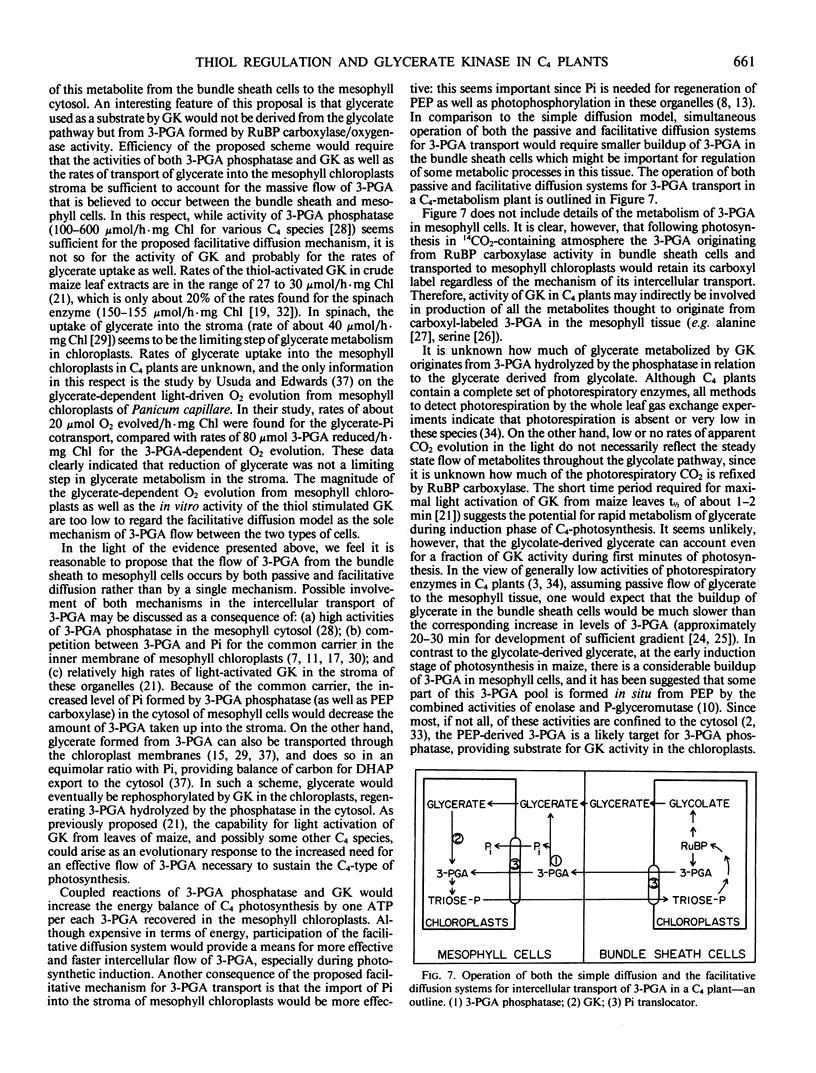
Abstract
We have recently reported that the activity of maize leaf glycerate kinase [EC 2.7.1.31] is regulated in vivo by the light/dark transition, possibly involving the ferredoxin/thioredoxin mechanism, and that the stimulating effect of light can be mimicked in vitro by incubation of crude leaf extract with reducing compounds (LA Kleczkowski, DD Randall 1985 Plant Physiol 79: 274-277). In the present study it was found that the time course of thiol activation of the enzyme was substantially dependent on the presence of some low molecular weight inhibitor(s) of activation found both in leaf extracts and mesophyll chloroplasts. Activity of glycerate kinase from maize as well as wheat leaves increased upon greening of etiolated plants and was correlated with the development of photosynthetic apparatus in these species. The maize enzyme was strongly activated by thiols at all stages of development from etiolated to green seedlings. Thiol activation of glycerate kinase was observed for a number of C4 plants, notably of the nicotinamide adenine dinucleotide phosphate-malic enzyme type, with the strongest effect found for the enzyme from leaf extracts of maize and sorghum (10- and 8-fold activation, respectively). Among the C3 species tested, only the enzyme from soybean leaves was affected under the same conditions (1.6-fold activation). This finding was reflected by an apparent lack of cross-reactivity between the enzyme from maize leaves and antibodies raised against purified spinach leaf glycerate kinase. We suggest that, in addition to its role as a final step of photorespiration in leaves, glycerate kinase from C4 species may serve as a part of the facilitative diffusion system for the intercellular transport of 3-phosphoglycerate. Simultaneous operation of both the passive and the facilitative diffusion mechanisms of 3-phosphoglycerate transport in C4 plants is postulated.
