Abstract
Backgrounds:
People with prior cancer diagnosis are more likely to have low muscle mass (LMM) than their cancer-free counterparts. Understanding the impact of LMM on prognosis of cancer survivors can be clinically important. Here we investigated if risks of all-cause and cardiovascular disease (CVD)-specific mortality differ by status of LMM in cancer survivors and a matched cohort without cancer history.
Methods:
We used cohort data from the 1999-2006 and 2011-2014 National Health and Nutrition Examination Survey. Participants included 946 adults surviving for at least 1 year since cancer diagnosis and a matched cohort (by age, sex, and race) without cancer history (N=1,857). LMM was defined by appendicular lean mass and body height (males<7.26 kg/m2, females<5.45 kg/m2). Death was ascertained via the National Death Index and cause of death was assessed via ICD-10. Multivariable Cox proportional hazards models were used to estimate adjusted hazard ratio (aHR) and 95% confidence interval (CI) of LMM.
Results:
The mean age of cancer survivors and matched cohort was 60.6 (SD=15.0) and 60.2 (SD=14.9) years, respectively. The median follow-up was 10.5 years for survivors and 10.9 years for matched cohort. Overall, 22.2% of cancer survivors and 19.7% of matched cohort had LMM, respectively. A total of 321 (33.9%) survivors and 495 (26.7%) participants in matched cohort died during follow-up. CVD-specific deaths were identified in 58 (6.1%) survivors and 122 (6.6%) participants in matched cohort. The multivariable Cox model suggested that LMM was positively associated with all-cause (aHR=1.73, 95% CI=1.31, 2.29) and CVD-specific (aHR=2.13, 95% CI=1.14, 4.00) mortality in cancer survivors. The associations between LMM and risk of all-cause (aHR=1.24, 95% CI=0.98, 1.56) and CVD-specific (aHR=1.21, 95% CI=0.75, 1.93) mortality were not statistically significant in the matched cohort.
Conclusion:
Cancer survivors with LMM have an increased risk of all-cause and CVD-specific mortality. Such increase appears to be larger than that in counterparts without cancer history.
Keywords: low muscle mass, cancer survivorship, epidemiology, nutrition, geriatric oncology
Introduction
Low muscle mass (LMM) is a strong predictor for burden of chronic health conditions, declined function, and unfavorable prognosis of disease [1]. A meta-analysis synthesizing data from 35 studies suggests that a substantial proportion of people are living with LMM (3%-36%), and its prevalence increases with age and comorbidity burden [2,3]. Many factors can contribute to development of LMM, including chronic inflammation, aging, and disturbed metabolic homeostasis [4–6].
Cancer is a leading cause of morbidity and mortality nationally and internationally, accounting for approximately 602,350 deaths in the United States and 10 million deaths globally in 2020 [7,8]. The pathogeneses of cancer involve the aforementioned biological processes that are important for development of LMM, and toxicities of cancer treatment can also impact immune system, process of senescence, and metabolic homeostasis [9–11]. This indicates that cancer survivors may face a higher burden of LMM than their healthier counterparts. For example, our prior study using the National Health and Nutrition Examination Survey (NHANES) data analyzed 2,483 older adults and found that 29% of older cancer survivors had sarcopenia, which was 38% higher than prevalence of sarcopenia (21%) in older adults without cancer history [5]. In addition, cancer, as well as toxicities of cancer treatment, can have the potential to interact with LMM and synergistically induce adverse biological events (e.g., inflammation, aging, and disturbed metabolism). Thus, cancer survivors living with LMM may have a worse outcome than survivors without LMM [12]. Specifically, among adults with prior cancer diagnosis, LMM does not only affect their physical function and mobility but also has the potential to influence their long-term survival and risk of severe health outcomes, such as fatal cardiovascular events—the events over 10% of cancer survivors die from [13]. Therefore, understanding the potential impacts of LMM on survival and cardiovascular disease (CVD)-specific death among cancer survivors is important, and such knowledge may help health practitioners more precisely predict prognosis, improve survival, and manage chronic diseases for cancer survivors.
Here we investigated the association of all-cause and CVD-specific mortality with LMM in cancer survivors and a non-cancer matched cohort using the NHANES data.
Methods
Data source and study population
The NHANES is a cross-sectional survey led by Centers for Disease Control and Prevention. The NHANES leverages questionnaires, physical examinations, and laboratory tests to measure health-related information of participants in the United States [14]. In this study, we used data from the 1999-2006 and 2011-2014 NHANES data which were linked to death certificate records from the National Death Index (NDI) and had relevant measures to define LMM. Participants with the following characteristics were treated as cancer survivors and included for this study: (1) underwent medical examinations to measure body composition, (2) had cancer history, (3) had survived for at least 1 year since cancer diagnosis (because acute effects of cancer treatment can substantially impact dietary behaviors and clinical measures), (4) had data on vital status, and (5) had no missing value in other covariates. A total of 946 cancer survivors were included for this study. In order to do a comparative analysis, we built another cohort (N=1,857), which was matched by age, sex, and race at a ratio of 1:2, from adults without cancer history in NHANES. Details regarding participant selection are present in Supplementary Figure.
Exposure and outcome of interest
LMM was the exposure of interest in our study. When conducting the 1999-2006 and 2011-2014 NHANES, research staff used whole body dual-energy X-ray absorptiometry (DEXA) scans (Hologic Scanner, QDR-4500, Bedford, MA, USA) to measure appendicular skeletal muscle mass (ASM) of study participants [15]. We then calculated ASM index (ASMI) as ASM/height (m)2; in this formula, height without shoes was measured at baseline by trained examiners using stadiometer. Based on criteria used in the European Working Group on Sarcopenia in Older People (EWGSOP), ASMI lower than 7.26 kg/m2 and 5.45 kg/m2 were used to define LMM for males and females, respectively [16].
The outcomes of interest included all-cause and cardiovascular disease (CVD)-specific mortality. Death was identified by linkage to the National Death Index (NDI) through December 31, 2015. The cause of death was ascertained by the International Classification of Diseases, Tenth Revision (ICD-10).
Other covariates
At baseline interview, demographic factors (age, sex, and race) were obtained by self-report. Age was categorized as a 4-level ordinal variable (<50, 50-64, 65-74, ≥75 years). Sex was categorized as female and male. In our study, race was treated as a categorical variable (white, black, and other). Education attainment was categorized as high school or less, attended college, and graduated from college. Participants who were single, never married, divorced, or widowed were treated as not married; participants who were married or living with partner were pooled as one category. Since some evidence suggests a potential link between smoking and LMM, we included smoking for the current analysis [17]. Participants were categorized as never smokers, current smokers (if ever smoked at least 100 cigarettes in life), and former smokers. Body fat mass was reflected by relative fat mass (RFM), which is a sex-specific measure based on height and waist circumference. RFM was calculated by the equation: (64 ─ (20× height/waist circumference) +12×sex) %; in this formula, waist circumference was measured by trained examiners at baseline, and sex equaled 0 for men and 1 for women. RFM was categorized into 3 levels to reflect low (female: <35%; male: <25%), moderate (female: 35–39.9%; male: 25–29.9%), and high (female: ≥40%; male: ≥30%) body fat [18]. Daily energy intake (kcal/day) was estimated based on food items measured in 24-hour food recall at baseline interview, and we categorized it as an ordinal variable to approximate quartiles (<1,364.0 kcal/day, 1,364.0 to 1,790.2 kcal/day, 1,790.3 to 2,339.9 kcal/day, and ≥ 2,340.0 kcal/day). Clinical evidence indicated that exercise training could attenuate or reverse muscle wasting [19], thus we included regular physical exercise as a confounder in this study; specifically, regular physical exercise was defined as moderate (only caused light sweating or a slight-to-moderate increase in breathing or heart rate) or vigorous (caused heavy sweating or large increases in breathing or heart rate) activities during the past 30 days. In our study, we incorporated 7 self-reported health conditions (chronic kidney disease, hypertension, diabetes mellitus, heart attack, stroke, coronary heart disease, and congestive heart failure) which could be associated with a higher risk of death or fatal cardiovascular events. Self-reported cancer-related information included time elapsed since cancer diagnosis (1-4, 5-9, and ≥10 years), history of more than one cancer, and cancer type (breast cancer, prostate cancer, colorectal cancer, melanoma, and other). Selection of study covariates was based on a priori knowledge regarding their relationship with exposure and outcomes of interest [5,17,20–22].
