Abstract
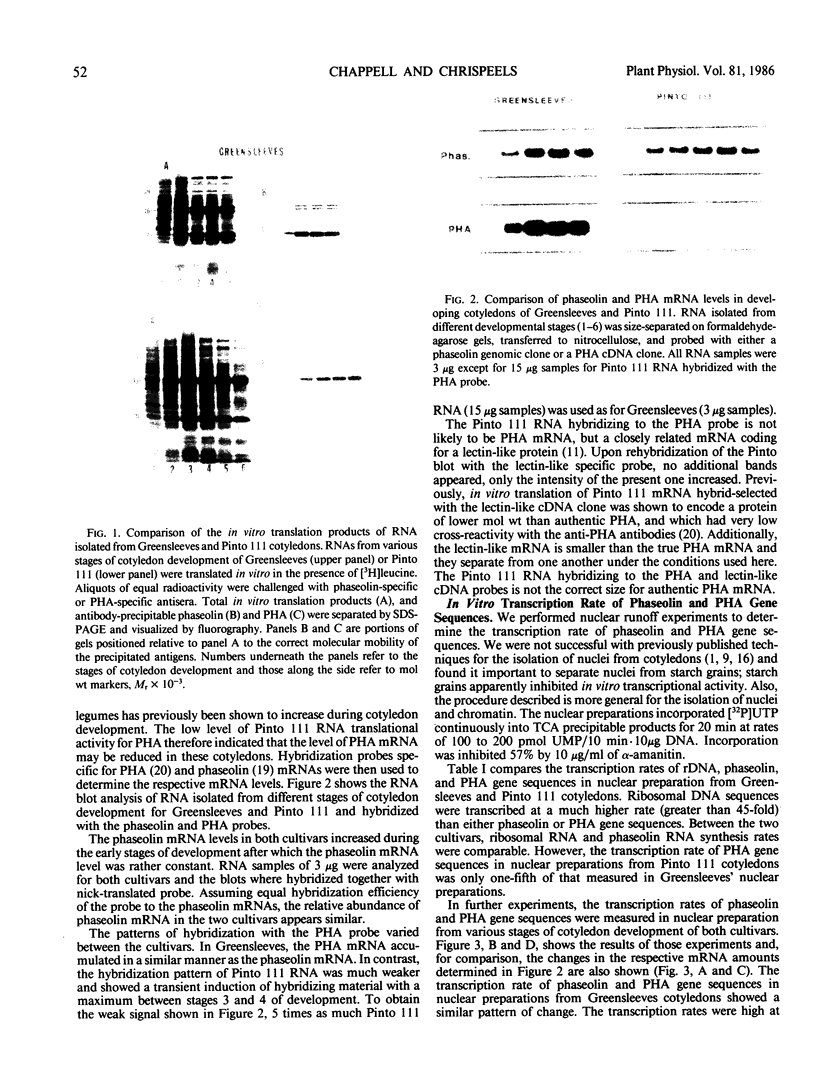
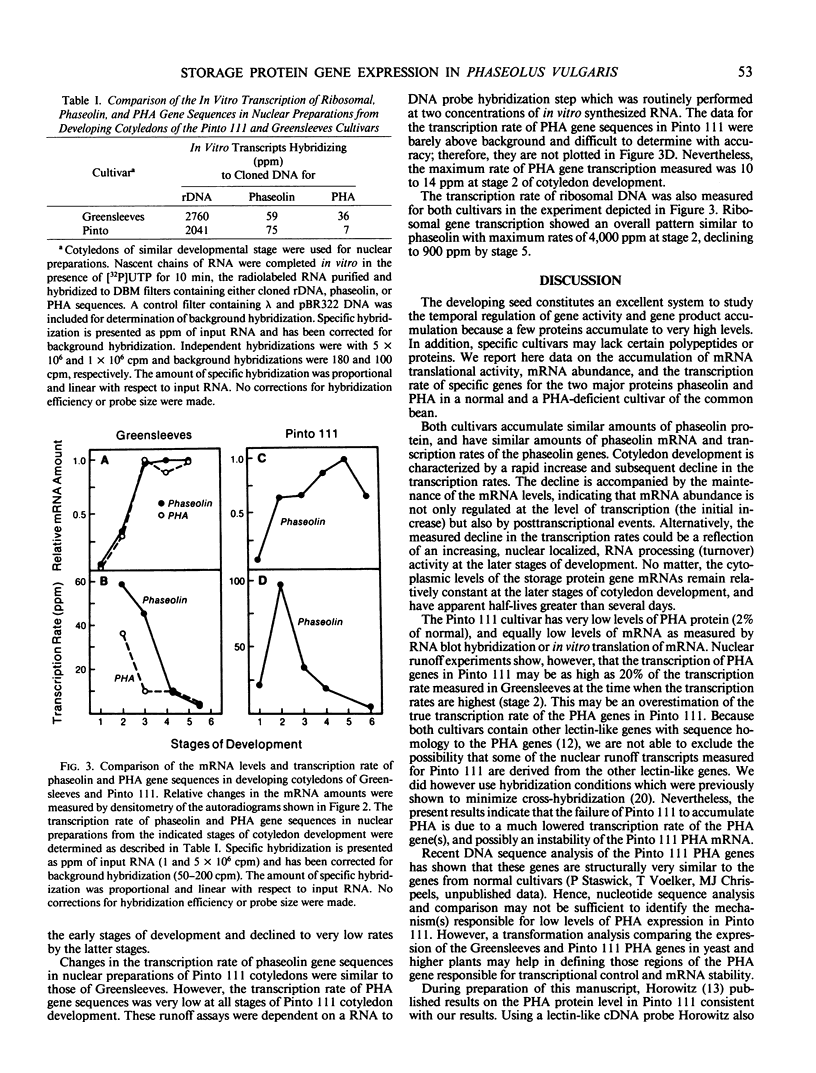
The expression of phaseolin and phytohemagglutinin (PHA) in the developing cotyledons of a normal (Greensleeves) and a PHA-deficient (Pinto 111) cultivar of Phaseolus vulgaris was investigated. Phaseolin mRNA translational activity and abundance were present at similar levels in both cultivars. In contrast, PHA mRNA translational activity and abundance in Pinto 111 were less than 1% of the levels measured in Greensleeves. Using nuclear runoff assays, the transcription rate of phaseolin gene sequences was similar in both cultivars. The transcription rate of PHA gene sequences in Pinto 111 was only 20% of that measured in Greensleeves. Comparison of the transcription rates with the relative mRNA amounts measured in RNA blot hybridizations indicated that the normally expressed storage protein gene mRNAs were very stable with half-lives greater than several days. Because a low level of PHA gene transcription in Pinto 111 was measurable but no PHA mRNA accumulated, these results suggest that the PHA deficiency in Pinto 111 is due to a reduced transcription rate and possibly an instability of the mRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach L. R., Spencer D., Randall P. J., Higgins T. J. Transcriptional and post-transcriptional regulation of storage protein gene expression in sulfur-deficient pea seeds. Nucleic Acids Res. 1985 Feb 11;13(3):999–1013. doi: 10.1093/nar/13.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cockburn A. F., Newkirk M. J., Firtel R. A. Organization of the ribosomal RNA genes of Dictyostelium discoideum: mapping of the nontranscribed spacer regions. Cell. 1976 Dec;9(4 Pt 1):605–613. doi: 10.1016/0092-8674(76)90043-x. