Abstract
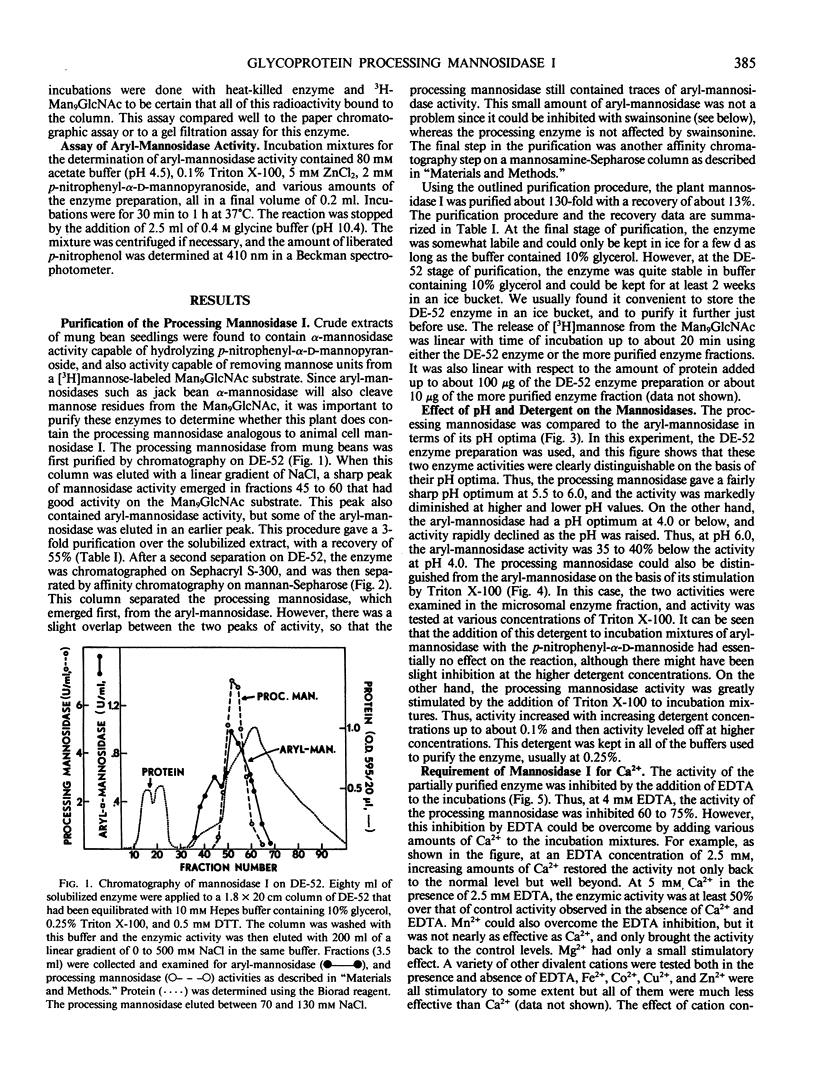
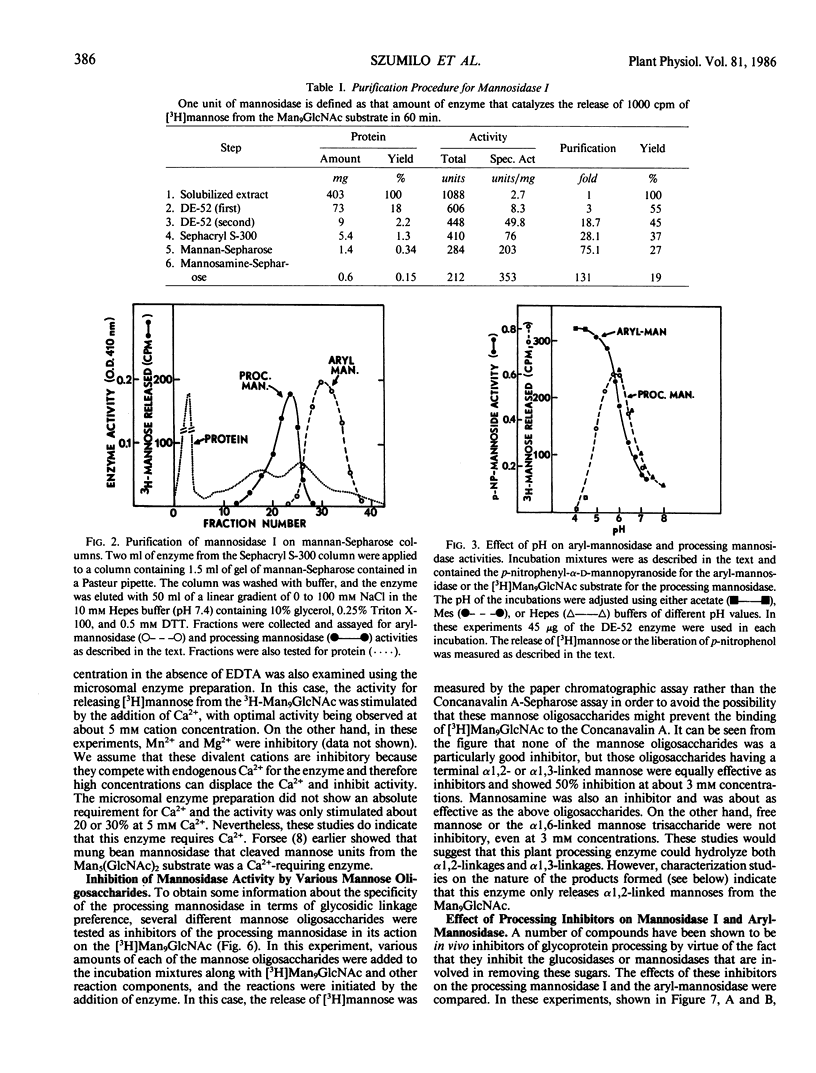
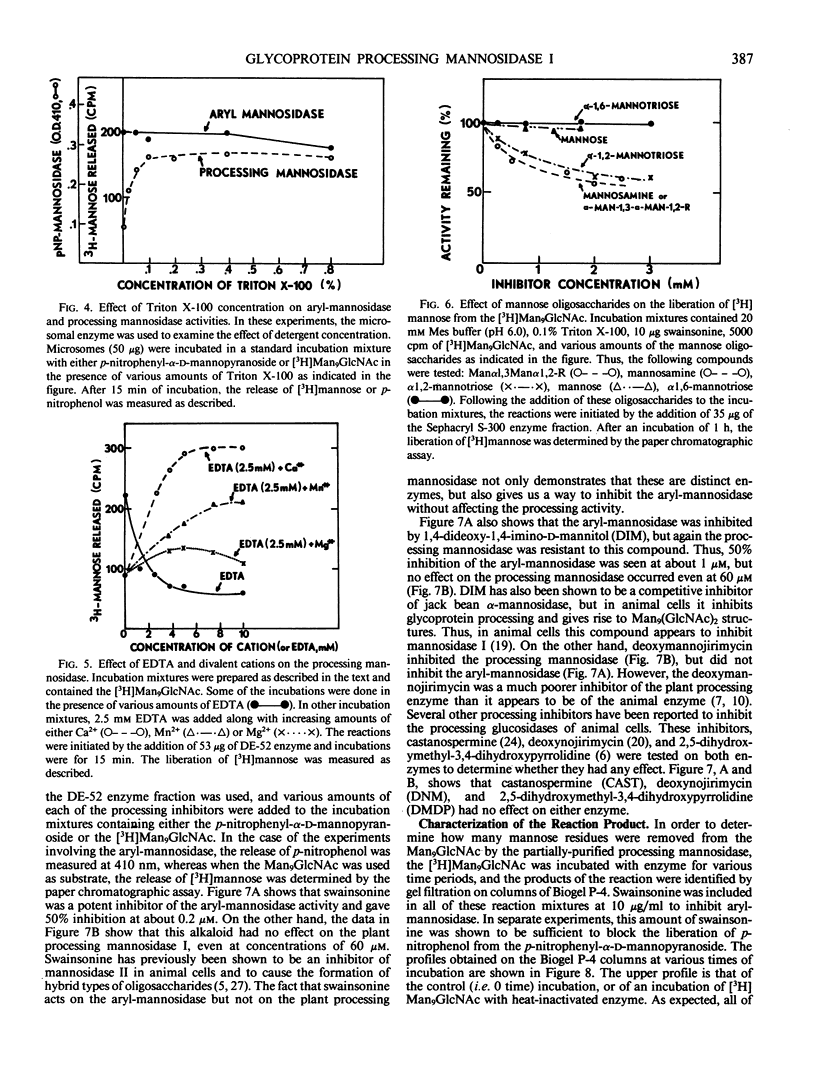
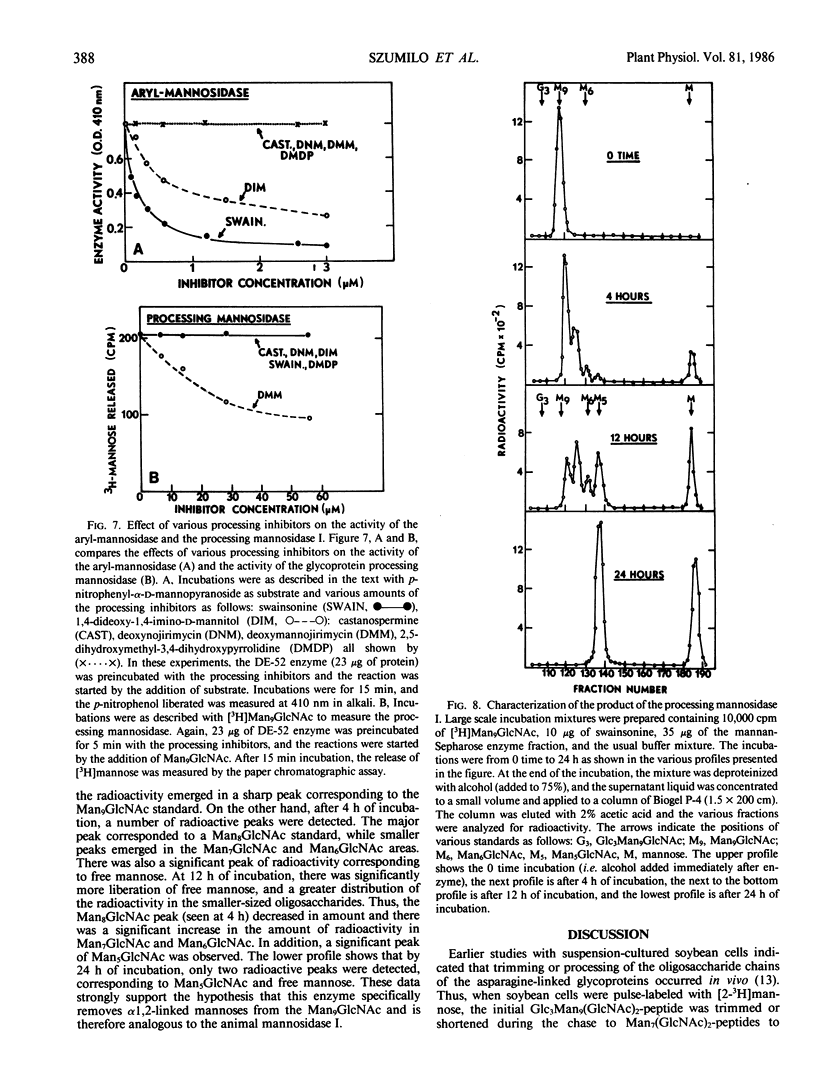
The microsomal fraction of mung bean seedlings contains mannosidase activities capable of hydrolyzing [3H]mannose from the [3H]Man9GlcNAc as well as for releasing mannose from p-nitrophenyl-α-d-mannopyranoside. The glycoprotein processing mannosidase was solubilized from the microsomes with 1.5% Triton X-100 and was purified 130-fold by conventional methods and also by affinity chromatography on mannan-Sepharose and mannosamine-Sepharose. The final enzyme preparation contained a trace of aryl-mannosidase, but this activity was inhibited by swainsonine whereas the processing enzyme was not. The pH optimum for the processing enzyme was 5.5 to 6.0, and activity was optimum in the presence of 0.1% Triton X-100. The enzyme was inhibited by ethylenediaminetetraacetate while Ca2+ was the most effective cation for reversing this inhibition. Mn2+ was considerably less effective than Ca2+ and Mg2+ was without effect. The processing mannosidase was inhibited by α1,2- and α1,3-linked mannose oligosaccharides (50% inhibition at 3 millimolar), whereas free mannose and α1,6-linked mannose oligosaccharides were ineffective. Mannosamine was also an inhibitor of this enzyme. The aryl-mannosidase and the processing mannosidase could also be distinguished by their susceptibility to various processing inhibitors. The