Abstract
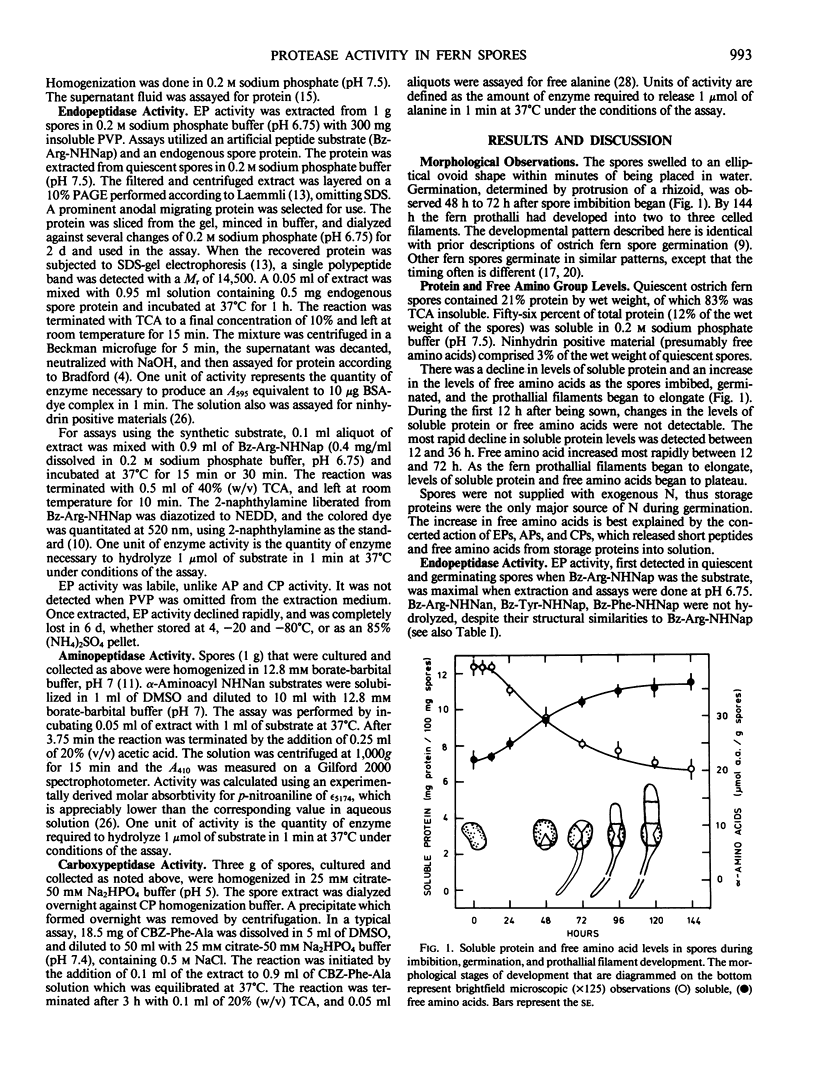
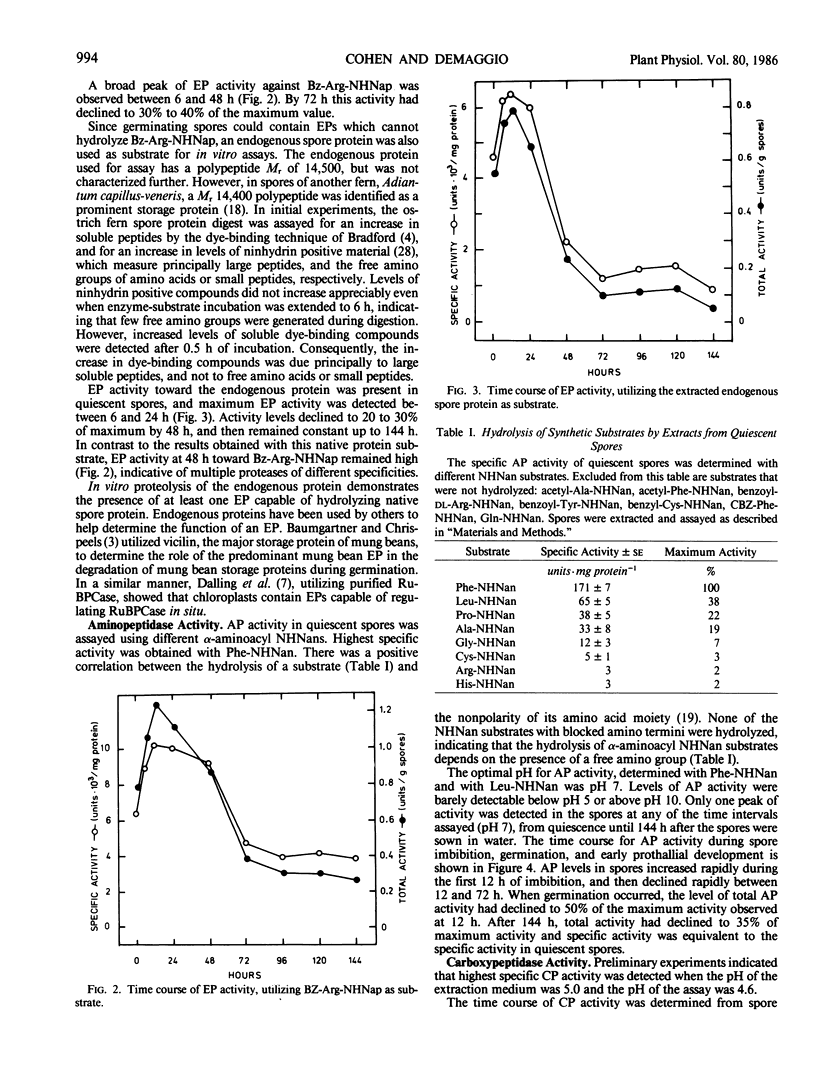
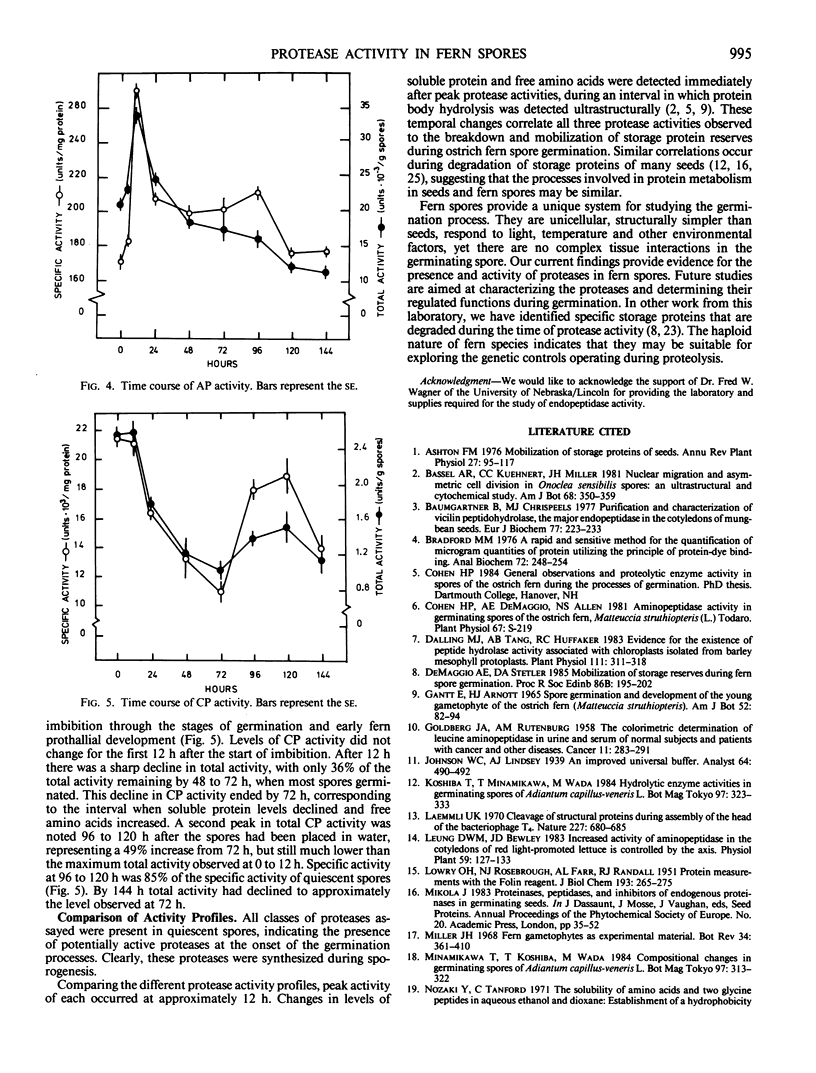
Protease activities were detected in quiescent and germinating spores of the ostrich fern (Matteuccia struthiopteris [L.] Todaro). Peak endopeptidase, aminopeptidase, and carboxypeptidase activities were detected 12 to 24 hours after spores began imbibing under light. There was a correlation between activities of proteases, the onset of a decline in levels of soluble protein, and an increase in levels of free amino acids. The earliest visible event of spore germination, breakage of the spore coat and protrusion of a rhizoid cell, was observed after peak protease activity, 48 to 72 hours after the start of imbibition. Results of this study demonstrate similarities in the pattern of protease activities during germination of ostrich fern spores to those of some seeds.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner B., Chrispeels M. J. Purification and characterization of vicilin peptidohydrolase, the major endopeptidase in the cotyledons of mung-bean seedlings. Eur J Biochem. 1977 Jul 15;77(2):223–233. doi: 10.1111/j.1432-1033.1977.tb11661.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- GANTT E., ARNOTT H. J. SPORE GERMINATION AND DEVELOPMENT OF THE YOUNG GAMETOPHYTE OF THE OSTRICH FERN (MATTEUCCIA STRUTHIOPTERIS). Am J Bot. 1965 Jan;52:82–94. [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUPPY H., WIESBAUER U., WINTERSBERGER E. [Amino acid-p-nitroanilide as a substrate for aminopeptidases and other proteolytic enzymes]. Hoppe Seylers Z Physiol Chem. 1962 Nov 15;329:278–288. doi: 10.1515/bchm2.1962.329.1.278. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Proteases and Peptidases of Castor Bean Endosperm: Enzyme Characterization and Changes during Germination. Plant Physiol. 1978 Nov;62(5):746–750. doi: 10.1104/pp.62.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]


