HCl buffers and at pH 8.6 to 9.0 in glycine-NaOH buffer using dl-glyceraldehyde or 3-pyridinecarboxaldehyde as substrate. NADP was a competitive inhibitor with respect to NADPH with a Ki of 60 micromolar. Glycerol was an uncompetitive inhibitor to dl-glyceraldehyde (K′i = 460 millimolar). The Euonymus enzyme was inhibited by sulfhydryl inhibitor, phenobarbital, and high concentrations of Li2SO4. Pyrazol and metal chelating agents inhibited the enzyme slightly. Enzyme activity was detected in the leaves and berries of Celastrus orbiculatus and several species of Euonymus. Probable function of this enzyme is to reduce d-galactose to galactitol, a characteristic metabolite in phloem sap of members of the Celastraceae family.
Full text
PDF

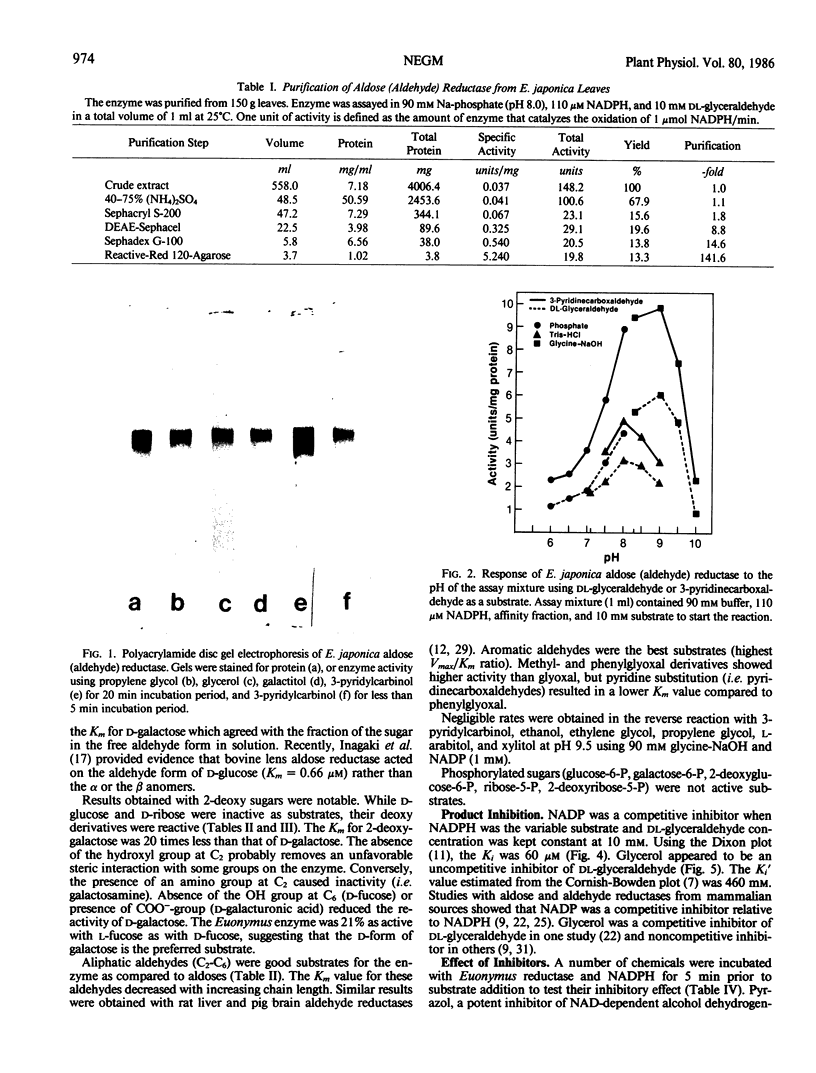
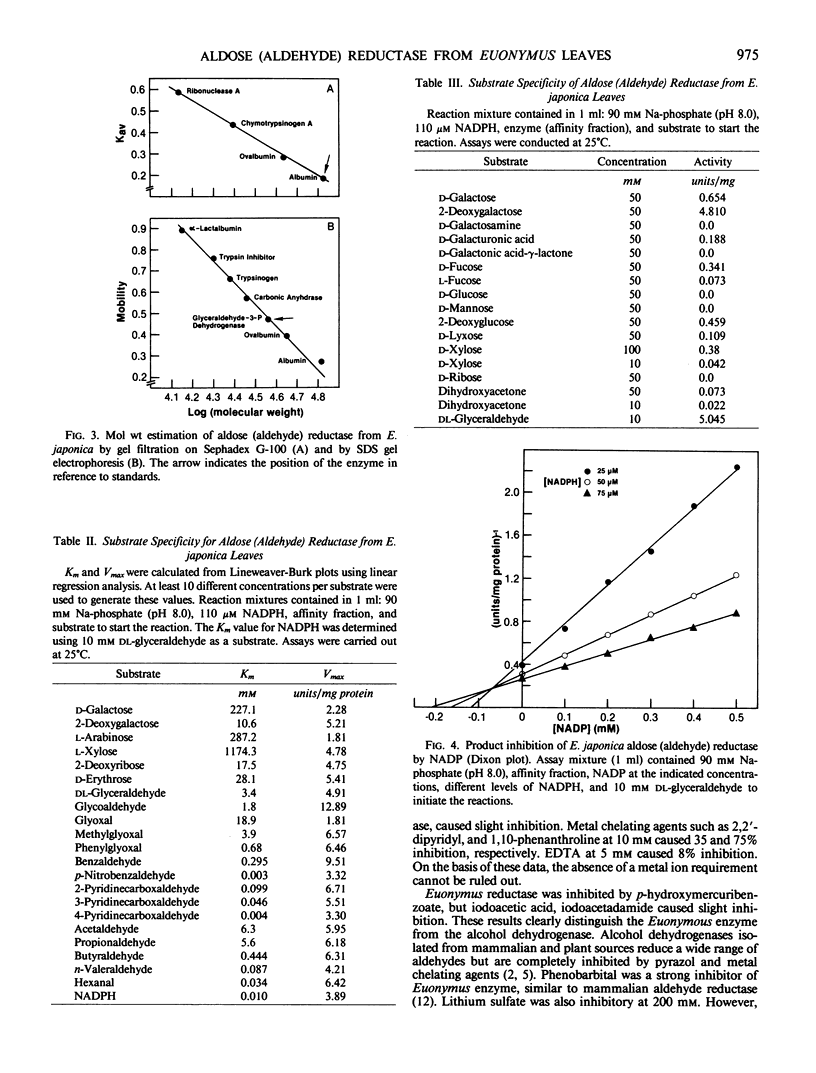
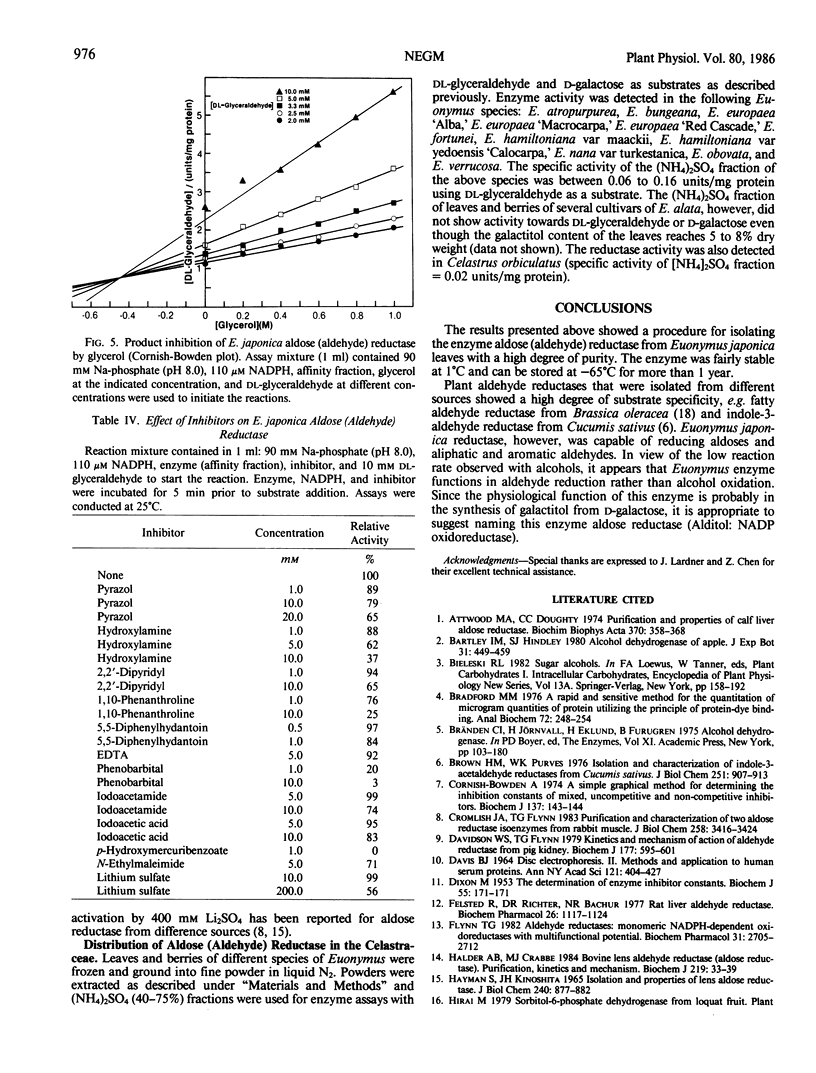

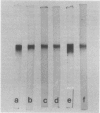
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwood M. A., Doughty C. C. Purification and properties of calf liver aldose reductase. Biochim Biophys Acta. 1974 Dec 29;370(2):358–368. doi: 10.1016/0005-2744(74)90097-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown H. M., Purves W. K. Isolation and characterization of indole-3-acetaldehyde reductases from Cucumis sativus. J Biol Chem. 1976 Feb 25;251(4):907–913. [PubMed] [Google Scholar]
- Cornish-Bowden A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J. 1974 Jan;137(1):143–144. doi: 10.1042/bj1370143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromlish J. A., Flynn T. G. Purification and characterization of two aldose reductase isoenzymes from rabbit muscle. J Biol Chem. 1983 Mar 10;258(5):3416–3424. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W. S., Flynn T. G. Kinetics and mechanism of action of aldehyde reductase from pig kidney. Biochem J. 1979 Feb 1;177(2):595–601. doi: 10.1042/bj1770595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsted R. L., Richter D. R., Bachur N. R. Rat liver aldehyde reductase. Biochem Pharmacol. 1977 Jun 15;26(12):1117–1124. doi: 10.1016/0006-2952(77)90054-5. [DOI] [PubMed] [Google Scholar]