Full text
PDF
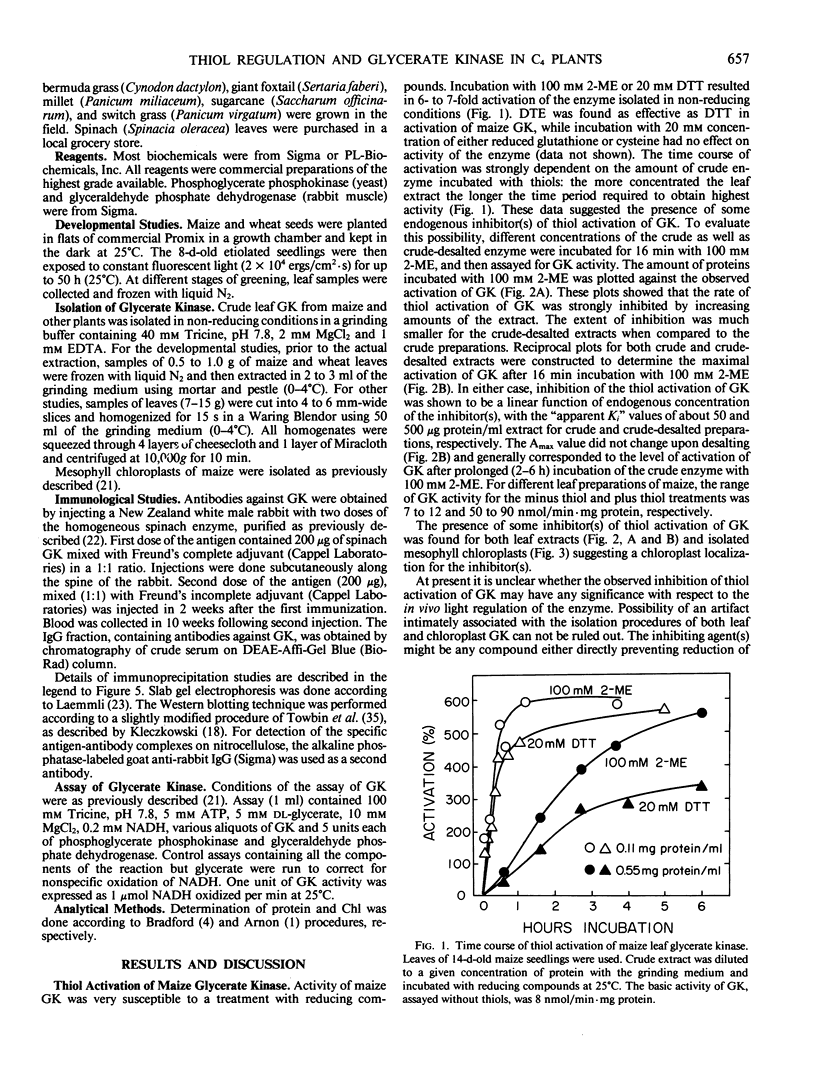
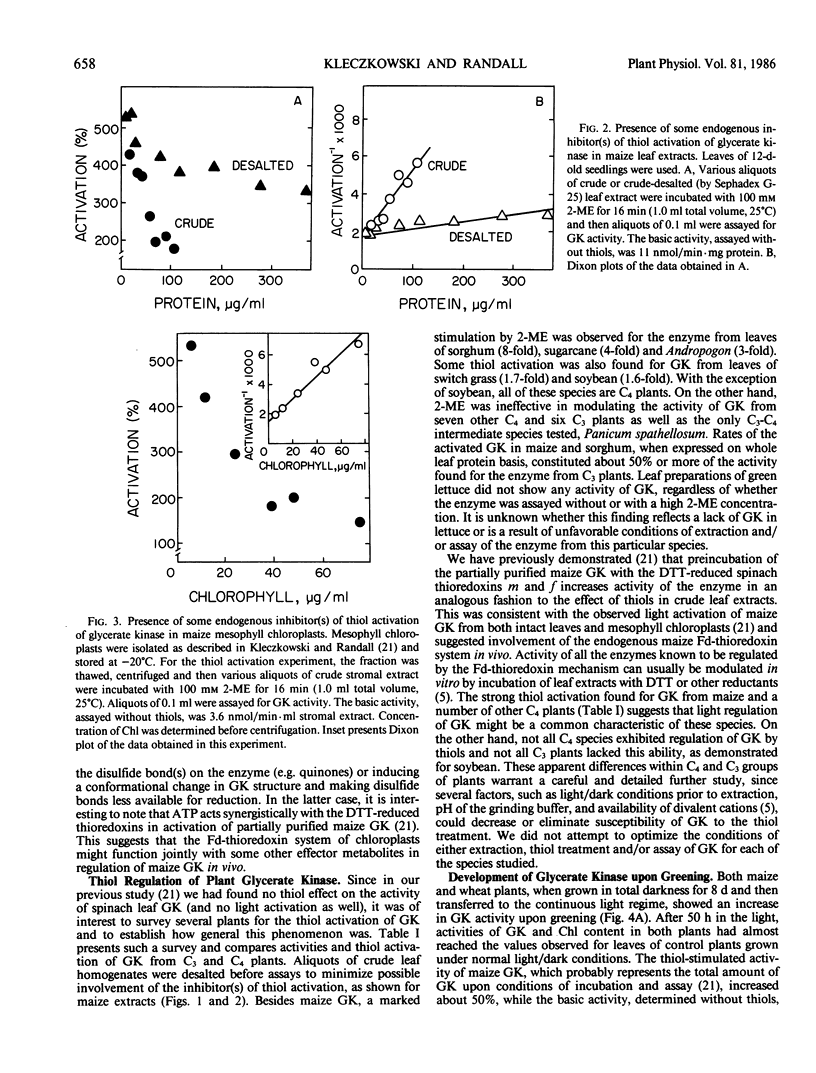

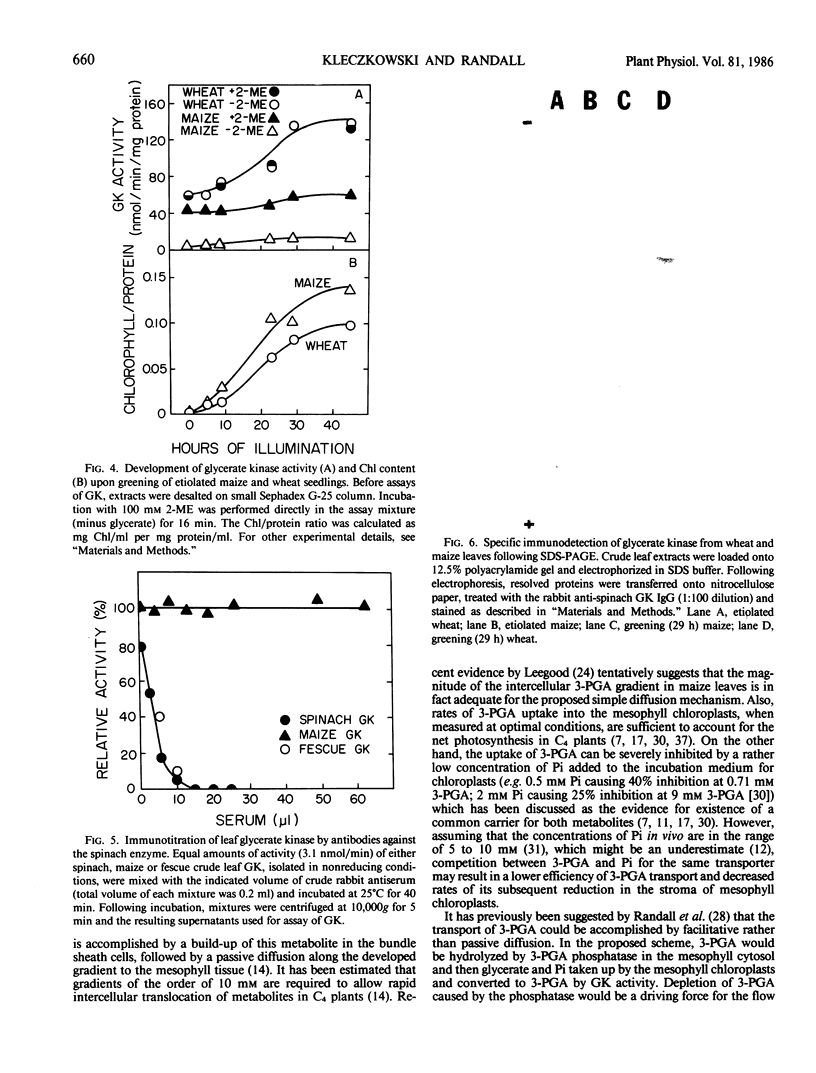

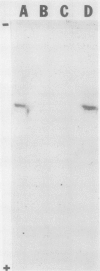
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chapman K. S., Berry J. A., Hatch M. D. Photosynthetic metabolism in bundle sheath cells of the C4 species Zea mays: Sources of ATP and NADPH and the contribution of photosystem II. Arch Biochem Biophys. 1980 Jul;202(2):330–341. doi: 10.1016/0003-9861(80)90435-x. [DOI] [PubMed] [Google Scholar]
- Day D. A., Hatch M. D. Transport of 3-phosphoglyceric acid, phosphoenolpyruvate, and inorganic phosphate in maize mesophyll chloroplasts,, and the effect of 3-phosphoglyceric acid on malate and phosphoenolpyruvate production. Arch Biochem Biophys. 1981 Oct 15;211(2):743–749. doi: 10.1016/0003-9861(81)90511-7. [DOI] [PubMed] [Google Scholar]
- Hatch M. D. Regulation of enzymes in C4 photosynthesis. Curr Top Cell Regul. 1978;14:1–27. [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D. Light and thiol activation of maize leaf glycerate kinase : the stimulating effect of reduced thioredoxins and ATP. Plant Physiol. 