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Vodkin L. O. An insertion sequence blocks the expression of a soybean lectin gene. Cell. 1983 Jun;33(2):465–475. doi: 10.1016/0092-8674(83)90428-2. [DOI] [PubMed] [Google Scholar]
- Hoffman L. M., Donaldson D. D. Characterization of two Phaseolus vulgaris phytohemagglutinin genes closely linked on the chromosome. EMBO J. 1985 Apr;4(4):883–889. doi: 10.1002/j.1460-2075.1985.tb03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. M., Ma Y., Barker R. F. Molecular cloning of Phaseolus vulgaris lectin mRNA and use of cDNA as a probe to estimate lectin transcript levels in various tissues. Nucleic Acids Res. 1982 Dec 11;10(23):7819–7828. doi: 10.1093/nar/10.23.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: I. ISOLATION OF NUCLEI AND ELIMINATION OF ENDOGENOUS RIBONUCLEASE ACTIVITY. Plant Physiol. 1980 Feb;65(2):305–308. doi: 10.1104/pp.65.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom J. L., Sun S. M., Hall T. C. Complete nucleotide sequence of a French bean storage protein gene: Phaseolin. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1897–1901. doi: 10.1073/pnas.80.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P., Chrispeels M. J. Expression of lectin genes during seed development in normal and phytohemagglutinin-deficient cultivars of Phaseolus vulgaris. J Mol Appl Genet. 1984;2(6):525–535. [PubMed] [Google Scholar]
- Vodkin L. O., Rhodes P. R., Goldberg R. B. cA lectin gene insertion has the structural features of a transposable element. Cell. 1983 Oct;34(3):1023–1031. doi: 10.1016/0092-8674(83)90560-3. [DOI] [PubMed] [Google Scholar]