Abstract
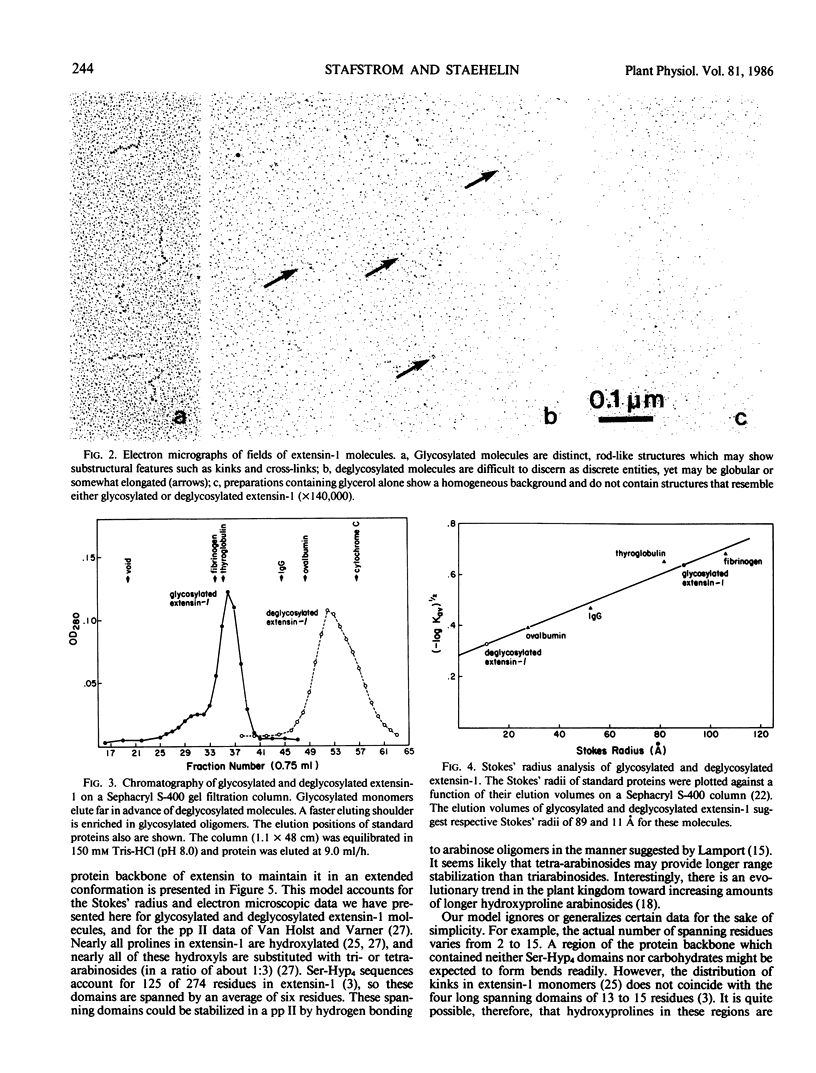
Monomers of the plant cell wall glycoprotein extensin are secreted into the wall where they become cross-linked to each other to form a rigid matrix. Expression of the extensin matrix is correlated with the inhibition of further cell elongation during normal development, with increased resistance to virulent pathogens and with other physiological responses characterized by wall strengthening. Carbohydrates make up about two-thirds of the mass of extensin. Arabinose oligomers linked to hydroxyproline residues represent 95% of the total carbohydrate with the remainder occurring as single residues of galactose linked to some serine residues. Electron microscopy of shadowed extensin shows the glycosylated form to be an easily visualized and highly elongated molecule. In contrast, extensin that has been deglycosylated with anhydrous hydrogen fluoride is difficult to resolve in the EM. Glycosylated extensin elutes from a gel filtration column much more rapidly than does the deglycosylated form, and from this analysis we have calculated respective Stokes' radii of 89 and 11 Ångstroms for these molecules. Others have shown that inhibition of extensin glycosylation has no effect on its secretion or insolubilization in the cell wall, but that this extensin cannot inhibit cell elongation. It is likely that carbohydrate moieties keep extensin in an extended conformation and that extensin must be in this conformation to form a cross-linked matrix that can function properly in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and Secretion of Hydroxyproline-containing Macromolecules in Carrots: II. In vivo Conversion of Peptidyl Proline to Peptidyl Hydroxyproline. Plant Physiol. 1970 Feb;45(2):223–227. doi: 10.1104/pp.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R., Karlsnes A. M. A possible role of hydroxyproline-containing proteins in the cessation of cell elongation. Plant Physiol. 1967 May;42(5):669–671. doi: 10.1104/pp.42.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Adair W. S., Mecham R. P., Heuser J. E. Chlamydomonas agglutinin is a hydroxyproline-rich glycoprotein. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5898–5901. doi: 10.1073/pnas.80.19.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Varner J. E. Selective inhibition of proline hydroxylation by 3,4-dehydroproline. Plant Physiol. 1983 Oct;73(2):324–328. doi: 10.1104/pp.73.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T., Lafitte C., Mazau D., Toppan A., Touzé A. Cell Surfaces in Plant-Microorganism Interactions: II. Evidence for the Accumulation of Hydroxyproline-rich Glycoproteins in the Cell Wall of Diseased Plants as a Defense Mechanism. Plant Physiol. 1979 Aug;64(2):320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T., Miller D. H. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971 Oct;48(4):454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T. The role of hydroxyproline-rich proteins in the extracellular matrix of plants. Symp Soc Dev Biol. 1974;30(0):113–130. doi: 10.1016/b978-0-12-612973-1.50011-5. [DOI] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Lamport D. T. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem. 1977 Oct;82(2):289–309. doi: 10.1016/0003-2697(77)90165-8. [DOI] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Hydroxyproline-rich cell wall protein (extensin): role in the cessation of elongation in excised pea epicotyls. Dev Biol. 1973 Jan;30(1):49–55. doi: 10.1016/0012-1606(73)90047-x. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Smith M. A. Characterization of Carrot Cell Wall Protein : I. EFFECT OF alpha, alpha'-DIPYRIDYL ON CELL WALL PROTEIN SYNTHESIS AND SECRETION IN INCUBATED CARROT DISCS. Plant Physiol. 1981 Oct;68(4):956–963. doi: 10.1104/pp.68.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom J. P., Staehelin L. A. Cross-linking patterns in salt-extractable extensin from carrot cell walls. Plant Physiol. 1986 May;81(1):234–241. doi: 10.1104/pp.81.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980 May;71(2):95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- van Holst G. J., Varner J. E. Reinforced Polyproline II Conformation in a Hydroxyproline-Rich Cell Wall Glycoprotein from Carrot Root. Plant Physiol. 1984 Feb;74(2):247–251. doi: 10.1104/pp.74.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]