Statistical analysis
First, we summarized distributions of study characteristics by status of LMM in cancer survivors and matched cohort. In time-to-event analysis, participants entered risk set at baseline interview and were followed until death or censoring. We then summarized number of death, person-year during follow-up, and mortality rate for cancer survivors and matched cohort by LMM status. Kaplan–Meier curves were used to visualize risk for all-cause and CVD-specific mortality by LMM in cancer survivors and matched cohort. Log-rank tests were performed to examine if risk of death varied by LMM. Age-adjusted and multivariable Cox proportional hazards regression models were used to estimate hazard ratio (HR) and 95% confidence interval (CI) of LMM in both study populations. When all-cause mortality was the outcome in the model, participants were censored if they were alive; when CVD-specific mortality was the outcome, participants were censored if they were alive or died from non-cardiovascular causes. The multivariable model adjusted for age, sex, race, education, marital status, smoking status, RFM, energy intake, regular exercise, burden of comorbidities, history of more than 1 cancer (for survivors), and time elapsed since cancer diagnosis (for survivors). We further corrected for NHANES sampling weight in multivariable Cox model to explore if adjusted HR (aHR) changed substantially. The proportional hazards assumption was assessed on the basis of scaled Schoenfeld residuals. There was no violation of this assumption for cancer survivors when outcome was CVD-specific mortality in the model. However, violation was noted for time elapsed since cancer diagnosis and smoking status when we investigated all-cause mortality for cancer survivors; we then fitted a stratified Cox model over strata of time elapsed since cancer diagnosis (1-4, 5-9, ≥10 year) and smoking status, and there was no violation afterwards. There was no violation of this assumption in matched cohort.
Subgroup analyses were based on age (<65 vs. ≥65 years), sex (female vs. male), RFM (low and moderate vs. high body fat), energy consumption (<1790.3 vs. ≥1790.3 kcal/day), burden of comorbidities (0 vs. ≥1 comorbidity), and survival time (1-6 vs. ≥7 years). An interaction term between these factors and LMM was generated and added into the model; a Wald test was used to assess if the interaction was significant. In these subgroup analyses, we incorporated RFM and energy consumption in subgroup analysis because they could impact the same downstream factor (e.g. metabolic homeostasis) [23–25] as LMM, indicating a potential that they could have an interaction with LMM in relation to outcomes of interest. In addition, sides effects of cancer adjuvant therapy might take a period of time (several months to several years) to go away completely [26], thus we conducted the subgroup analysis by survival time.
Two sets of sensitivity analyses were conducted. In the first set of sensitivity analysis, we applied restricted cubic splines for dose-response analysis exploring association between ASMI and outcomes of interest. Specifically, we employed the same multivariable Cox proportional hazards regression model in which we treated ASMI as the independent variable in the model. In dose-response curve, we used ASMI=8.10 kg/m2 as the reference because this value was close to the 75th percentile of ASMI in both cancer survivors and matched cohort. We assessed the non-linearity by contrasting the model fit using restricted cubic splines with a model fit assuming linearity for ASMI by likelihood ratio test [27]. In the second set of sensitivity analysis, we excluded participants with health conditions (chronic kidney disease, heart failure, and diabetes) that could affect hydration status; the reason is that measurement of body composition can be affected by hydration status of the lean soft tissue [28].
The proportion of missing covariates was less than 10%, thus we did not use imputation to handle that in analysis. Two-sided values of p<0.05 were considered to be statistically significant. Statistical analyses were conducted with SAS v9.4 (SAS Institute Inc., Cary, NC) and STATA 17.0 (College Station, TX: StataCorp, LLP).
Results
In this study, the mean age of cancer survivors and matched cohort was 60.6 (SD=15.0) and 60.2 (SD=14.9) years, respectively. In cancer survivors (Table 1), 22.2% (N=210) had LMM, and prevalence of LMM was slightly lower in the matched cohort (19.7%, N=365). Distributions of sex and race were similar between cancer survivors and the matched cohort; specifically, over half of participants were female (cancer survivors: 57.1%; matched cohort: 57.2%) and about three-fourths were white (cancer survivors: 72.4%; matched cohort: 72.0%). Overall, participants with an older age had a higher prevalence of LMM, males were more likely to have LMM than females, and white participants had a higher prevalence of LMM than black participants (Table 1). More detailed distributions of study characteristics are present in Table 1.
Table 1.
Summary of study characteristics
| Cancer survivors (N=946) |
Matched cohort (N=1,857) |
|||||
|---|---|---|---|---|---|---|
| Overall N (col%) |
No LMM N (row%) |
With LMM N (row%) |
Overall N (col%) |
No LMM N (row%) |
With LMM N (row%) |
|
| Age (year) | ||||||
| <50 | 227 (24.0) | 199 (87.7) | 28 (12.3) | 454 (24.5) | 404 (89.0) | 50 (11.0) |
| 50-64 | 312 (33.0) | 254 (81.4) | 58 (18.6) | 624 (33.6) | 531 (85.1) | 93 (14.9) |
| 65-74 | 209 (22.1) | 166 (79.4) | 43 (20.6) | 411 (22.1) | 319 (77.6) | 92 (22.4) |
| ≥75 | 198 (20.9) | 117 (59.1) | 81 (40.9) | 368 (19.8) | 238 (64.7) | 130 (35.3) |
| Sex | ||||||
| Female | 540 (57.1) | 433 (80.2) | 107 (19.8) | 1062 (57.2) | 892 (84.0) | 170 (16.0) |
| Male | 406 (42.9) | 303 (74.6) | 103 (25.4) | 795 (42.8) | 600 (75.5) | 195 (24.5) |
| Race | ||||||
| White | 685 (72.4) | 515 (75.2) | 170 (24.8) | 1337 (72.0) | 1043 (78.0) | 294 (22.0) |
| Black | 122 (12.9) | 108 (88.5) | 14 (11.5) | 242 (13.0) | 223 (92.2) | 19 (7.9) |
