Abstract
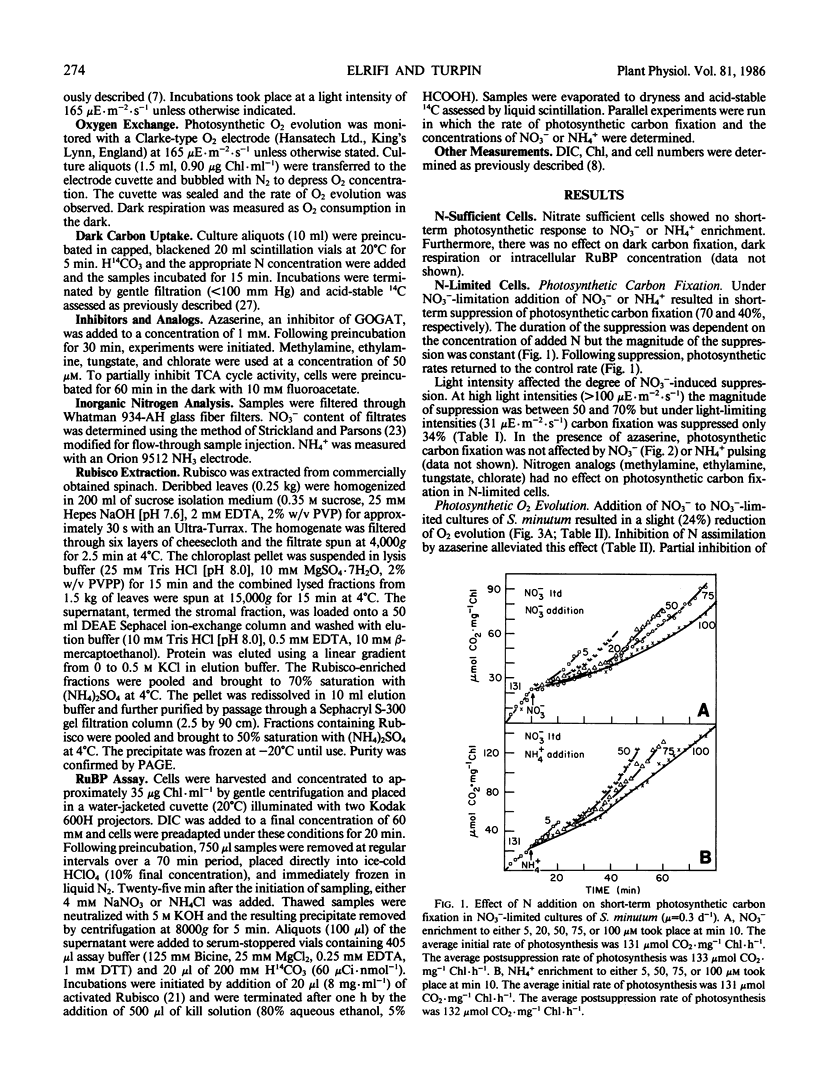
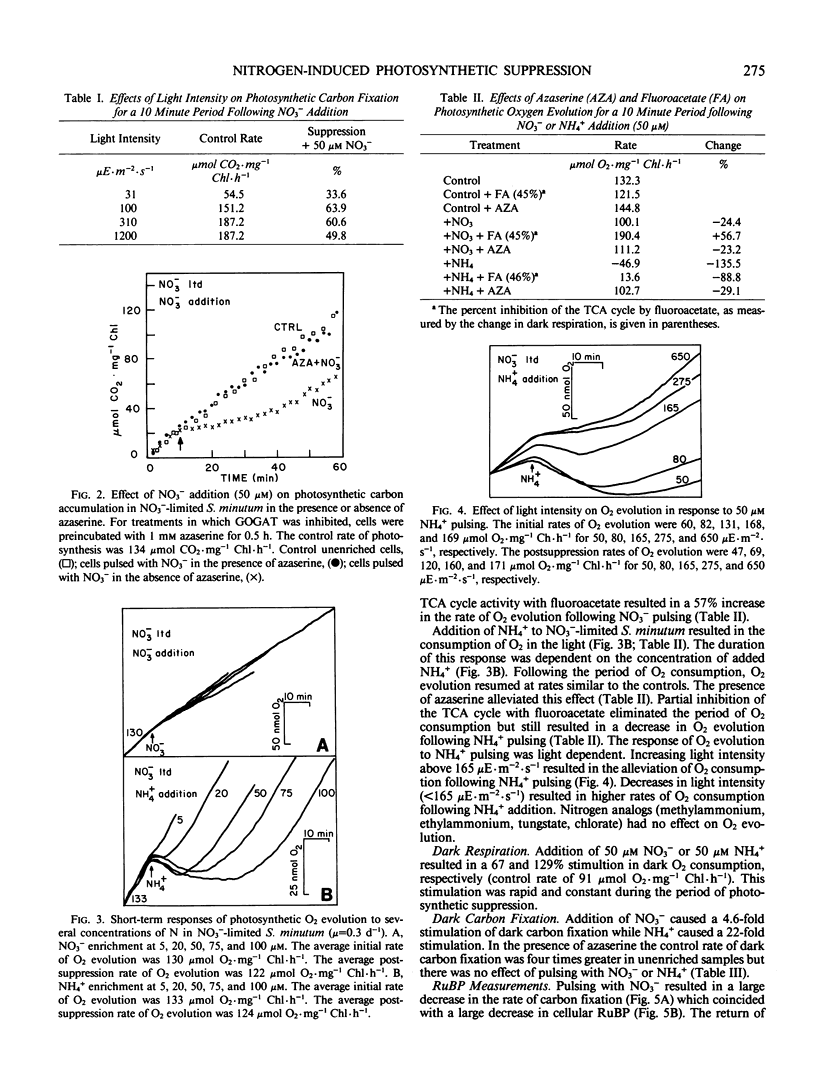
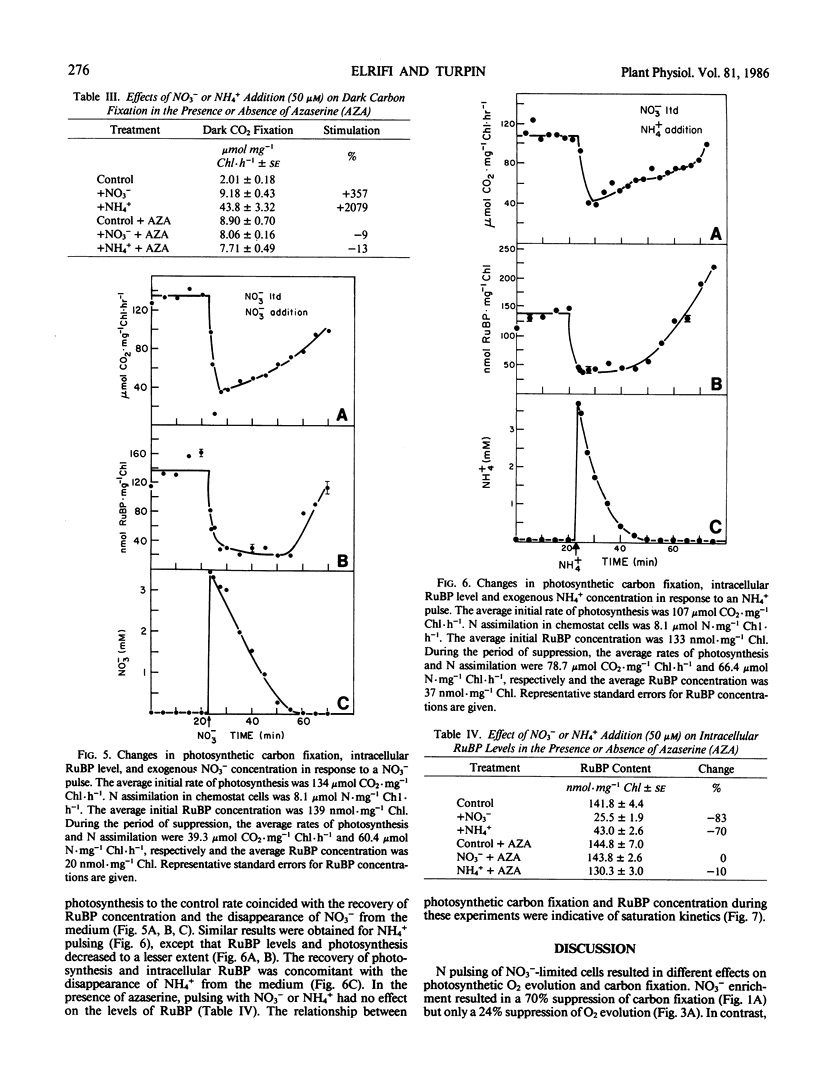
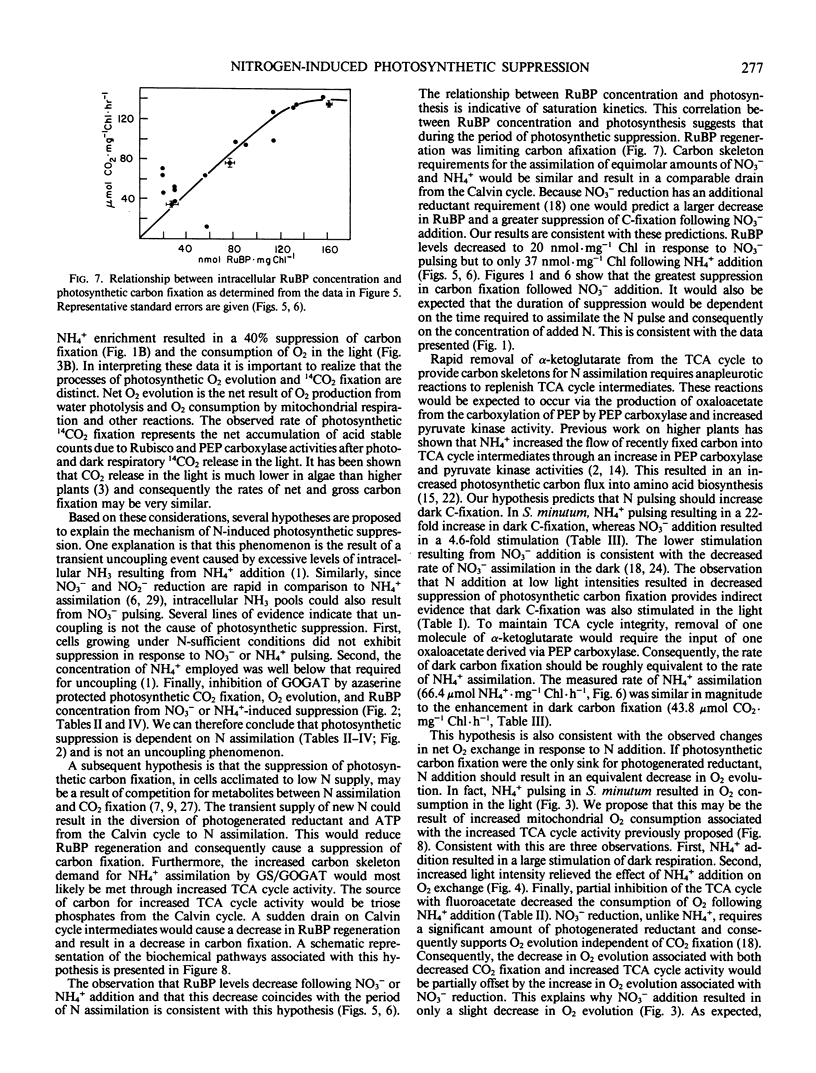
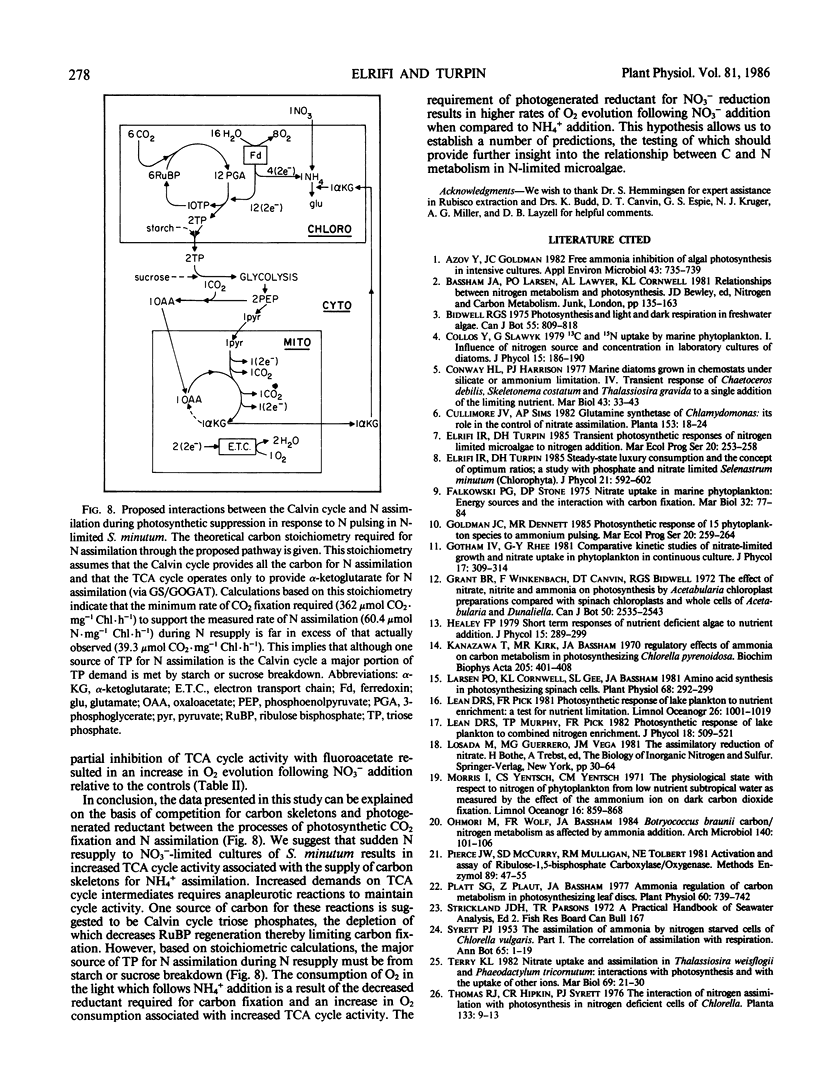
Nitrate-limited chemostat cultures of Selenastrum minutum Naeg. Collins (Chlorophyta) were used to determine the effects of nitrogen addition on photosynthesis, dark respiration, and dark carbon fixation. Addition of NO3− or NH4+ induced a transient suppression of photosynthetic carbon fixation (70 and 40% respectively). Intracellular ribulose bisphosphate levels decreased during suppression and recovered in parallel with photosynthesis. Photosynthetic oxygen evolution was decreased by N-pulsing under saturating light (650 microeinsteins per square meter per second). Under subsaturating light