Abstract
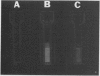
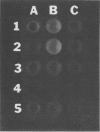
Two methods were developed for the detection of altered ureide metabolism in legume nodules. Both techniques are based on the positive correlation between the presence of high xanthine dehydrogenase (EC 1.2.1.37) specific activity in nodules and the ability of those nodules to produce the ureides, allantoin and allantoic acid. In the first method, nodulated legumes are treated for 2 weeks with a soil drench of allopurinol. After allopurinol treatment, leaves of N2-fed, ureide-producing legumes, soybean, cowpea, and lima bean, became very chlorotic. Leaves of KNO3− or NH4Cl-fed ureide-producing legumes were unaffected by the allopurinol treatment. Leaves of the amide-producing legumes, alfalfa, clover, peak, and lupin, were unaffected by the allopurinol treatment with N2, KNO3, or NH4Cl as nitrogen source. These experiments showed that long-term allopurinol treatments are useful in differentiating between ureide- and amide-producing legumes when effectively nodulated. A second method was developed for the rapid, qualitative estimation of xanthine dehydrogenase activity in legume nodules. This method utilizes pterin, an alternate substrate for xanthine dehydrogenase. Xanthine dehydrogenase hydroxylates pterin in the presence of NAD+ to produce isoxanthopterin. When exposed to long wave ultraviolet light (365 nanometers), isoxanthopterin emits blue fluorescence. When nodules of ureide-producing legumes were sliced in half and placed in microtiter plate wells containing NAD+ and pterin, isoxanthopterin was observed after 6 hours of incubation at room temperature. Allopurinol prevented isoxanthopterin production. When slices of amide-producing legume nodules were placed in wells with pterin and NAD+, no blue fluorescence was observed. The production of NADH by xanthine dehydrogenase does not interfere with the fluorescence of isoxanthopterin. These observations agree with the high specific activity of xanthine dehydrogenase in nodules of ureide-producing legumes and the low activity measured in amide-producing nodules. The wild soybean, Glycine soja Sieb. and Zucc., was examined for ureide synthesis. Stems of wild soybean plants had a high ureide abundance with N2 as sole nitrogen source when nodulated with either Rhizobium fredii or Bradyrhizobium japonicum. Ureide abundance declined when nitrate or ammonium was added to the nutrient solution. Nodule slices of these plants produced isoxanthopterin when incubated with pterin. Nodule crude extracts of G. soja had high levels of xanthine dehydrogenase activity. Both Glycine max and G. soja plants were found to produce ureides when plants were inoculated with fast-growing R. fredii. The two methods described here can be used to discriminate ureide producers from amide producers as well as detect nitrogen-fixing legumes which have altered ureide metabolism. A nodulated legume that lacks xanthine dehydrogenase activity as demonstrated by the pterin assay cannot produce ureides since ureide synthesis has been shown to require xanthine dehydrogenase activity both in vivo and in vitro. A nodulated legume that remains green during allopurinol treatment also lacks ureide synthesis since the leaves of ureide-producing legumes are very chlorotic following allopurinol treatment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christensen T. M., Jochimsen B. U. Enzymes of ureide synthesis in pea and soybean. Plant Physiol. 1983 May;72(1):56–59. doi: 10.1104/pp.72.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORREST H. S., GLASSMAN E., MITCHELL H. K. Conversion of 2-amino-4-hydroxypteridine to isoxanthopterin in D. Melanogaster. Science. 1956 Oct 19;124(3225):725–726. doi: 10.1126/science.124.3225.725. [DOI] [PubMed] [Google Scholar]
- Herridge D. F., Atkins C. A., Pate J. S., Rainbird R. M. Allantoin and Allantoic Acid in the Nitrogen Economy of the Cowpea (Vigna unguiculata [L.] Walp.). Plant Physiol. 1978 Oct;62(4):495–498. doi: 10.1104/pp.62.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F. Relative abundance of ureides and nitrate in plant tissues of soybean as a quantitative assay of nitrogen fixation. Plant Physiol. 1982 Jul;70(1):1–6. doi: 10.1104/pp.70.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. R., Hanus F. J., Russell S. A., Evans H. J. Determination of the hydrogenase status of individual legume nodules by a methylene blue reduction assay. Appl Environ Microbiol. 1985 Aug;50(2):537–539. doi: 10.1128/aem.50.2.537-539.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W., Volk R. J. Evaluation of the Relative Ureide Content of Xylem Sap as an Indicator of N(2) Fixation in Soybeans: GREENHOUSE STUDIES. Plant Physiol. 1980 Oct;66(4):720–725. doi: 10.1104/pp.66.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Atkins C. A., White S. T., Rainbird R. M., Woo K. C. Nitrogen Nutrition and Xylem Transport of Nitrogen in Ureide-producing Grain Legumes. Plant Physiol. 1980 May;65(5):961–965. doi: 10.1104/pp.65.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Allantoin and Allantoic Acid in Tissues and Stem Exudate from Field-grown Soybean Plants. Plant Physiol. 1979 Mar;63(3):478–480. doi: 10.1104/pp.63.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Blevins D. G., Randall D. D. Allantoic Acid Synthesis in Soybean Root Nodule Cytosol via Xanthine Dehydrogenase. Plant Physiol. 1980 Jun;65(6):1203–1206. doi: 10.1104/pp.65.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Blevins D. G., Randall D. D. Purification and properties of soybean nodule xanthine dehydrogenase. Arch Biochem Biophys. 1982 Nov;219(1):39–46. doi: 10.1016/0003-9861(82)90131-x. [DOI] [PubMed] [Google Scholar]
- Triplett E. W. Intercellular nodule localization and nodule specificity of xanthine dehydrogenase in soybean. Plant Physiol. 1985 Apr;77(4):1004–1009. doi: 10.1104/pp.77.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Lending C. R., Gumpf D. J., Ware C. F. Production, characterization, and applications of monoclonal antibodies reactive with soybean nodule xanthine dehydrogenase. Plant Physiol. 1986 Apr;80(4):965–971. doi: 10.1104/pp.80.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P., Boylan K. L., Maxwell C. A., Heichel G. H., Hardman L. L. Transport and Partitioning of CO(2) Fixed by Root Nodules of Ureide and Amide Producing Legumes. Plant Physiol. 1985 Aug;78(4):774–778. doi: 10.1104/pp.78.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]