aryl-mannosidase was inhibited by swainsonine and 1,4-dideoxy-1,4-imino-d-mannitol but not by deoxymannojirimycin or other inhibitors, while the processing mannosidase was only inhibited by deoxymannojirimycin. The processing mannosidase was incubated for long periods with [3H]Man9GlcNAc and the products were identified by gel filtration. Even after a 24 hour incubation, the only two radioactive products were Man5GlcNAc and free mannose. Thus, this enzyme appears to be similar to the animal processing enzyme, mannosidase I, and is apparently a specific α1,2-mannosidase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. D., Tsai D., Schachter H. Control of glycoprotein synthesis. The in vitro synthesis by hen oviduct membrane preparations of hybrid asparagine-linked oligosaccharides containing 5 mannose residues. J Biol Chem. 1984 Jun 10;259(11):6984–6990. [PubMed] [Google Scholar]
- Chrispeels M. J., Vitale A. Abnormal processing of the modified oligosaccharide side chains of phytohemagglutinin in the presence of swainsonine and deoxynojirimycin. Plant Physiol. 1985 Aug;78(4):704–709. doi: 10.1104/pp.78.4.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D., Legler G., Tlusty A., McDowell W., Schwarz R. The effect of deoxymannojirimycin on the processing of the influenza viral glycoproteins. Arch Biochem Biophys. 1984 Dec;235(2):579–588. doi: 10.1016/0003-9861(84)90232-7. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Mitchell M., Sanford B. A., Fellows L. E., Evans S. V. The pyrrolidine alkaloid, 2,5-dihydroxymethyl-3,4-dihydroxypyrrolidine, inhibits glycoprotein processing. J Biol Chem. 1984 Oct 25;259(20):12409–12413. [PubMed] [Google Scholar]