- Flynn T. G. Aldehyde reductases: monomeric NADPH-dependent oxidoreductases with multifunctional potential. Biochem Pharmacol. 1982 Sep 1;31(17):2705–2712. doi: 10.1016/0006-2952(82)90123-x. [DOI] [PubMed] [Google Scholar]
- HAYMAN S., KINOSHITA J. H. ISOLATION AND PROPERTIES OF LENS ALDOSE REDUCTASE. J Biol Chem. 1965 Feb;240:877–882. [PubMed] [Google Scholar]
- Halder A. B., Crabbe M. J. Bovine lens aldehyde reductase (aldose reductase). Purification, kinetics and mechanism. Biochem J. 1984 Apr 1;219(1):33–39. doi: 10.1042/bj2190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K., Miwa I., Okuda J. Affinity purification and glucose specificity of aldose reductase from bovine lens. Arch Biochem Biophys. 1982 Jun;216(1):337–344. doi: 10.1016/0003-9861(82)90219-3. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Enzymatic synthesis of fatty alcohols in Brassica oleracea. Arch Biochem Biophys. 1971 Feb;142(2):701–709. doi: 10.1016/0003-9861(71)90536-4. [DOI] [PubMed] [Google Scholar]
- Negm F. B., Loescher W. H. Characterization and Partial Purification of Aldose-6-phosphate Reductase (Alditol-6-Phosphate:NADP 1-Oxidoreductase) from Apple Leaves. Plant Physiol. 1981 Jan;67(1):139–142. doi: 10.1104/pp.67.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negm F. B., Marlow G. C. Partial Purification and Characterization of d-Ribose-5-phosphate Reductase from Adonis vernalis L. Leaves. Plant Physiol. 1985 Aug;78(4):758–761. doi: 10.1104/pp.78.4.