| Other | 139 (14.7) | 113 (81.3) | 26 (18.7) | 278 (15.0) | 226 (81.3) | 52 (18.7) |
| Education | ||||||
| High school or less | 437 (46.2) | 334 (76.4) | 103 (23.6) | 958 (51.6) | 757 (79.0) | 201 (21.0) |
| Attended college | 263 (27.8) | 205 (78.0) | 58 (22.1) | 468 (25.2) | 386 (82.5) | 82 (17.5) |
| Graduated from college | 246 (26.0) | 197 (80.1) | 49 (19.9) | 431 (23.2) | 349 (81.0) | 82 (19.0) |
| Marital status | ||||||
| Not married | 326 (34.5) | 252 (77.3) | 74 (22.7) | 647 (34.8) | 503 (77.7) | 144 (22.3) |
| Married or living with partner | 620 (65.5) | 484 (78.1) | 136 (21.9) | 1210 (65.2) | 989 (81.7) | 221 (18.3) |
| Smoking status | ||||||
| Never | 404 (42.7) | 327 (80.9) | 77 (19.1) | 923 (49.7) | 770 (83.4) | 153 (16.6) |
| Current | 190 (20.1) | 144 (75.8) | 46 (24.2) | 330 (17.8) | 245 (74.2) | 85 (25.8) |
| Former | 352 (37.2) | 265 (75.3) | 87 (24.7) | 604 (32.5) | 477 (79.0) | 127 (21.0) |
| RFM * | ||||||
| Low | 145 (15.3) | 69 (47.6) | 76 (52.4) | 275 (14.8) | 135 (49.1) | 140 (50.9) |
| Moderate | 289 (30.6) | 204 (70.6) | 85 (29.4) | 560 (30.2) | 416 (74.3) | 144 (25.7) |
| High | 512 (54.1) | 463 (90.4) | 49 (9.6) | 1,022 (55.0) | 941 (92.1) | 81 (7.9) |
| Energy intake (kcal/day) | ||||||
| <1,364.0 | 236 (25.0) | 169 (71.6) | 67 (28.4) | 488 (26.3) | 389 (79.7) | 99 (20.3) |
| 1,364.0-1,790.2 | 237 (25.0) | 176 (74.3) | 61 (25.7) | 465 (25.0) | 374 (80.4) | 91 (19.6) |
| 1,790.3-2,339.9 | 236 (25.0) | 190 (80.5) | 46 (19.5) | 501 (27.0) | 393 (78.4) | 108 (21.6) |
| ≥2,340.0 | 237 (25.0) | 201 (84.8) | 36 (15.2) | 403 (21.7) | 336 (83.4) | 67 (16.6) |
| Regular exercise | ||||||
| No | 403 (42.6) | 302 (74.9) | 101 (25.1) | 812 (43.7) | 640 (78.8) | 172 (21.2) |
| Yes | 543 (57.4) | 434 (79.9) | 109 (20.1) | 1,045 (56.3) | 852 (81.5) | 193 (18.5) |
| No. comorbidities | ||||||
| 0 | 417 (44.1) | 317 (76.0) | 100 (24.0) | 963 (51.9) | 765 (79.4) | 198 (20.6) |
| 1 | 318 (33.6) | 257 (80.8) | 61 (19.2) | 580 (31.2) | 478 (82.4) | 102 (17.6) |
| ≥2 | 211 (22.3) | 162 (76.8) | 49 (23.2) | 314 (16.9) | 249 (79.3) | 65 (20.7) |
| Chronic kidney disease | ||||||
| No | 911 (96.3) | 710 (77.9) | 201 (22.1) | 1804 (97.2) | 1454 (80.6) | 350 (19.4) |
| Yes | 35 (3.7) | 26 (74.3) | 9 (25.7) | 53 (2.9) | 38 (71.7) | 15 (28.3) |
| Hypertension | ||||||
| No | 507 (53.6) | 386 (76.1) | 121 (23.9) | 1114 (60.0) | 877 (78.7) | 237 (21.3) |
| Yes | 439 (46.4) | 350 (79.7) | 89 (20.3) | 743 (40.0) | 615 (82.8) | 128 (17.2) |
| Diabetes mellitus | ||||||
| No | 795 (84.0) | 611 (76.9) | 184 (23.1) | 1639 (88.3) | 1311 (80.0) | 328 (20.0) |
| Yes | 151 (16.0) | 125 (82.8) | 26 (17.2) | 218 (11.7) | 181 (83.0) | 37 (17.0) |
| Heart attack | ||||||
| No | 877 (92.7) | 685 (78.1) | 192 (21.9) | 1759 (94.7) | 1419 (80.7) | 340 (19.3) |
| Yes | 69 (7.3) | 51 (73.9) | 18 (26.1) | 98 (5.3) | 73 (74.5) | 25 (25.5) |
| Stroke | ||||||
| No | 891 (94.2) | 698 (78.3) | 193 (21.7) | 1774 (95.5) | 1429 (80.6) | 345 (19.5) |
| Yes | 55 (5.8) | 38 (69.1) | 17 (30.9) | 83 (4.5) | 63 (75.9) | 20 (24.1) |
| Coronary heart disease | ||||||
| No | 871 (92.1) | 681 (78.2) | 190 (21.8) | 1745 (94.0) | 1404 (80.5) | 341 (19.5) |
| Yes | 75 (7.9) | 55 (73.3) | 20 (26.7) | 112 (6.0) | 88 (78.6) | 24 (21.4) |
| Congestive heart failure | ||||||
| No | 900 (95.1) | 701 (77.9) | 199 (22.1) | 1795 (96.7) | 1446 (80.6) | 349 (19.4) |
| Yes | 46 (4.9) | 35 (76.1) | 11 (23.9) | 62 (3.3) | 46 (74.2) | 16 (25.8) |
| History of more than 1 cancer | ||||||
| No | 860 (90.9) | 676 (78.6) | 184 (21.4) | N/A | N/A | N/A |
| Yes | 86 (9.1) | 60 (69.8) | 26 (30.2) | N/A | N/A | N/A |
| Time elapsed since cancer diagnosis (year) | ||||||
| 1-4 | 310 (32.8) | 245 (79.0) | 65 (21.0) | N/A | N/A | N/A |
| 5-9 | 234 (24.7) | 184 (78.6) | 50 (21.4) | N/A | N/A | N/A |
| ≥10 | 402 (42.5) | 307 (76.4) | 95 (23.6) | N/A | N/A | N/A |
| Cancer type | ||||||
| Breast | 134 (14.2) | 103 (76.9) | 31 (23.1) | N/A | N/A | N/A |
| Prostate | 119 (12.6) | 84 (70.6) | 35 (29.4) | N/A | N/A | N/A |
| Colorectal | 55 (5.8) | 42 (76.4) | 13 (23.6) | N/A | N/A | N/A |
| Melanoma | 57 (6.0) | 47 (82.5) | 10 (17.5) | N/A | N/A | N/A |
| Other | 581 (61.4) | 460 (79.2) | 121 (20.8) | N/A | N/A | N/A |
Abbreviation: RFM: relative fat mass
Cutoffs of relative fat mass were as follows—low: female: <35%, male: <25%; moderate: female: 35–39.9%, male: 25–29.9%; high: female: ≥40%, male: ≥30%.
In our study, the median follow-up time was 10.5 years for survivors and 10.9 years for the matched cohort. A total of 321 (33.9%) cancer survivors and 495 (26.7%) participants in the matched cohort died during follow-up. CVD-specific deaths were observed in 58 (6.1%) survivors and 122 (6.6%) participants in the matched cohort. Kaplan-Meier curves suggested that participants with LMM had a higher risk of all-cause mortality (Figure 1A) than those without LMM (log-rank ps<0.01), and all-cause mortality of cancer survivors was higher than that of matched cohort (log-rank ps<0.01). CVD-specific mortality (Figure 1B) was also higher in participants with LMM (log-rank ps<0.01), but its risk was not significantly different between cancer survivors and matched cohort (log-rank ps>0.05). Mortality of major types of cancer (breast cancer, prostate cancer, colorectal cancer, and melanoma) are present in Supplementary Table 1. In age-adjusted Cox proportional hazards regression models (Table 2), positive associations between LMM and risk of death were observed in our study populations regardless of prior cancer history, although the age-adjusted associations were only significant for all-cause mortality. In the multivariable Cox models (Table 2), LMM was associated with a higher all-cause (aHR=1.73, 95% CI=1.31-2.29) and CVD-specific mortality (aHR=2.13, 95% CI=1.14-4.00) in cancer survivors. In the matched cohort, association between LMM and all-cause mortality was marginally significant (aHR=1.24, 95% CI=0.98-1.56), and the association for CVD-specific mortality was non-significant (aHR=1.21, 95% CI=0.75-1.93). Correction for NHANES sampling weight did not substantially change the results.
Figure 1.
