Abstract
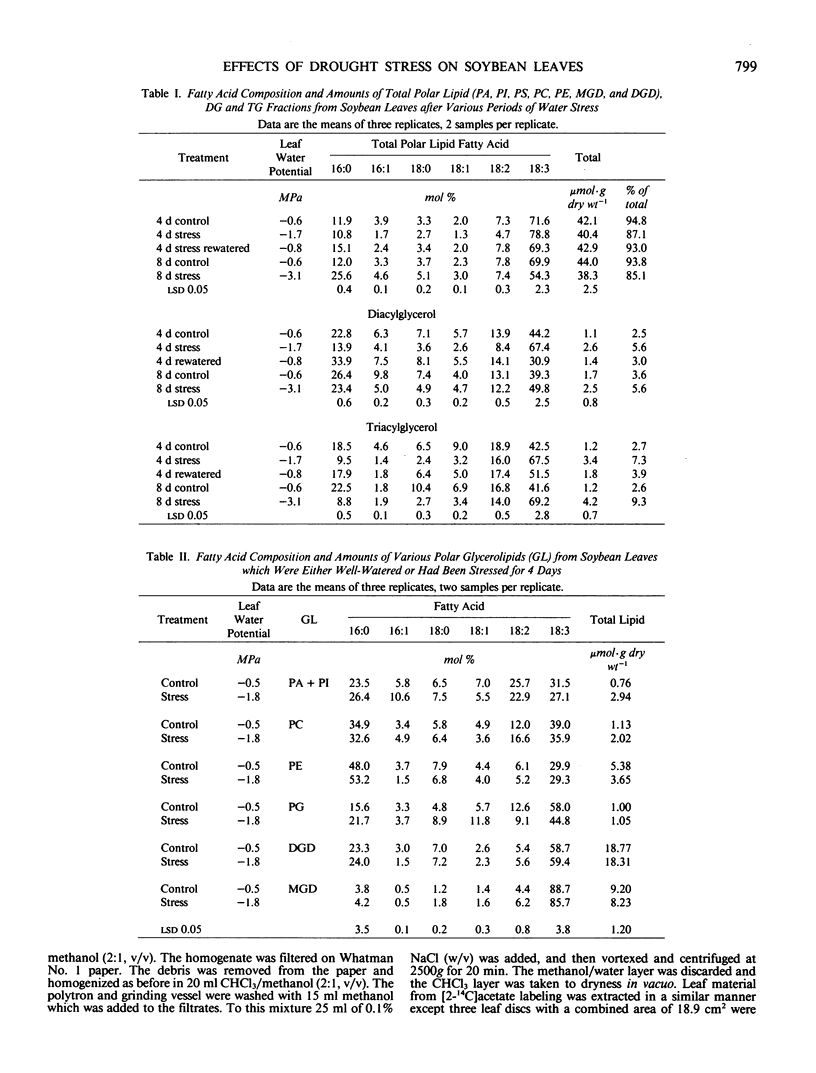
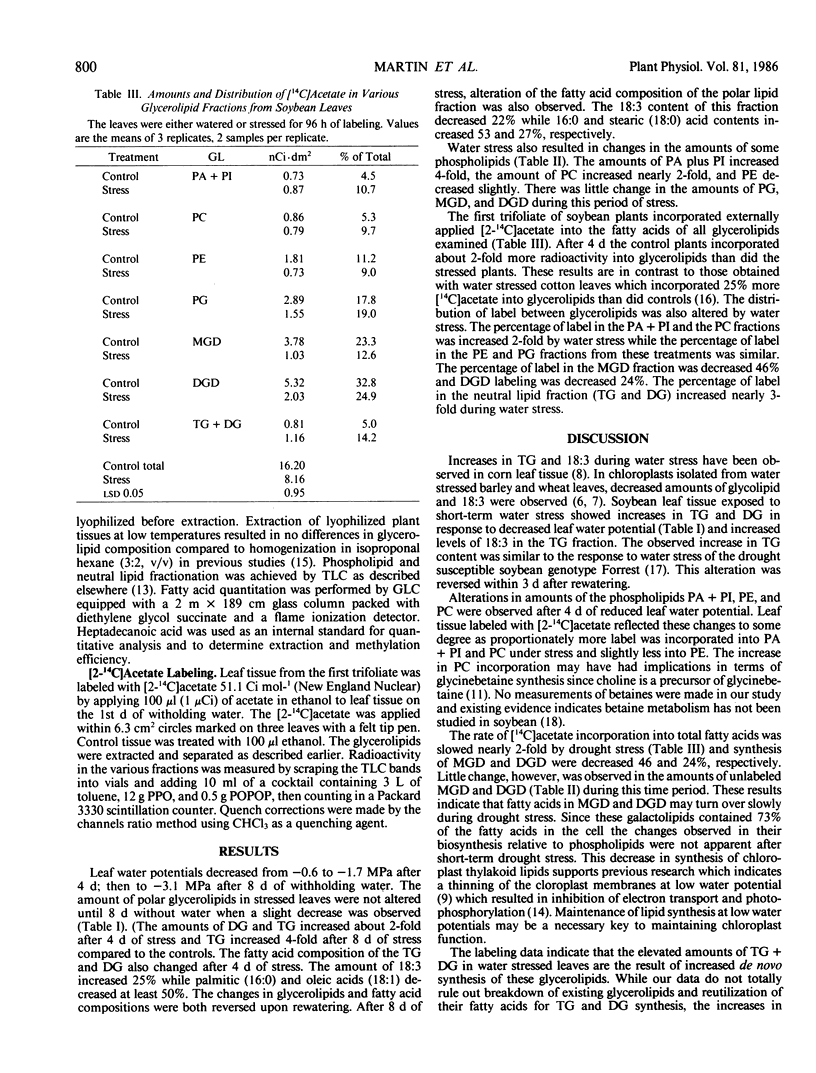
Soybean (Glycine max [L.] Merr.) plants with the first trifoliate leaf fully expanded were exposed to 4 and 8 days of water stress. Leaf water potentials dropped from −0.6 megapascal to −1.7 megapascals after 4 days of stress; then to −3.1 megapascals after 8 days without water. All of the plants recovered when rewatered. The effects of short-term drought stress on triacylglycerol, diacylglycerol, phospholipid, and galactolipid metabolism in the first trifoliate leaves was determined. Leaf triacylglycerol and diacylglycerol content increased 2-fold during the first 4 days of stress and returned to control levels 3 days after rewatering. The polar lipid fraction, which contained phospholipids and galactolipids, changed little during this time. The linolenic acid (18:3) content of the triacylglycerol and diacylglycerol increased 25% during stress and the polar lipid 18:3 content decreased 15%. The pattern of glycerolipid labeling, after applying [2-14C]acetate to intact leaves was altered by water stress. After 4 days of water stress the radioactivity of phosphatidic acid + phosphatidylinositol, phosphatidylcholine, triacylglycerol, and diacylglycerol increased between 4 and 9% (compared to control plans) while radioactivity of phosphatidylethanolamine, monogalactosyldiglyceride, and digalactosyldiglyceride decreased 2 to 11%. These data indicated that increased levels of triacylglycerol and diacylglycerol observed during water stress were attributed to de novo synthesis rather than breakdown or reutilization of existing glycerolipids and fatty acids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. S. Leaf water potentials measured with a pressure chamber. Plant Physiol. 1967 Jan;42(1):133–137. doi: 10.1104/pp.42.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck R. W., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: III. Differing Inhibition of Electron Transport and Photophosphorylation. Plant Physiol. 1974 Mar;53(3):474–479. doi: 10.1104/pp.53.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]
- Weete J. D., Leek G. L., Peterson C. M., Currie H. E., Branch W. D. Lipid and Surface Wax Synthesis in Water-stressed Cotton Leaves. Plant Physiol. 1978 Nov;62(5):675–677. doi: 10.1104/pp.62.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]


