Abstract
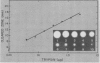
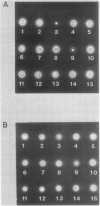
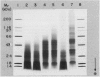
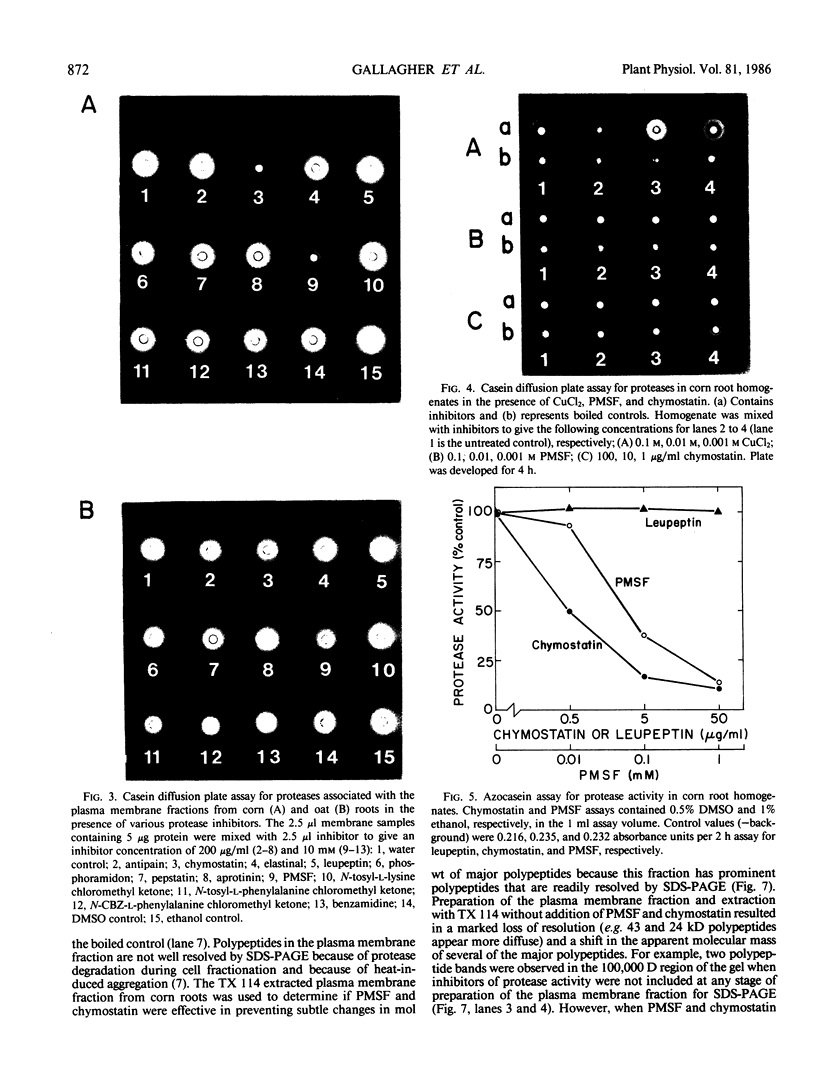
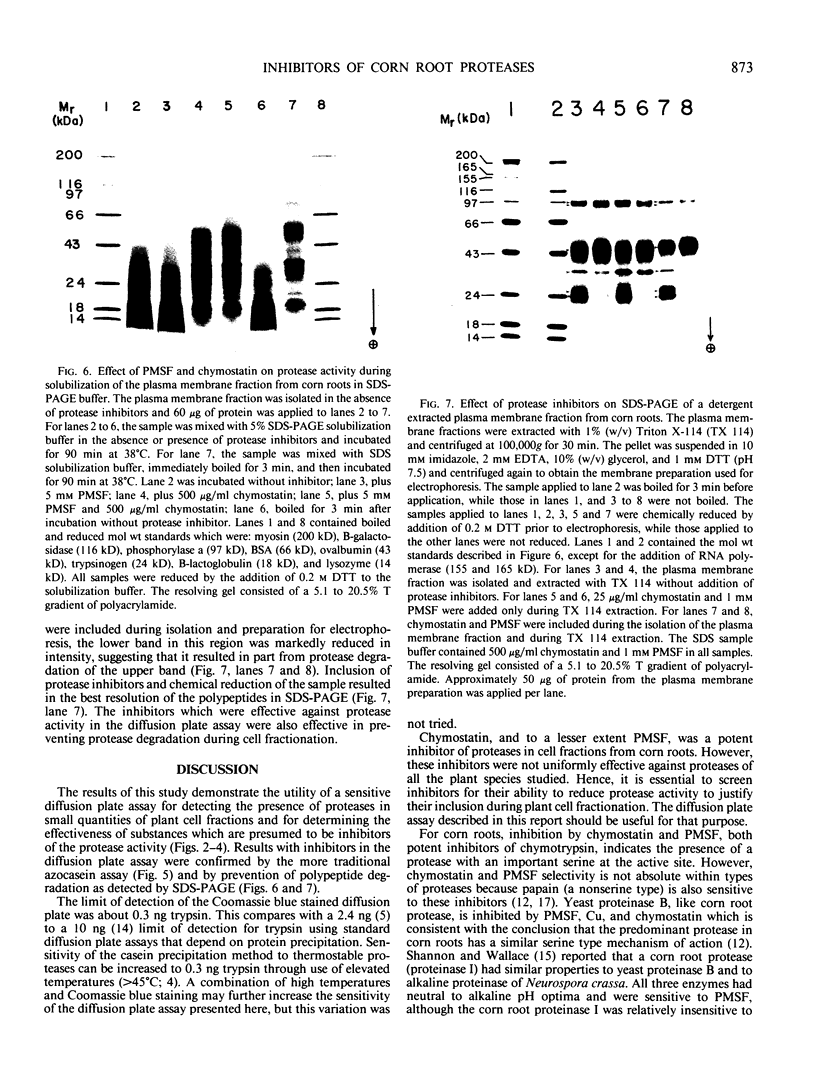
Proteolytic activity was detected, using a sensitive radial diffusion plate assay, in the plasma membrane fractions of corn (Zea mays L.) roots and from roots of several other plant species. The proteases could be effectively inhibited in corn with phenylmethane sulfonyl fluoride or chymostatin. Protease activity of oat roots, however, was not significantly reduced by these inhibitors. The results of diffusion plate assay were confirmed with the less sensitive azocasein assay using crude cell homogenates. Chymostatin and phenylmethane sulfonyl fluoride were effective in preventing protease degradation of polypeptides as revealed by electrophoresis. The diffusion plate assay uses a permanent support for a 0.75 millimeter thick agarose slab containing 200 micrograms per milliliter casein. By staining the fixed and dried gel with Coomassie blue R-250, proteolytic activity was visualized as a cleared area around the sample well with a detection limit of about 0.3 nanograms trypsin. The diffusion plate assay should prove useful for screening inhibitors of proteases where limited amounts of material are available, such as with plant cell fractions or highly purified proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison R., Scarborough G. A. Solubilization and purification of the Neurospora plasma membrane H+-ATPase. J Biol Chem. 1981 Dec 25;256(24):13165–13171. [PubMed] [Google Scholar]
- Alpi A., Beevers H. Proteinases and enzyme stability in crude extracts of castor bean endosperm. Plant Physiol. 1981 Mar;67(3):499–502. doi: 10.1104/pp.67.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. The many forms and functions of cellular proteinases. Fed Proc. 1980 Jan;39(1):9–14. [PubMed] [Google Scholar]
