Abstract
Background
People with advanced kidney disease treated with dialysis experience mortality rates from cardiovascular disease that are substantially higher than for the general population. Studies that have assessed the benefits of statins (HMG CoA reductase inhibitors) report conflicting conclusions for people on dialysis and existing meta‐analyses have not had sufficient power to determine whether the effects of statins vary with severity of kidney disease. Recently, additional data for the effects of statins in dialysis patients have become available. This is an update of a review first published in 2004 and last updated in 2009.
Objectives
To assess the benefits and harms of statin use in adults who require dialysis (haemodialysis or peritoneal dialysis).
Search methods
We searched the Cochrane Renal Group's Specialised Register to 29 February 2012 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs that compared the effects of statins with placebo, no treatment, standard care or other statins on mortality, cardiovascular events and treatment‐related toxicity in adults treated with dialysis were sought for inclusion.
Data collection and analysis
Two or more authors independently extracted data and assessed study risk of bias. Treatment effects were summarised using a random‐effects model and subgroup analyses were conducted to explore sources of heterogeneity. Treatment effects were expressed as mean difference (MD) for continuous outcomes and risk ratios (RR) for dichotomous outcomes together with 95% confidence intervals (CI).
Main results
The risk of bias was high in many of the included studies. Random sequence generation and allocation concealment was reported in three (12%) and four studies (16%), respectively. Participants and personnel were blinded in 13 studies (52%), and outcome assessors were blinded in five studies (20%). Complete outcome reporting occurred in nine studies (36%). Adverse events were only reported in nine studies (36%); 11 studies (44%) reported industry funding.
We included 25 studies (8289 participants) in this latest update; 23 studies (24 comparisons, 8166 participants) compared statins with placebo or no treatment, and two studies (123 participants) compared statins directly with one or more other statins. Statins had little or no effect on major cardiovascular events (4 studies, 7084 participants: RR 0.95, 95% CI 0.88 to 1.03), all‐cause mortality (13 studies, 4705 participants: RR 0.96, 95% CI 0.90 to 1.02), cardiovascular mortality (13 studies, 4627 participants: RR 0.94, 95% CI 0.84 to 1.06) and myocardial infarction (3 studies, 4047 participants: RR 0.87, 95% CI 0.71 to 1.07); and uncertain effects on stroke (2 studies, 4018 participants: RR 1.29, 95% CI 0.96 to 1.72).
Risks of adverse events from statin therapy were uncertain; these included effects on elevated creatine kinase (5 studies, 3067 participants: RR 1.25, 95% CI 0.55 to 2.83) or liver function enzymes (4 studies, 3044 participants; RR 1.09, 95% CI 0.41 to 1.25), withdrawal due to adverse events (9 studies, 1832 participants: RR 1.04, 95% CI 0.87 to 1.25) or cancer (2 studies, 4012 participants: RR 0.90, 95% CI 0.72 to 1.11). Statins reduced total serum cholesterol (14 studies, 1803 participants; MD ‐44.86 mg/dL, 95% CI ‐55.19 to ‐34.53) and low‐density lipoprotein cholesterol (12 studies, 1747 participants: MD ‐39.99 mg/dL, 95% CI ‐52.46 to ‐27.52) levels. Data comparing statin therapy directly with another statin were sparse.
Authors' conclusions
Statins have little or no beneficial effects on mortality or cardiovascular events and uncertain adverse effects in adults treated with dialysis despite clinically relevant reductions in serum cholesterol levels.
Plain language summary
Does statin therapy improve survival or reduce risk of heart disease in people on dialysis?
Adults with severe kidney disease who are treated with dialysis have high risks of developing heart disease. Statin treatment reduces risks of death and complications of heart disease in the general population.
In 2009 we identified 14 studies, enrolling 2086 patients, and found that while statins were generally safe and reduced cholesterol levels, they did not prevent death or clinical cardiac events in people treated with dialysis. This latest update analysed a total or 25 studies (8289 patients), and included the results from two new large studies. We found that statins lowered cholesterol in people treated with dialysis but did not prevent death, heart attack, or stroke.
