Abstract
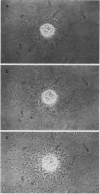
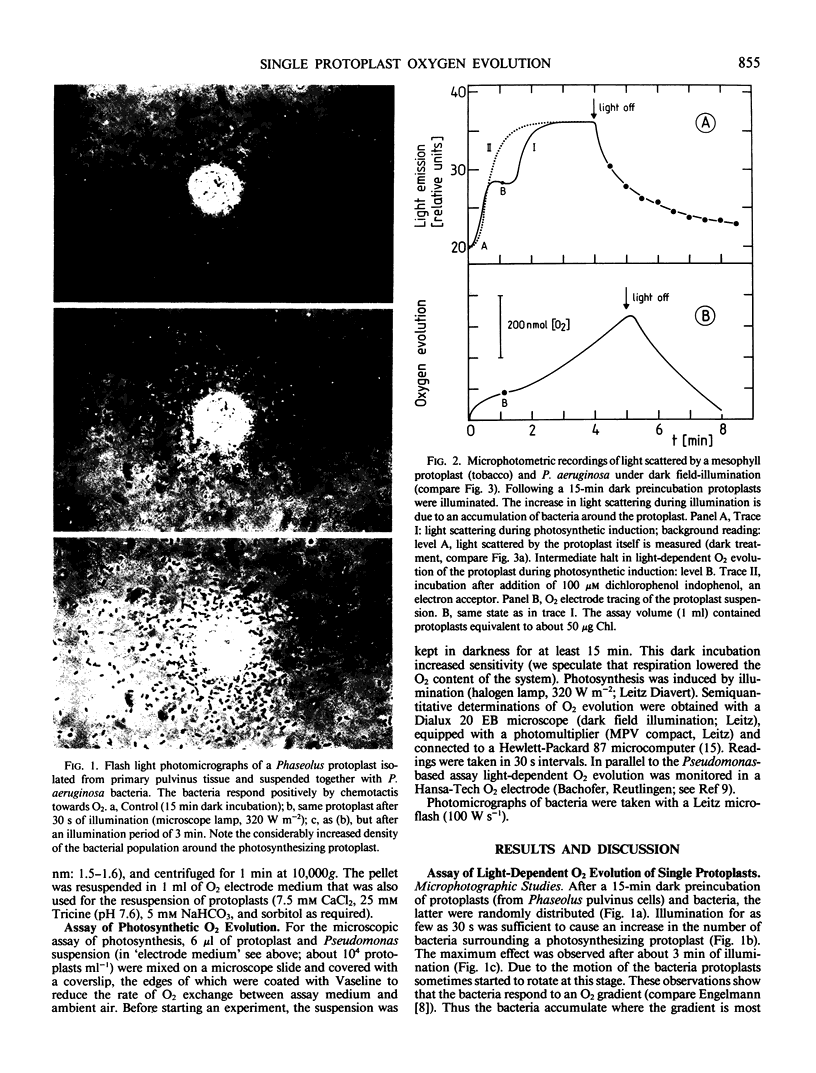
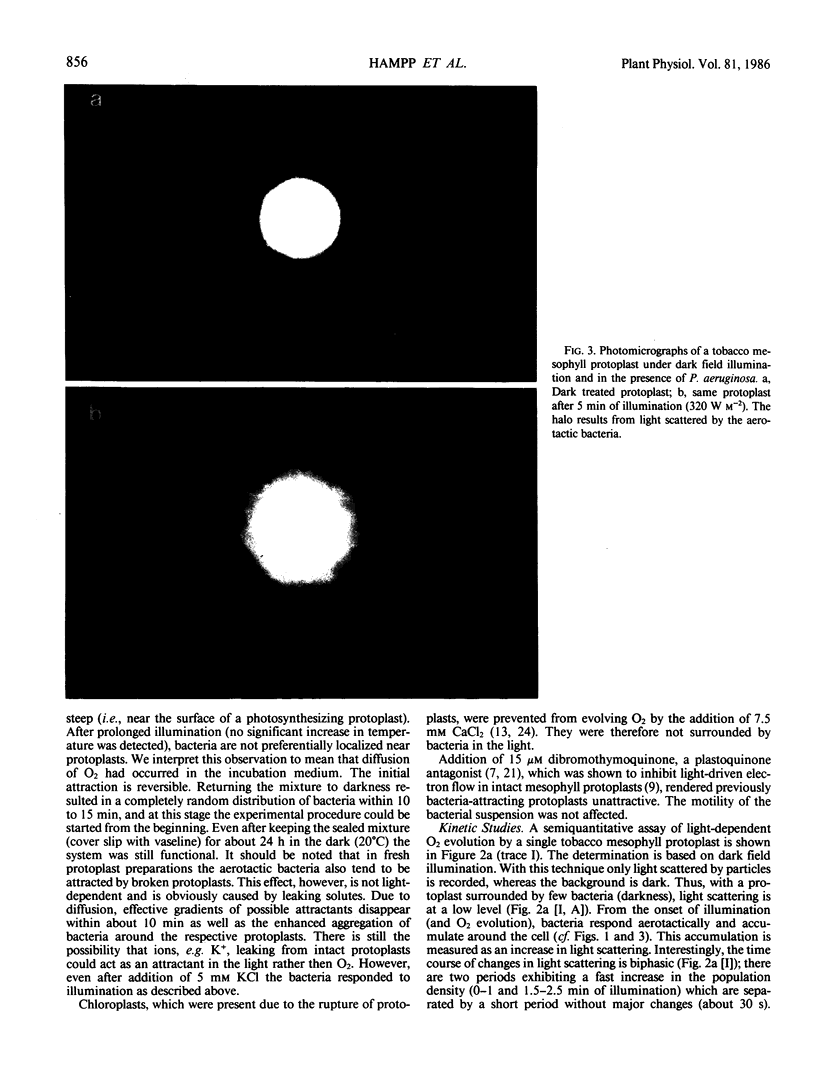
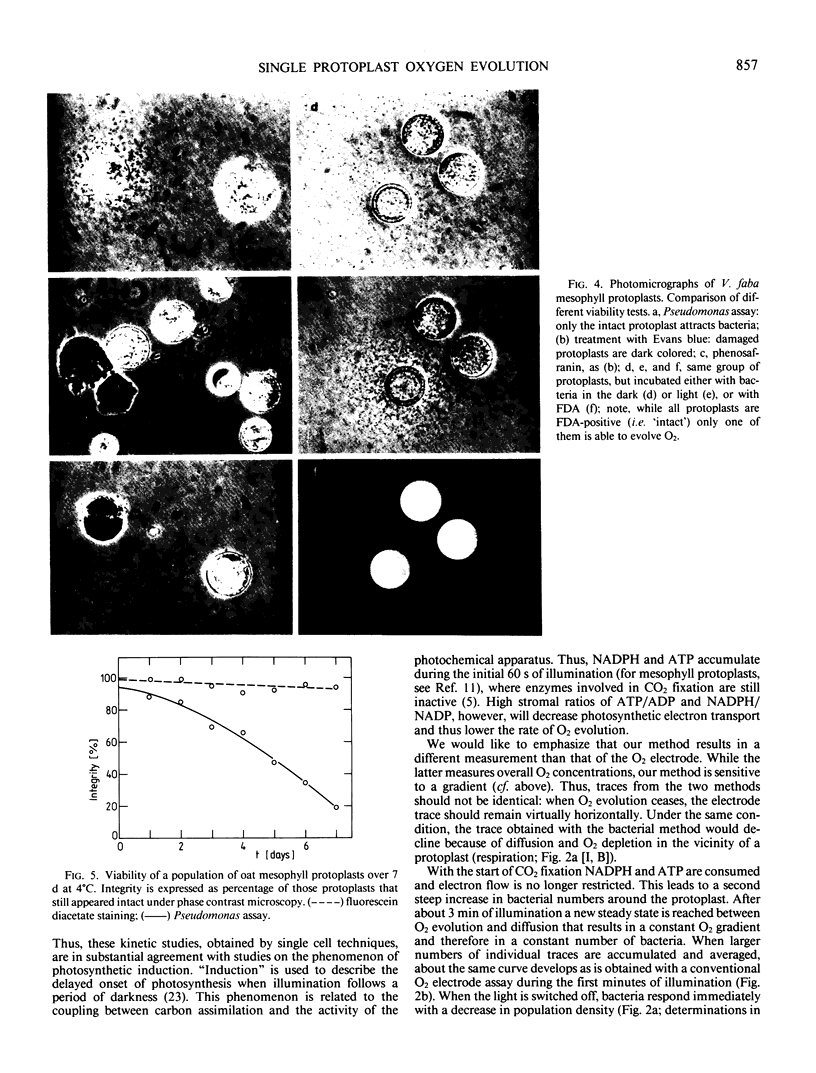
A semiquantitative assay for light-dependent O2 evolution by a single mesophyll protoplast is described. The assay indicator is the density of aerotactic bacteria (Pseudomonas aeruginosa, ATCC 10145; `Engelmann experiment') attracted to the protoplast. Quantification is by dark field microphotometry. The sensitivity is about 50 femtomoles O2 per protoplast per minute. The results demonstrate the biphasic nature of O2 evolution of a single protoplast during photosynthetic induction. Computerized data acquisition yields traces which, until a steady state of photosynthetic O2 evolution is reached, are identical to ordinary O2 electrode traces.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Bruening G. The use of an abrasive in the isolation of cowpea leaf protoplasts which support the multiplication of cowpea mosaic virus. Virology. 1975 Mar;64(1):272–276. doi: 10.1016/0042-6822(75)90099-9. [DOI] [PubMed] [Google Scholar]
- Crowther D., Hind G. Partial characterization of cyclic electron transport in intact chloroplasts. Arch Biochem Biophys. 1980 Oct 15;204(2):568–577. doi: 10.1016/0003-9861(80)90069-7. [DOI] [PubMed] [Google Scholar]
- Draber W., Trebst A., Harth E. On a new inhibitor of photosynthetic electron-transport in isolated chloroplasts. Z Naturforsch B. 1970 Oct;25(10):1157–1159. doi: 10.1515/znb-1970-1018. [DOI] [PubMed] [Google Scholar]
- Outlaw W. H., Springer S. A., Tarczynski M. C. Histochemical technique : a general method for quantitative enzyme assays of single cell ;extracts' with a time resolution of seconds and a reading precision of femtomoles. Plant Physiol. 1985 Mar;77(3):659–666. doi: 10.1104/pp.77.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A., Reider M. L., Fletcher J. S. Relationship between Vital Staining and Subculture Growth during the Senescence of Plant Tissue Cultures. Plant Physiol. 1982 Oct;70(4):1228–1230. doi: 10.1104/pp.70.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz W., Stitt M., Heldt H. W. Enzymic determination of metabolites in the subcellular compartments of spinach protoplasts. Plant Physiol. 1980 Jul;66(1):187–193. doi: 10.1104/pp.66.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U., Vienken J., Halfmann J., Emeis C. C. Electrofusion: a novel hybridization technique. Adv Biotechnol Processes. 1985;4:79–150. [PubMed] [Google Scholar]