- Cowan D. A., Daniel R. M. A modification for increasing the sensitivity of the casein-agar plate assay: a simple semiquantitative assay for thermophilic and mesophilic proteases. J Biochem Biophys Methods. 1982 Apr;6(1):31–37. doi: 10.1016/0165-022x(82)90023-9. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowenstein H., Ingild A. A micromethod for determination of proteolytic enzymes in the pH range of 2.8 to 4.8. Radial enzyme diffusion into skim milk-containing agarose gel. Anal Biochem. 1976 Mar;71(1):204–208. doi: 10.1016/0003-2697(76)90028-2. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pringle J. R. Methods for avoiding proteolytic artefacts in studies of enzymes and other proteins from yeasts. Methods Cell Biol. 1975;12:149–184. doi: 10.1016/s0091-679x(08)60956-5. [DOI] [PubMed] [Google Scholar]
- Satoh S., Fujii T. A membrane-bound protease in microsomes of spinach callus. Plant Physiol. 1985 Jun;78(2):267–271. doi: 10.1104/pp.78.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher G. F., Schill W. B. Radial diffusion in gel for micro determination of enzymes. II. Plasminogen activator, elastase, and nonspecific proteases. Anal Biochem. 1972 Jul;48(1):9–26. doi: 10.1016/0003-2697(72)90165-0. [DOI] [PubMed] [Google Scholar]
- Shannon J. D., Wallace W. Isolation and characterisation of peptide hydrolases from the maize root. Eur J Biochem. 1979 Dec 17;102(2):399–408. doi: 10.1111/j.1432-1033.1979.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Low-molecular-weight enzyme inhibitors of microbial origin. Annu Rev Microbiol. 1982;36:75–99. doi: 10.1146/annurev.mi.36.100182.000451. [DOI] [PubMed] [Google Scholar]
- Wu F. S., Wang M. Y. Extraction of proteins for sodium dodecyl sulfate-polyacrylamide gel electrophoresis from protease-rich plant tissues. Anal Biochem. 1984 May 15;139(1):100–103. doi: 10.1016/0003-2697(84)90394-4. [DOI] [PubMed] [Google Scholar]