Evidence for side‐effects was incomplete, and potential harms from statin therapy remain uncertain. Current study data did not address whether statin treatment should be stopped when a person starts dialysis, although the benefits associated with continued treatment are likely to be small. Limited information was available for people treated with peritoneal dialysis, suggesting that more research is needed in this setting.
Summary of findings
for the main comparison.
| Statin versus placebo or no treatment for dialysis patients | |||||
|
Patient or population: adults with chronic kidney disease Settings: dialysis Intervention: statin therapy Comparison: placebo or no treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk/year/1000 treated | Corresponding risk/year/1000 treated | ||||
| Placebo or no treatment | Statin | ||||
| Major cardiovascular events | 150 per 1000 | 143 per 1000 (7 fewer) (132 to 155) (18 fewer to 5 more) | RR 0.95 (0.88 to 1.03) | 7804 (4) | ⊕⊕⊕⊕ high |
| All‐cause mortality | 200 per 1000 | 192 per 1000 (8 fewer) (176 to 208) (24 fewer to 8 more) | RR 0.96 (0.90 to 1.02) | 4705 (13) | ⊕⊕⊕ moderate |
| Cardiovascular mortality | 100 per 1000 | 94 per 1000 (6 fewer) (82 to 105) (18 fewer to 5 more) | RR 0.94 (0.84 to 1.06) | 4627 (13) | ⊕⊕⊕ moderate |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | |||||
Absolute approximate events rates of outcomes per year were derived from previously observational cohort studies. Absolute numbers of people on dialysis with cardiovascular or mortality events avoided or incurred per 1000 treated were estimated using these assumed risks together with the estimated relative risks and 95% confidence intervals (Herzog 1998; Trivedi 2009; Weiner 2006; Wetmore 2009)
Background
Description of the condition
Although cardiovascular mortality is decreasing, events among dialysis patients remains 20‐ to 30‐times higher than for the general population (Foley 2007; Herzog 2011; USRDS 2011). Elevated circulating lipid levels is one of several factors, that also include hypertension, diabetes, and smoking, that have been implicated in the increased cardiovascular risk associated with chronic kidney disease (CKD) (Ganesh 2001; Jungers 1997; Mallamaci 2002).
How the intervention might work
Clinical studies conducted in the general population, and in people with established cardiovascular disease, have found a strong, consistent and independent association between lipid lowering, primarily low‐density lipoprotein (LDL) cholesterol, and the risk of all‐cause and cardiovascular mortality (Law 1994; Rossouw 1990). A linear proportional reduction in the risk of major vascular events equal to approximately 20% per 1 mmol/L (39 mg/dL) reduction in LDL cholesterol has been reported (Baigent 2005). Optimal lowering of serum lipid levels has been anticipated to lower cardiovascular and overall mortality for people treated with dialysis.
Why it is important to do this review
Study data for the benefits of lipid lowering in people on dialysis are increasingly conflicted. Our previous review (Navaneethan 2009a) identified little or no benefit from statin therapy on mortality, although one study reported fewer major cardiovascular events in people with diabetes on dialysis (4D Study 2004). The Study for Heart and Renal Protection (SHARP Study 2010, completed since our last review update), which included 3023 people on dialysis, reported that benefits for lipid‐lowering therapy extended to people with advanced kidney disease on dialysis, whereas the AURORA Study 2005 (A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events) conducted with 2776 adults on haemodialysis, found no clear benefit for statin therapy in this population. An advisory committee to the US Food and Drug Administration that considered SHARP Study 2010 study data did not recommend lipid‐lowering using simvastatin/ezetimibe in people on dialysis, citing insufficient evidence (FDA 2011).
In light of conflicting information on the benefits of statin therapy to inform clinical practice and policy in people on dialysis, together with new study data, we conducted an update of our earlier review (Navaneethan 2009a) to evaluate the benefits and harms of statin therapy in people on dialysis.