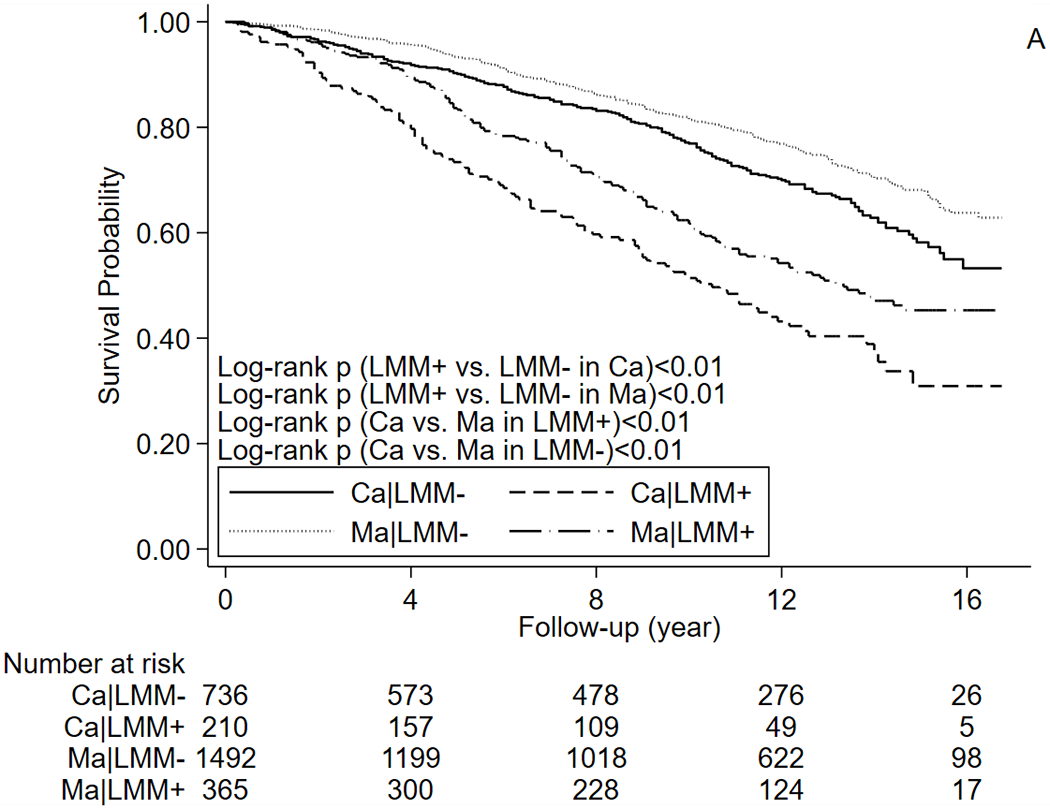
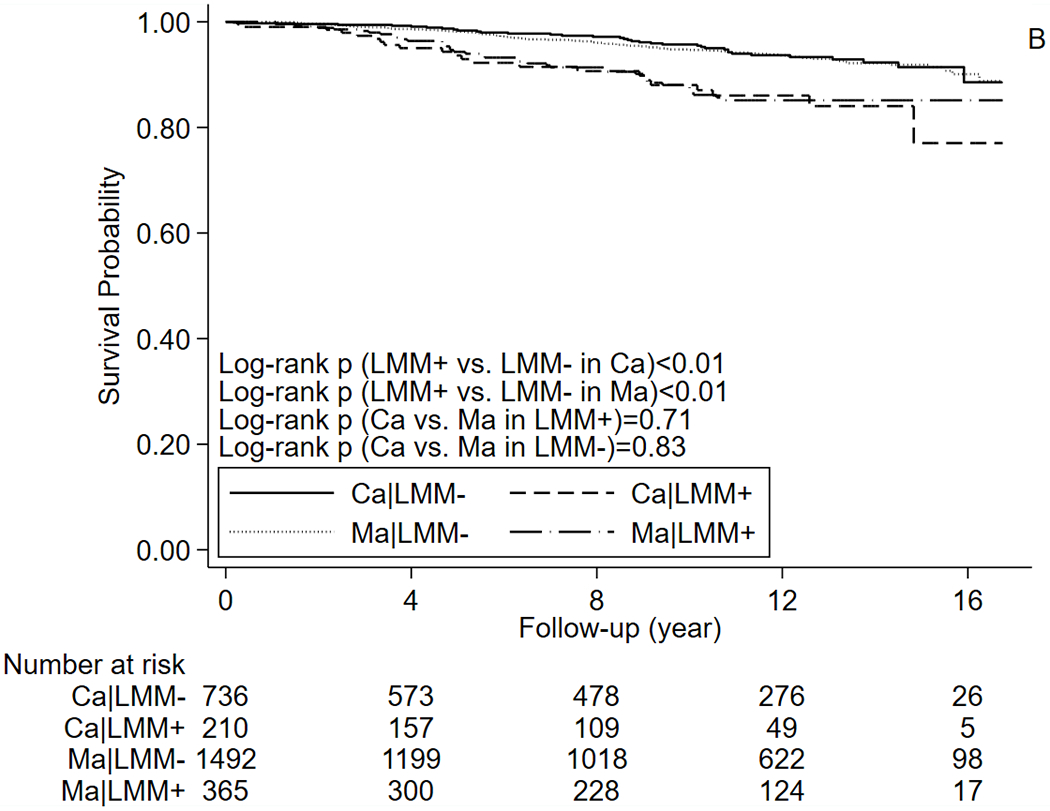
Kaplan–Meier curves for (A) All-cause mortality (B) CVD-specific mortality. Abbreviations: Ca: cancer survivors, CVD: cardiovascular disease, Ma: matched cohort, LMM: low muscle mass.
Table 2.
Association between LMM and risk of all-cause and CVD-specific mortality in cancer survivors and matched cohort
| No. death/person-years | Mortality rate (per 1,000 person-years) (95% CI) | Age-adjusted HR (95% CI) | aHR (95% CI)† | aHR (95% CI)‡ | |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Cancer survivors (N=946) | |||||
| Without LMM (N=736) | 208/6,862.7 | 30.3 (26.5, 34.7) | REF | REF | REF |
| With LMM (N=210) | 113/1,686.3 | 67.0 (55.7, 80.6) | 1.63 (1.29, 2.06) | 1.73 (1.31, 2.29) | 1.66 (1.21, 2.28) |
| overall: 321/8548.9 | overall: 37.5 (33.7, 41.9) | ||||
| Matched cohort (N=1,857) | |||||
| Without LMM (N=1,492) | 333/14,667.1 | 22.7 (20.4, 25.3) | REF | REF | REF |
| With LMM (N=365) | 162/3,386.8 | 47.8 (41.0, 55.8) | 1.29 (1.06, 1.57) | 1.24 (0.98, 1.56) | 1.24 (0.95, 1.63) |
| overall: 495/18,053.9 | overall: 27.4 (25.1, 29.9) | ||||
| CVD-specific mortality | |||||
| Cancer survivors (N=946) | |||||
| Without LMM (N=736) | 36/6,862.7 | 5.2 (3.8, 7.3) | REF | REF | REF |
| With LMM (N=210) | 22/1,686.3 | 13.0 (8.6, 19.8) | 1.64 (0.95, 2.81) | 2.13 (1.14, 4.00) | 2.46 (1.27, 4.74) |
| overall: 58/8,548.9 | overall: 6.8 (5.2, 8.8) | ||||
| Matched cohort (N=1,857) | |||||
| Without LMM (N=1,492) | 82/14,667.1 | 5.6 (4.5, 6.9) | REF | REF | REF |
| With LMM (N=365) | 40/3,386.8 | 11.8 (8.7, 16.1) | 1.25 (0.85, 1.85) | 1.21 (0.75, 1.93) | 1.22 (0.72, 2.06) |
| overall: 122/18,053.9 | overall: 6.8 (5.7, 8.1) |
Abbreviations: aHR: adjusted hazard ratio, CI: confidence interval, CVD: cardiovascular disease
The model adjusted for age, sex, race, education, marital status, smoking status, relative fat mass, energy intake, physical exercise, burden of comorbidities, history of more than 1 cancer (for survivors). For cancer survivors, the model was stratified by time elapse since cancer diagnosis and smoking status when outcome was all-cause mortality.