intensities (<165 microeinsteins per square meter per second) NH4+ addition resulted in O2 consumption in the light which was alleviated by the presence of the tricarboxylic acid cycle inhibitor fluoroacetate. Addition of NO3− or NH4+ resulted in a large stimulation of dark respiration (67 and 129%, respectively) and dark carbon fixation (360 and 2080%, respectively). The duration of N-induced perturbations was dependent on the concentration of added N. Inhibition of glutamine 2-oxoglutarate aminotransferase by azaserine alleviated all these effects. It is proposed that suppression of photosynthetic carbon fixation in response to N pulsing was the result of a competition for metabolites between the Calvin cycle and nitrogen assimilation. Carbon skeletons required for nitrogen assimilation would be derived from tricarboxylic acid cycle intermediates. To maintain tricarboxylic acid cycle activity triose phosphates would be exported from the chloroplast. This would decrease the rate of ribulose bisphosphate regeneration and consequently decrease net photosynthetic carbon accumulation. Stoichiometric calculations indicate that the Calvin cycle is one source of triose phosphates for N assimilation; however, during transient N resupply the major demand for triose phosphates must be met by starch or sucrose breakdown. The effects of N-pulsing on O2 evolution, dark respiration, and dark C-fixation are shown to be consistent with this model.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azov Y., Goldman J. C. Free ammonia inhibition of algal photosynthesis in intensive cultures. Appl Environ Microbiol. 1982 Apr;43(4):735–739. doi: 10.1128/aem.43.4.735-739.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Larsen P. O., Cornwell K. L., Gee S. L., Bassham J. A. Amino Acid Synthesis in Photosynthesizing Spinach Cells : EFFECTS OF AMMONIA ON POOL SIZES AND RATES OF LABELING FROM CO(2). Plant Physiol. 1981 Aug;68(2):292–299. doi: 10.1104/pp.68.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., McCurry S. D., Mulligan R. M., Tolbert N. E. Activation and assay of ribulose-1,5-bisphosphate carboxylase/oxygenase. Methods Enzymol. 1982;89(Pt 500):47–55. doi: 10.1016/s0076-6879(82)89011-3. [DOI] [PubMed] [Google Scholar]
- Platt S. G. Ammonia regulation of carbon metabolism in photosynthesizing leaf discs. Plant Physiol. 1977 Nov;60(5):739–742. doi: 10.1104/pp.60.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]