1985 Sep;79(1):274–277. doi: 10.1104/pp.79.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D., Zahler W. L. The substrate specificity, kinetics, and mechanism of glycerate-3-kinase from spinach leaves. Arch Biochem Biophys. 1985 Jan;236(1):185–194. doi: 10.1016/0003-9861(85)90618-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morot-Gaudry J. F. Oxygen effect on photosynthetic and glycolate pathways in young maize leaves. Plant Physiol. 1980 Dec;66(6):1079–1084. doi: 10.1104/pp.66.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON R., TOLBERTNE, KEARNEY P. C. Formation of serine and glyceric acid by the glycolate pathway. Arch Biochem Biophys. 1962 Jul;98:154–163. doi: 10.1016/0003-9861(62)90161-3. [DOI] [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E., Gremel D. 3-Phosphoglycerate Phosphatase in Plants: II. Distribution, Physiological Considerations, and Comparison with P-Glycolate Phosphatase. Plant Physiol. 1971 Oct;48(4):480–487. doi: 10.1104/pp.48.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P. Transport of Glycerate across the Envelope Membrane of Isolated Spinach Chloroplasts. Plant Physiol. 1982 Oct;70(4):1032–1038. doi: 10.1104/pp.70.4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpho M. E., Edwards G. E. Inhibition of 3-Phosphoglycerate-Dependent O(2) Evolution by Phosphoenolpyruvate in C(4) Mesophyll Chloroplasts of Digitaria sanguinalis (L.) Scop. Plant Physiol. 1984 Nov;76(3):711–718. doi: 10.1104/pp.76.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Schmitt M. R., Edwards G. E. Glycerate kinase from leaves of C3 plants. Arch Biochem Biophys. 1983 Jul 1;224(1):332–341. doi: 10.1016/0003-9861(83)90217-5. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D., Goodchild D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 Sep;114(3):489–498. doi: 10.1042/bj1140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H., Edwards G. E. Localization of glycerate kinase and some enzymes for sucrose synthesis in c(3) and c(4) plants. Plant Physiol. 1980 May;65(5):1017–1022. doi: 10.1104/pp.65.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]