Abstract
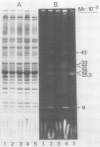
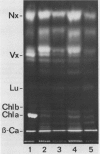
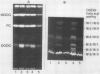
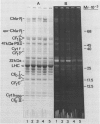
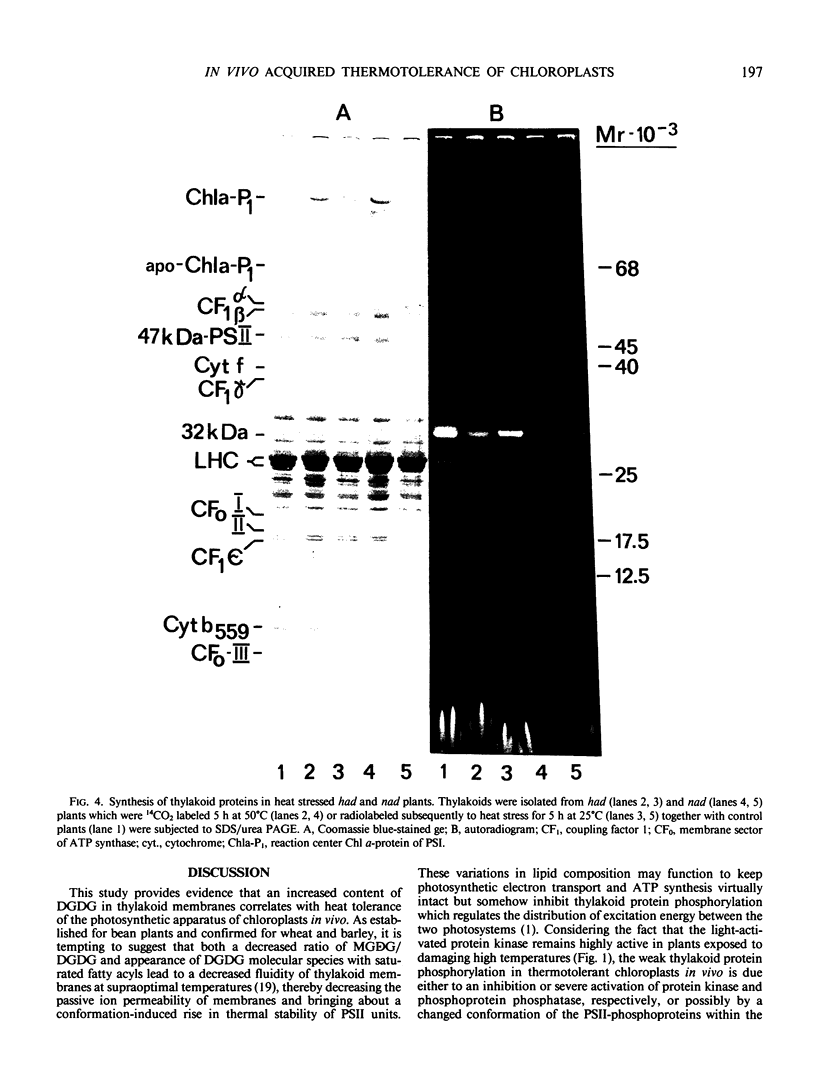
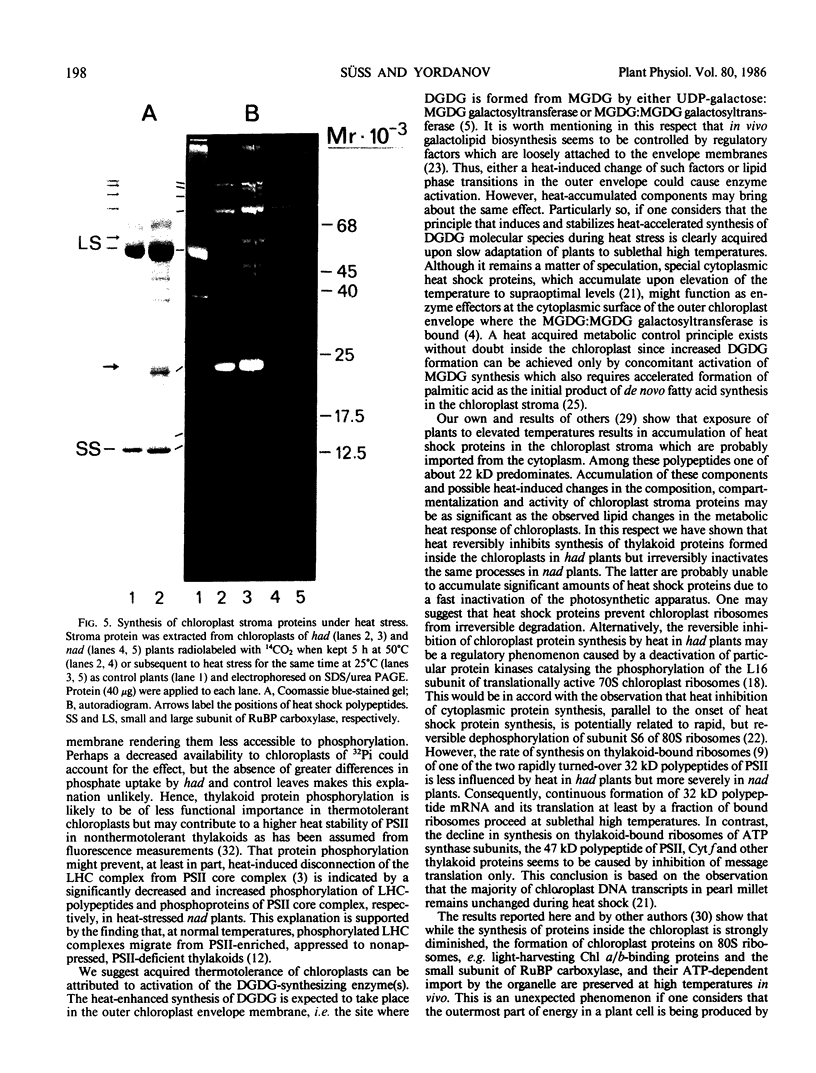
Thermotolerance of photosynthetic light reactions in vivo is correlated with a decrease in the ratio of monogalactosyl diacylglycerol to digalactosyl diacylglycerol and an increased incorporation into thylakoid membranes of saturated digalactosyl diacylglycerol species. Although electron transport remains virtually intact in thermotolerant chloroplasts, thylakoid protein phosphorylation is strongly inhibited. The opposite is shown for thermosensitive chloroplasts in vivo. Heat stress causes reversible and irreversible inactivation of chloroplast protein synthesis in heat-adapted and nonadapted plants, respectively, but doe not greatly affect formation of rapidly turned-over 32 kilodalton proteins of photosystem II. The formation on cytoplasmic ribosomes and import by chloroplasts of thylakoid and stroma proteins remain preserved, although decreased in rate, at supraoptimal temperatures. Thermotolerant chloroplasts accumulate heat shock proteins in the stroma among which 22 kilodalton polypeptides predominate. We suggest that interactions of heat shock proteins with the outer chloroplast envelope membrane might enhance formation of digalactosyl diacylglycerol species. Furthermore, a heat-induced recompartmentalization of the chloroplast matrix that ensures effective transport of ATP from thylakoid membranes towards those sites inside the chloroplast and the cytoplasm where photosynthetically indispensable components and heat shock proteins are being formed is proposed as a metabolic strategy of plant cells to survive and recover from heat stress.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. A., Dorne A. J., Joyard J., Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J Biol Chem. 1983 Nov 10;258(21):13281–13286. [PubMed] [Google Scholar]