- Elbein A. D., Solf R., Dorling P. R., Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsee W. T. Characterization of microsomal and cytosolic alpha-1,2-mannosidases from mung bean hypocotyls. Arch Biochem Biophys. 1985 Oct;242(1):48–57. doi: 10.1016/0003-9861(85)90478-3. [DOI] [PubMed] [Google Scholar]
- Forsee W. T., Schutzbach J. S. Purification and characterization of a phospholipid-dependent alpha-mannosidase from rabbit liver. J Biol Chem. 1981 Jul 10;256(13):6577–6582. [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Legler G., Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature. 1984 Feb 23;307(5953):755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- Harpaz N., Schachter H. Control of glycoprotein synthesis. Bovine colostrum UDP-N-acetylglucosamine:alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. Separation from UDP-N-acetylglucosamine:alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase II, partial purification, and substrate specificity. J Biol Chem. 1980 May 25;255(10):4885–4893. [PubMed] [Google Scholar]
- Hori H., Elbein A. D. Processing of N-linked oligosaccharides in soybean cultured cells. Arch Biochem Biophys. 1983 Feb 1;220(2):415–425. doi: 10.1016/0003-9861(83)90431-9. [DOI] [PubMed] [Google Scholar]
- Hori H., James D. W., Jr, Elbein A. D. Isolation and characterization of the Glc3Man9GlcNAc2 from lipid-linked oligosaccharides of plants. Arch Biochem Biophys. 1982 Apr 15;215(1):12–21. doi: 10.1016/0003-9861(82)90273-9. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Opheim D. J., Touster O. Lysosomal alpha-D-mannosidase of rat liver. Purification and comparison with the golgi and cytosolic alpha-D-mannosidases. J Biol Chem. 1978 Feb 25;253(4):1017–1023. [PubMed] [Google Scholar]
- Oppenheimer C. L., Hill R. L. Purification and characterization of a rabbit liver alpha 1 goes to 3 mannoside beta 1 goes to 2 N-acetylglucosaminyltransferase. J Biol Chem. 1981 Jan 25;256(2):799–804. [PubMed] [Google Scholar]
- Palamarczyk G., Mitchell M., Smith P. W., Fleet G. W., Elbein A. D. 1,4-Dideoxy-1,4-imino-D-mannitol inhibits glycoprotein processing and mannosidase. Arch Biochem Biophys. 1985 Nov 15;243(1):35–45. doi: 10.1016/0003-9861(85)90771-4. [DOI] [PubMed] [Google Scholar]
- Saunier B., Kilker R. D., Jr, Tkacz J. S., Quaroni A., Herscovics A. Inhibition of N-linked complex oligosaccharide formation by 1-deoxynojirimycin, an inhibitor of processing glucosidases. J Biol Chem. 1982 Dec 10;257(23):14155–14161. [PubMed] [Google Scholar]
- Staneloni R. J., Leloir L. F. The biosynthetic pathway of the asparagine-linked oligosaccharides of glycoproteins. CRC Crit Rev Biochem. 1982 Apr;12(4):289–326. doi: 10.1080/10409238209104422. [DOI] [PubMed] [Google Scholar]