The model adjusted for the same set of covariate and was corrected for NHANES sampling weight
In subgroup analyses for cancer survivors (Table 3), we found that LMM significantly interacted with RFM in relation to all-cause mortality. Specifically, impact of LMM on all-cause mortality was stronger in cancer survivors with lower value of RFM (low and moderate RFM: aHR[LMM+ vs. LMM−]=2.19, 95% CI=1.54, 3.12; high RFM: aHR[LMM+ vs. LMM−]=1.02, 95% CI=0.66, 1.56; p-interaction=0.02). In subgroup analysis for all-cause mortality, although effect size of LMM was substantially larger in males (female: aHR[LMM+ vs. LMM−]=1.20, 95% CI=0.79-1.81; male: aHR[LMM+ vs. LMM−]=1.89, 95% CI=1.35-2.64; p-interaction=0.06), the analytical results only indicated a marginally significant interaction for sex. No significant interaction was observed for cancer survivors when outcome was CVD-specific mortality. We did not identify any significant interaction in subgroup analyses for the matched cohort.
Table 3.
Association between LMM and risk of mortality in subgroups of cancer survivors and matched cohort
| Cancer survivors (N=946) |
Matched cohort (N=1,857) |
|||||
|---|---|---|---|---|---|---|
| No. death/Total (%) | aHR (95% CI)† | p-interaction | No. death/Total (%) | aHR (95% CI)† | p-interaction | |
| All-cause mortality | ||||||
| Age (years) | ||||||
| <65 | 71/539 (13.2) | 2.28 (1.27, 4.10) | 0.17 | 77/1,078 (7.1) | 1.19 (0.60, 2.33) | 0.31 |
| ≥65 | 250/407 (61.4) | 1.79 (1.36, 2.37) | 418/779 (53.7) | 1.70 (1.35, 2.15) | ||
| Sex | ||||||
| Female | 142/540 (26.3) | 1.20 (0.79, 1.81) | 0.06 | 193/1,062 (18.2) | 1.20 (0.82, 1.73) | 0.63 |
| Male | 179/406 (44.1) | 1.89 (1.35, 2.64) | 302/795 (38.0) | 1.32 (1.00, 1.75) | ||
| RFM * | ||||||
| Low and moderate | 156/434 (35.9) | 2.19 (1.54, 3.12) | 0.02 | 212/835 (25.4) | 1.18 (0.88, 1.59) | 0.08 |
| High | 165/512 (32.2) | 1.02 (0.66, 1.56) | 283/1,022 (27.7) | 1.38 (1.00, 1.91) | ||
| Energy intake (kcal/day) | ||||||
| <1790.3 | 197/473 (41.7) | 1.47 (1.08, 2.01) | 0.41 | 291/953 (30.5) | 1.22 (0.91, 1.64) | 0.61 |
| ≥1790.3 | 124/473 (26.2) | 1.46 (0.94, 2.26) | 204/904 (22.6) | 1.28 (0.91, 1.80) | ||
| Comorbidity burden | ||||||
| 0 | 90/417 (21.6) | 1.34 (0.81, 2.24) | 0.55 | 167/963 (17.3) | 1.27 (0.87, 1.85) | 0.65 |
| 1-7 | 231/529 (43.7) | 1.64 (1.21, 2.22) | 328/894 (36.7) | 1.35 (1.03, 1.79) | ||
| Survival time (year) | ||||||
| 1-6 | 149/441 (33.8) | 1.28 (0.86, 1.90) | 0.52 | N/A | N/A | N/A |
| ≥7 | 172/505 (34.1) | 1.63 (1.16, 2.29) | N/A | N/A | ||
| CVD-specific mortality | ||||||
| Age (years) | ||||||
| <65 | 7/539 (1.3) | 1.13 (0.10, 12.99) | 0.52 | 17/1,078 (1.6) | 1.62 (0.33, 7.92) | 0.89 |
| ≥65 | 51/407 (12.5) | 2.71 (1.46, 5.04) | 105/779 (13.5) | 1.74 (1.10, 2.75) | ||
| Sex | ||||||
| Female | 21/540 (3.9) | 4.17 (1.40, 12.42) | 0.50 | 43/1,062 (4.1) | 1.23 (0.56, 2.67) | 0.42 |
| Male | 37/406 (9.1) | 1.35 (0.63, 2.90) | 79/795 (9.9) | 1.41 (0.82, 2.42) | ||
| RFM * | ||||||
| Low and moderate | 22/434 (5.1) | 1.93 (0.72, 5.19) | 0.80 | 47/835 (5.6) | 1.42 (0.75, 2.67) | 1.00 |
| High | 36/512 (7.0) | 2.25 (1.02, 4.97) | 75/1,022 (7.3) | 1.25 (0.66, 2.35) | ||
| Energy intake (kcal/day) | ||||||
| <1790.3 | 31/473 (6.6) | 1.68 (0.76, 3.73) | 0.73 | 70/953 (7.4) | 1.24 (0.69, 2.21) | 0.57 |
| ≥1790.3 | 27/473 (5.7) | 1.85 (0.71, 4.77) | 52/904 (5.8) | 1.73 (0.87, 3.43) | ||
| Comorbidity burden | ||||||
| 0 | 12/417 (2.9) | 1.16 (0.28, 4.82) | 0.39 | 32/963 (3.3) | 1.77 (0.76, 4.10) | 0.54 |
| 1-7 | 46/529 (8.7) | 2.12 (1.07, 4.17) | 90/894 (10.1) | 1.29 (0.76, 2.20) | ||
| Survival time (year) | ||||||
| 1-6 | 27/441 (6.1) | 2.17 (0.87, 5.37) | 0.34 | N/A | N/A | N/A |
| ≥7 | 31/505 (6.1) | 1.72 (0.75, 3.98) | N/A | N/A | ||
Abbreviations: aHR: adjusted hazard ratio, CI: confidence interval, RFM: relative fat mass
Cutoffs of relative fat mass were as follows—low: female: <35%, male: <25%; moderate: female: 35–39.9%, male: 25–29.9%; high: female: ≥40%, male: ≥30%.
The model treated LMM as the exposure of interest and adjusted for age, sex, race, education, marital status, smoking status, relative fat mass, energy intake, physical exercise, burden of comorbidities, history of more than 1 cancer (for survivors), and time elapsed since cancer diagnosis (for survivors). In each set of subgroup, factors used for stratification were not included in the multivariable model.
The dose-response curves suggested that risk of all-cause mortality (Figure 2A) declined in both cancer survivors and matched cohort as ASMI increased. Specifically, the whole curve of cancer survivors was statistically significant and it declined more drastically than that of the matched cohort, and the curve of the matched cohort became non-significant when ASMI was higher than 8.10 kg/m2. When outcome was CVD-specific mortality (Figure 2B), the dose-response curves declined in both cancer survivors and the matched cohort as ASMI increased. However, in CVD-specific mortality analysis, the dose-response relationship was significant only when ASMI was lower than approximately 6.80 kg/m2 for cancer survivors; this relationship was significant for the matched cohort when ASMI was lower than approximately 5.00 kg/m2. The likelihood ratio tests did not support non-linearity (ps>0.05). In analysis excluding participants with chronic kidney disease, heart failure, and diabetes (Supplementary Table 2), the association pattern did not change substantially for the match cohort; for cancer survivors, the association of all-cause mortality was still positive and significant, but the positive HR of CVD-specific mortality turned non-significant, which could be caused by the reduction in sample size.