- Joyard J., Douce R., Siebertz H. P., Heinz E. Distribution of radioactive lipids between envelopes and thylakoids from chloroplasts labelled in vivo. Eur J Biochem. 1980;108(1):171–176. doi: 10.1111/j.1432-1033.1980.tb04709.x. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Staehelin L. A., Arntzen C. J. Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch Biochem Biophys. 1983 Apr 15;222(2):527–541. doi: 10.1016/0003-9861(83)90551-9. [DOI] [PubMed] [Google Scholar]
- Scharf K. D., Nover L. Heat-shock-induced alterations of ribosomal protein phosphorylation in plant cell cultures. Cell. 1982 Sep;30(2):427–437. doi: 10.1016/0092-8674(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Siebertz H. P., Heinz E., Joyard J., Douce R. Labelling in vivo and in vitro of molecular species of lipids from chloroplast envelopes and thylakoids. Eur J Biochem. 1980;108(1):177–185. doi: 10.1111/j.1432-1033.1980.tb04710.x. [DOI] [PubMed] [Google Scholar]
- Steinback K. E., Bose S., Kyle D. J. Phosphorylation of the light-harvesting chlorophyll-protein regulates excitation energy distribution between photosystem II and photosystem I. Arch Biochem Biophys. 1982 Jun;216(1):356–361. doi: 10.1016/0003-9861(82)90221-1. [DOI] [PubMed] [Google Scholar]
- Süss K. H. Identification of chloroplast thylakoid phosphoproteins. Evidence for the absence of phosphoryl-polypeptide intermediates in the ATPase complex. Biochem Biophys Res Commun. 1981 Sep 30;102(2):724–729. doi: 10.1016/s0006-291x(81)80192-1. [DOI] [PubMed] [Google Scholar]
- Trebst A. Measurement of Hill reactions and photoreduction. Methods Enzymol. 1972;24:146–165. doi: 10.1016/0076-6879(72)24065-4. [DOI] [PubMed] [Google Scholar]
- Vierling E., Key J. L. Ribulose 1,5-Bisphosphate Carboxylase Synthesis during Heat Shock. Plant Physiol. 1985 May;78(1):155–162. doi: 10.1104/pp.78.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]