- Staneloni R. J., Tolmasky M. E., Petriella C., Ugalde R. A., Leloir L. F. Presence in a plant of a compound similar to the dolichyl diphosphate oligosaccharide of animal tissue. Biochem J. 1980 Oct 1;191(1):257–260. doi: 10.1042/bj1910257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle D. P. Increased levels of UMP synthase protein and mRNA in pyrazofurin-resistant rat hepatoma cells. J Biol Chem. 1983 Jun 25;258(12):7707–7713. [PubMed] [Google Scholar]
- Szumilo T., Elbein A. D. A simple and reliable assay for glycoprotein-processing glycosidases. Anal Biochem. 1985 Nov 15;151(1):32–40. doi: 10.1016/0003-2697(85)90049-1. [DOI] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tulsiani D. R., Hubbard S. C., Robbins P. W., Touster O. alpha-D-Mannosidases of rat liver Golgi membranes. Mannosidase II is the GlcNAcMAN5-cleaving enzyme in glycoprotein biosynthesis and mannosidases Ia and IB are the enzymes converting Man9 precursors to Man5 intermediates. J Biol Chem. 1982 Apr 10;257(7):3660–3668. [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. Swainsonine causes the production of hybrid glycoproteins by human skin fibroblasts and rat liver Golgi preparations. J Biol Chem. 1983 Jun 25;258(12):7578–7585. [PubMed] [Google Scholar]
- Vitale A., Chrispeels M. J. Transient N-acetylglucosamine in the biosynthesis of phytohemagglutinin: attachment in the Golgi apparatus and removal in protein bodies. J Cell Biol. 1984 Jul;99(1 Pt 1):133–140. doi: 10.1083/jcb.99.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]