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien M. M., Schofield P. J. Polyol-pathway enzymes of human brain. Partial purification and properties of aldose reductase and hexonate dehydrogenase. Biochem J. 1980 Apr 1;187(1):21–30. doi: 10.1042/bj1870021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner A. H. Gel protein stains: a rapid procedure. Methods Enzymol. 1984;104:439–441. doi: 10.1016/s0076-6879(84)04110-0. [DOI] [PubMed] [Google Scholar]
- Rumpho M. E., Edwards G. E., Loescher W. H. A pathway for photosynthetic carbon flow to mannitol in celery leaves : activity and localization of key enzymes. Plant Physiol. 1983 Dec;73(4):869–873. doi: 10.1104/pp.73.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaff C. M., Doughty C. C. Physical and kinetic properties of homogenous bovine lens aldose reductase. J Biol Chem. 1976 May 10;251(9):2696–2702. [PubMed] [Google Scholar]
- Sheys G. H., Arnold W. J., Watson J. A., Hayashi J. A., Doughty C. C. Aldose reductase from Rhodotorula. I. Purification and properties. J Biol Chem. 1971 Jun 25;246(12):3824–3827. [PubMed] [Google Scholar]
- Thompson S. T., Cass K. H., Stellwagen E. Blue dextran-sepharose: an affinity column for the dinucleotide fold in proteins. Proc Natl Acad Sci U S A. 1975 Feb;72(2):669–672. doi: 10.1073/pnas.72.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Touster Resolution and partial characterization of two aldehyde reductases of mammalian liver. J Biol Chem. 1977 Apr 25;252(8):2545–2550. [PubMed] [Google Scholar]
- Turner A. J., Tipton K. F. The purification and properties of an NADPH-linked aldehyde reductase from pig brain. Eur J Biochem. 1972 Oct;30(2):361–368. doi: 10.1111/j.1432-1033.1972.tb02106.x. [DOI] [PubMed] [Google Scholar]
- Wermuth B., Bürgisser H., Bohren K., von Wartburg J. P. Purification and characterization of human-brain aldose reductase. Eur J Biochem. 1982 Oct;127(2):279–284. doi: 10.1111/j.1432-1033.1982.tb06867.x. [DOI] [PubMed] [Google Scholar]