Figure 2.
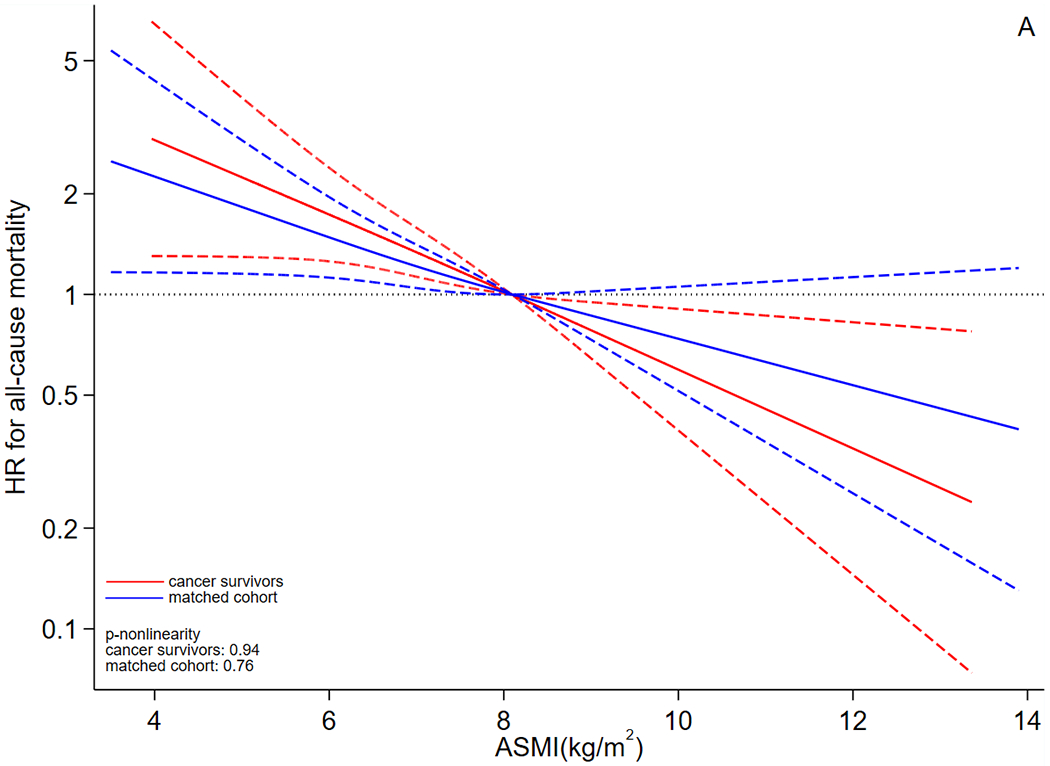
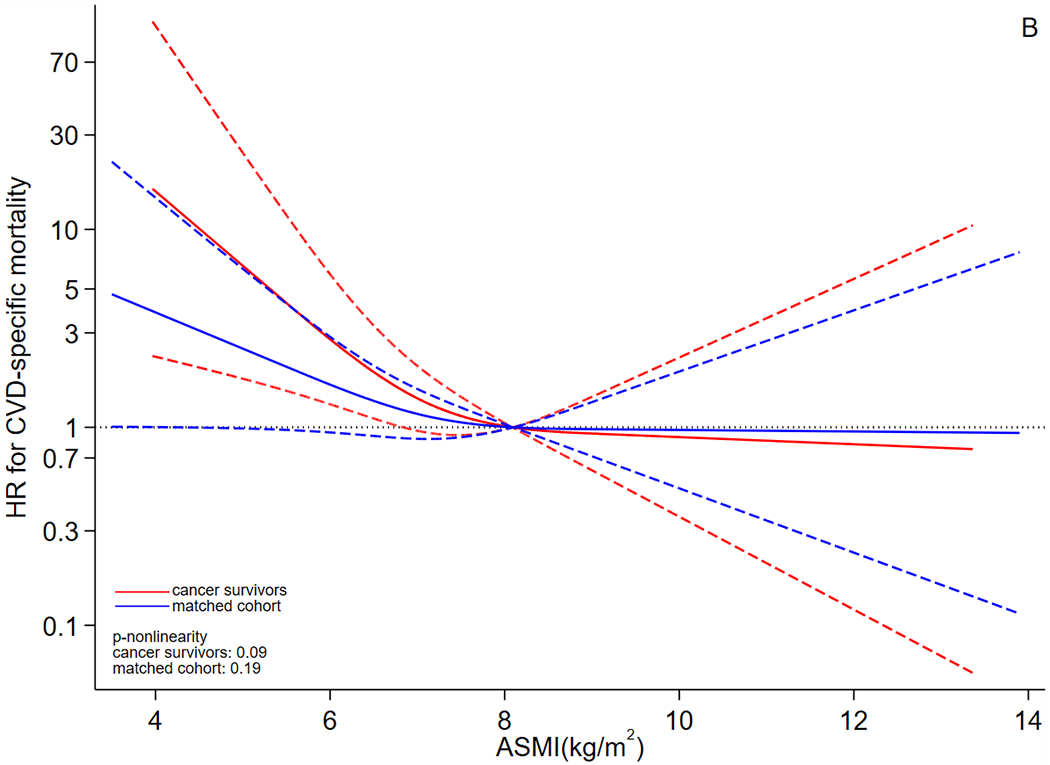
Dose-response curves for association between ASMI and risk of (A) all-cause and (B) CVD-specific mortality. Abbreviations: ASMI: appendicular skeletal muscle mass index, CVD: cardiovascular disease, HR: hazard ratio. The y axis was log-transformed for better visualization. The dose-response analysis employed the restricted cubic spline for ASMI and adjusted for the same set of covariates as the primary Cox model. Red lines represent cancer survivors and blue lines represent matched cohort. The solid lines are the fitted lines and dash lines are the 95% confidence intervals. The dotted lines are reference lines. ASMI=8.10 kg/m2 was used as the reference in the curve.
Discussion
Our study shows that LMM is associated with a higher risk of all-cause and CVD-specific mortality in cancer survivors. The primary multivariable Cox proportional hazards regression models, along with the dose-response curves, show a potential that the magnitude of association between LMM and death is stronger for cancer survivors than adults without cancer history, suggesting that cancer survivors may be more sensitive to skeletal muscle loss than their cancer-free counterparts. The subgroup analysis indicates that the relationship between LMM and risk of death is significantly positive in cancer survivors with lower RFM but the association in cancer survivors with higher RFM is almost null, and we have a speculation for this phenomenon. In clinical practice, both adipose tissue and skeletal muscles at abdominal area can be measured by cross-sectional imaging at the third lumbar level [29]. Thus, estimation of RFM, which incorporated waist circumferences, could be a mixed measure of body fat and skeletal muscle; this suggests that cancer survivors with larger RFM value may also have higher mass of skeletal muscle in abdominal area, antagonizing the adverse effects of LMM on survival [30]. This speculation is consistent with prior studies using body mass index (BMI) as a measure of adipose tissue which find that adults with prior cancer diagnosis can have a better survival if they have a higher BMI, since patients with BMI≥25 kg/m2 may also have higher muscle mass than their normal-weight counterparts [30]. Moreover, the magnitude of association between LMM and all-cause mortality in male cancer survivors is stronger than that of female cancer survivors, although the analysis only indicates a marginally significant interaction between sex and LMM. At the population level, some adverse lifestyle behaviors (e.g. smoking, insufficient fruit and vegetable consumption, and heavy alcohol drinking) [31,32], which are more common in males, may interact with LMM, synergistically disturb homeostasis, and lead to a much higher risk of death among male cancer survivors; however, this hypothesis should be examined in future studies with larger sample sizes. We did not obtain statistical evidence to support interaction between LMM and body fat or sex among participants without cancer history; one reason could be that adverse impacts of cancer treatment and pre-existing factors relevant to carcinogenesis (e.g., inflammation) make cancer survivors heterogeneous from their cancer-free counterparts, which leads to distinct outcomes in interaction test.
Several meta-analyses assessed to what extent risk of death in cancer patients varied by muscle mass. For example, a meta-analysis [33] summarized evidence from 39 studies and found that perioperative sarcopenia was associated with a shorter survival in gastric cancer patients. Another meta-analysis [34] synthesized data from 38 published articles and reported that cancer patients with LMM mass at diagnosis had a higher risk of death. Compared to the aforementioned studies, ours explored the research question from a different vision by focusing on adults with prior cancer diagnosis who had survived for at least 1 year since diagnosis, making our outcomes more translatable in cancer care continuum during survivorship period. Muscle mass measured during perioperative period can be different from mass measured during the survivorship period because cancer treatment has the potential to cause muscle mass loss [35]. Additionally, using perioperative or pre-treatment muscle mass in time-to-event analysis usually incorporates short-term mortality because some cancer patients may die within a short time after surgery [36]. Therefore, utilizing body composition measured at least 1 year after cancer diagnosis for our study population can preclude impact of the aforementioned effects in our analysis and better represents association pattern between LMM and mortality in the long-term survivorship period.
Several mechanisms may explain the positive association of all-cause and CVD-specific death with LMM in cancer survivors. First, there is a strong connection between LMM and systemic inflammation. Laboratory evidence suggests that pro-inflammatory cytokines secreted from tumors, such as interleukin-1, interleukin-6, and tumor necrosis factor-α, may induce loss of appetite, degradation of myofibrillar proteins, and reduction of protein synthesis, which ultimately result in muscle wasting [37,38]. Our prior study, which included 2,483 older adults living with chronic diseases, found that blood c-reactive protein and systemic immune-inflammation index were positively associated with sarcopenia [5]. At the population level, inflammation has been found to be associated with risk of death and adverse cardiovascular outcomes in adults with prior cancer diagnosis [39–41]. Second, LMM is an indicator of progressive withdrawal of anabolism and an increased catabolism [6]; these unfavorable metabolic events are strongly linked to frailty, functional decline, and organ failure in human [42,43], which are associated with shorter life expectancy. In addition, in people who received cancer treatment, LMM has been found to be associated with treatment toxicity (e.g. cardiotoxicity) whose burden can worsen prognosis and increase mortality risk [44,45]. For example, a meta-analysis [46] synthesizing data from 48 studies (4,803 study participants) reported that cancer patients with LMM had a 100-150% relative increase in risk of treatment toxicity.
Our study has some strengths in design and analysis. First, LMM was measured by DEXA, and vital status and cause of death were measured by linkage to NDI and ICD codes, respectively; these methods ensured validity of exposure and outcome of interest in measurement. Second, we assessed the association pattern of LMM in two different populations with similar demographic distribution, which makes the outcomes comparable between these two groups. In addition to primary Cox proportional hazards regression models, we applied restricted cubic splines which depicted the association from a dose-response perspective. However, several limitations should be noted when interpreting the results. For example, all cancer-related information, including cancer type and age at diagnosis, was self-reported, and this approach can be less valid compared to medical record review or registry data. Moreover, cancer adjuvant therapy is a factor that may induce cardiotoxicity during and after treatment [47], thus multivariable models without adjusting for cancer treatment may incorporate residual confounding in analysis.
In conclusion, cancer survivors with LMM have a poorer prognosis than survivors without LMM and may have a higher risk of fatal cardiovascular events. Health practitioners should consider monitoring body composition in cancer care continuum to improve prognosis and cardiovascular health. Future cohort studies incorporating body composition and cancer-specific measures (e.g. stage, cancer treatment, and histological type) will be needed to more precisely assess impact of LMM on adverse health outcomes (e.g. death, cardiovascular events, etc.) of cancer survivors.
Supplementary Material
Acknowledgement:
The authors thank the NHANES participants for their contributions of the data.
Funding source:
The study was internally funded through the University of Florida Health Cancer Center.
Footnotes
Conflict of interest: None of the authors had any conflict of interest.
Ethical compliance: We used a publicly de-identified dataset that does not require IRB approval.
References
- [1].Prado CM, Purcell SA, Alish C, Pereira SL, Deutz NE, Heyland DK, Goodpaster BH, Tappenden KA, Heymsfield SB, Implications of low muscle mass across the continuum of care: a narrative review, Ann Med. 50 (2018) 675–693. 10.1080/07853890.2018.1511918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R, Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies, J Diabetes Metab Disord. 16 (2017) 21. 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peterson SJ, Braunschweig CA, Prevalence of Sarcopenia and Associated Outcomes in the Clinical Setting, Nutr Clin Pract. 31 (2016) 40–48. 10.1177/0884533615622537. [DOI] [PubMed] [Google Scholar]
- [4].Wilkinson DJ, Piasecki M, Atherton PJ, The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans, Ageing Res Rev. 47 (2018) 123–132. 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Karanth SD, Washington C, Cheng T-YD, Zhou D, Leeuwenburgh C, Braithwaite D, Zhang D, Inflammation in Relation to Sarcopenia and Sarcopenic Obesity among Older Adults Living with Chronic Comorbidities: Results from the National Health and Nutrition Examination Survey 1999-2006, Nutrients. 13 (2021) 3957. 10.3390/nu13113957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Narici MV, Maffulli N, Sarcopenia: characteristics, mechanisms and functional significance, Br Med Bull. 95 (2010) 139–159. 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- [7].An Update on Cancer Deaths in the United States. https://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm [Last accessed April 2022], (n.d.).
- [8].Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer [last accessed April 2022], (n.d.).
- [9].Gebauer J, Higham C, Langer T, Denzer C, Brabant G, Long-Term Endocrine and Metabolic Consequences of Cancer Treatment: A Systematic Review, Endocr Rev. 40 (2019) 711–767. 10.1210/er.2018-00092. [DOI] [PubMed] [Google Scholar]
- [10].Dougan M, Understanding and Overcoming the Inflammatory Toxicities of Immunotherapy, Cancer Immunol Res. 8 (2020) 1230–1235. 10.1158/2326-6066.CIR-20-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang D, Leeuwenburgh C, Zhou D, Gong Y, Pahor M, Licht JD, Braithwaite D, Analysis of Biological Aging and Risks of All-Cause and Cardiovascular Disease-Specific Death in Cancer Survivors, JAMA Netw Open. 5 (2022) e2218183. 10.1001/jamanetworkopen.2022.18183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Santilli V, Bernetti A, Mangone M, Paoloni M, Clinical definition of sarcopenia, Clin Cases Miner Bone Metab. 11 (2014) 177–180. [PMC free article] [PubMed] [Google Scholar]
- [13].Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP, Zaorsky NG, A population-based study of cardiovascular disease mortality risk in US cancer patients, Eur Heart J. 40 (2019) 3889–3897. 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, Carroll MD, Hirsch R, Schober S, Johnson CL, The National Health and Nutrition Examination Survey: Sample Design, 1999-2006, Vital Health Stat 2. (2012) 1–39. [PubMed] [Google Scholar]
- [15].Goodman MJ, Ghate SR, Mavros P, Sen S, Marcus RL, Joy E, Brixner DI, Development of a practical screening tool to predict low muscle mass using NHANES 1999-2004, J Cachexia Sarcopenia Muscle. 4 (2013) 187–197. 10.1007/s13539-013-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel J-P, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People, Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People, Age Ageing. 39 (2010) 412–423. 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rom O, Kaisari S, Aizenbud D, Reznick AZ, Sarcopenia and smoking: a possible cellular model of cigarette smoke effects on muscle protein breakdown, Ann N Y Acad Sci. 1259 (2012) 47–53. 10.1111/j.1749-6632.2012.06532.x. [DOI] [PubMed] [Google Scholar]
- [18].Woolcott OO, Bergman RN, Defining cutoffs to diagnose obesity using the relative fat mass (RFM): Association with mortality in NHANES 1999-2014, Int J Obes (Lond). 44 (2020) 1301–1310. 10.1038/s41366-019-0516-8. [DOI] [PubMed] [Google Scholar]
- [19].Bowen TS, Schuler G, Adams V, Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training, J Cachexia Sarcopenia Muscle. 6 (2015) 197–207. 10.1002/jcsm.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cho YJ, Lim Y-H, Yun JM, Yoon H-J, Park M, Sex- and age-specific effects of energy intake and physical activity on sarcopenia, Sci Rep. 10 (2020) 9822. 10.1038/s41598-020-66249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Roh E, Choi KM, Health Consequences of Sarcopenic Obesity: A Narrative Review, Front Endocrinol (Lausanne). 11 (2020) 332. 10.3389/fendo.2020.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Swan L, Warters A, O’Sullivan M, Socioeconomic Inequality and Risk of Sarcopenia in Community-Dwelling Older Adults, Clin Interv Aging. 16 (2021) 1119–1129. 10.2147/CIA.S310774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Karakelides H, Nair KS, Sarcopenia of aging and its metabolic impact, Curr Top Dev Biol. 68 (2005) 123–148. 10.1016/S0070-2153(05)68005-2. [DOI] [PubMed] [Google Scholar]
- [24].Segal KR, Lacayanga I, Dunaif A, Gutin B, Pi-Sunyer FX, Impact of body fat mass and percent fat on metabolic rate and thermogenesis in men, Am J Physiol. 256 (1989) E573–579. 10.1152/ajpendo.1989.256.5.E573. [DOI] [PubMed] [Google Scholar]
- [25].Roberts SB, Rosenberg I, Nutrition and aging: changes in the regulation of energy metabolism with aging, Physiol Rev. 86 (2006) 651–667. 10.1152/physrev.00019.2005. [DOI] [PubMed] [Google Scholar]
- [26].Chemotherapy Side Effects. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/chemotherapy-side-effects.html [Last accessed Nov 2022], (n.d.).
- [27].Shepherd BE, Rebeiro PF, Caribbean, Central and South America network for HIV epidemiology, Brief Report: Assessing and Interpreting the Association Between Continuous Covariates and Outcomes in Observational Studies of HIV Using Splines, J Acquir Immune Defic Syndr. 74 (2017) e60–e63. 10.1097/QAI.0000000000001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Toomey CM, McCormack WG, Jakeman P, The effect of hydration status on the measurement of lean tissue mass by dual-energy X-ray absorptiometry, Eur J Appl Physiol. 117 (2017) 567–574. 10.1007/s00421-017-3552-x. [DOI] [PubMed] [Google Scholar]
- [29].Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC, Optimal body size adjustment of L3 CT skeletal muscle area for sarcopenia assessment, Sci Rep. 11 (2021) 279. 10.1038/s41598-020-79471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Caan BJ, Cespedes Feliciano EM, Kroenke CH, The Importance of Body Composition in Explaining the Overweight Paradox in Cancer-Counterpoint, Cancer Res. 78 (2018) 1906–1912. 10.1158/0008-5472.CAN-17-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim M, Choi KS, Suh M, Jun JK, Chuck KW, Park B, Risky Lifestyle Behaviors among Gastric Cancer Survivors Compared with Matched Non-cancer Controls: Results from Baseline Result of Community Based Cohort Study, Cancer Res Treat. 50 (2018) 738–747. 10.4143/crt.2017.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhu Z, Zhang D, Wang JH-Y, Qiao Y, Liu Y, Braithwaite D, Is education or income associated with insufficient fruit and vegetable intake among cancer survivors? A cross-sectional analysis of 2017 BRFSS data, BMJ Open. 10 (2020) e041285. 10.1136/bmjopen-2020-041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kamarajah SK, Bundred J, Tan BHL, Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis, Gastric Cancer. 22 (2019) 10–22. 10.1007/s10120-018-0882-2. [DOI] [PubMed] [Google Scholar]
- [34].Shachar SS, Williams GR, Muss HB, Nishijima TF, Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review, Eur J Cancer. 57 (2016) 58–67. 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- [35].Aversa Z, Costelli P, Muscaritoli M, Cancer-induced muscle wasting: latest findings in prevention and treatment, Ther Adv Med Oncol. 9 (2017) 369–382. 10.1177/1758834017698643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vogelsang RP, Bojesen RD, Hoelmich ER, Orhan A, Buzquurz F, Cai L, Grube C, Zahid JA, Allakhverdiiev E, Raskov HH, Drakos I, Derian N, Ryan PB, Rijnbeek PR, Gögenur I, Prediction of 90-day mortality after surgery for colorectal cancer using standardized nationwide quality-assurance data, BJS Open. 5 (2021) zrab023. 10.1093/bjsopen/zrab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lee SJ, Park YJ, Cartmell KB, Sarcopenia in cancer survivors is associated with increased cardiovascular disease risk, Support Care Cancer. 26 (2018) 2313–2321. 10.1007/s00520-018-4083-7. [DOI] [PubMed] [Google Scholar]
- [38].Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC, TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation, Am J Physiol Endocrinol Metab. 282 (2002) E336–347. 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- [39].Villaseñor A, Flatt SW, Marinac C, Natarajan L, Pierce JP, Patterson RE, Postdiagnosis C-reactive protein and breast cancer survivorship: findings from the WHEL study, Cancer Epidemiol Biomarkers Prev. 23 (2014) 189–199. 10.1158/1055-9965.EPI-13-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shafique K, Proctor MJ, McMillan DC, Qureshi K, Leung H, Morrison DS, Systemic inflammation and survival of patients with prostate cancer: evidence from the Glasgow Inflammation Outcome Study, Prostate Cancer Prostatic Dis. 15 (2012) 195–201. 10.1038/pcan.2011.60. [DOI] [PubMed] [Google Scholar]
- [41].Calvillo-Argüelles O, Jaiswal S, Shlush LI, Moslehi JJ, Schimmer A, Barac A, Thavendiranathan P, Connections Between Clonal Hematopoiesis, Cardiovascular Disease, and Cancer: A Review, JAMA Cardiol. 4 (2019) 380–387. 10.1001/jamacardio.2019.0302. [DOI] [PubMed] [Google Scholar]
- [42].Avgerinou C, Sarcopenia: why it matters in general practice, Br J Gen Pract. 70 (2020) 200–201. 10.3399/bjgp20X709253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wilson D, Jackson T, Sapey E, Lord JM, Frailty and sarcopenia: The potential role of an aged immune system, Ageing Res Rev. 36 (2017) 1–10. 10.1016/j.arr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- [44].Antoun S, Borget I, Lanoy E, Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer, Curr Opin Support Palliat Care. 7 (2013) 383–389. 10.1097/SPC.0000000000000011. [DOI] [PubMed] [Google Scholar]
- [45].Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB, Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment, Clin Cancer Res. 15 (2009) 2920–2926. 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- [46].Surov A, Pech M, Gessner D, Mikusko M, Fischer T, Alter M, Wienke A, Low skeletal muscle mass is a predictor of treatment related toxicity in oncologic patients. A meta-analysis, Clin Nutr. 40 (2021) 5298–5310. 10.1016/j.clnu.2021.08.023. [DOI] [PubMed] [Google Scholar]
- [47].Conway A, McCarthy AL, Lawrence P, Clark RA, The prevention, detection and management of cancer treatment-induced cardiotoxicity: a meta-review, BMC Cancer. 15 (2015) 366. 10.1186/s12885-015-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