Objectives
To evaluate the benefits (reductions in all‐cause mortality, cardiovascular mortality, major cardiovascular events, myocardial infarction and stroke) and harms (liver or muscle damage, or cancer) of statins compared with placebo, no treatment, or another statin in adults who require dialysis.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCT) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable method) of at least 8 weeks' duration that evaluated the benefits and harms of statins in adults treated with haemodialysis or peritoneal dialysis were included. The first periods of randomised cross‐over studies were also included. Studies of fewer than eight weeks' duration were excluded because they were unlikely to enable detection of mortality or cardiovascular outcomes related to statin therapy (Briel 2006).
Types of participants
Inclusion criteria
Adults treated with dialysis (haemodialysis and peritoneal dialysis) irrespective of pre‐existing cardiovascular disease or statin therapy were included.
Exclusion criteria
Studies in children were excluded. Studies including adults with CKD not treated with dialysis and recipients of a kidney transplant are the subject of other related reviews (Navaneethan 2009b; Navaneethan 2009c; updates in press (Palmer 2013a; Palmer 2013b)).
Types of interventions
We included studies that compared statins with placebo, no treatment or standard care, or another statin. We excluded studies that compared a statin with a second non‐statin regimen, including fibrate therapy.
Types of outcome measures
Primary outcomes
Major cardiovascular events
All‐cause mortality
Cardiovascular mortality
Fatal and non‐fatal myocardial infarction
Fatal and non‐fatal stroke
-
Adverse events attributable to interventions
Elevated creatine kinase
Elevated liver function enzymes
Withdrawal due to adverse events
Cancer.
Secondary outcomes
Lipid parameters (mg/dL)
-
Serum lipid levels
Total cholesterol
LDL cholesterol
Triglycerides
High‐density lipoprotein (HDL) cholesterol
Search methods for identification of studies
Electronic searches
2013 update
We searched the Cochrane Renal Group's Specialised Register to 29 February 2012 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of relevant clinical practice guidelines, review articles and studies.
Letters seeking information about unpublished or incomplete RCTs to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
Two authors independently screened all abstracts retrieved by electronic searches to identify potentially relevant citations for detailed study in full text format. Studies that might have included relevant data or information on studies involving HMG Co‐A reductase inhibitors were retained initially. Studies published in non‐English language journals were translated before assessment for inclusion.
Data extraction and management
Two authors independently extracted data from the eligible studies using standard data extraction forms. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was included. Any further information required from the original author was requested and any relevant information obtained was included in the review. Disagreements were resolved in consultation with a third author.
Data entry was carried out by the same two authors. Treatment effects were summarised using the random‐effects model but the fixed effects model was also analysed to ensure robustness of the model chosen and susceptibility to outliers. For dichotomous outcomes (cardiovascular events, mortality, and adverse events) treatment effects were summarised as relative risk (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used (lipid parameters), treatment effects were summarised using the mean difference (MD).
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011; Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Dichotomous outcomes (e.g. fatal and non‐fatal heart attack and stroke) were expressed as risk ratios (RR) with 95% confidence intervals (CI). Risk differences (RD) with 95% confidence intervals were calculated for adverse effects. Continuous outcomes were calculated as mean differences (MD) with 95% CI.
Dealing with missing data
Where applicable, study authors were contacted for further information or missing data. Data obtained in this manner were included in our analyses.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
This update included all studies identified in the Cochrane Renal Group's Specialised Register, which is updated regularly with published and unpublished reports identified in congress proceedings. This reduces the risk of publication bias. All reports of a single study were reviewed to ensure that all outcomes were reported to reduce the risk of selection bias.
Data synthesis
We summarised evidence quality together with absolute treatment effects for mortality and cardiovascular events based on estimated baseline risks using Grading of Recommendations Assessment Development and Evaluation (GRADE) guidelines (Table 1; Guyatt 2008). Absolute numbers of people on dialysis with cardiovascular events or adverse events avoided or incurred were estimated using the risk estimate for the outcome (and associated 95% confidence interval) obtained from the corresponding meta‐analysis together with the absolute population risk estimated from previously published observational studies (Herzog 1998; Trivedi 2009; Weiner 2006; Wetmore 2009).
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses to explore potential sources of heterogeneity in modifying estimates of the effects of statins in the studies. We planned subgroup analyses according to participant type, intervention, or study‐related characteristics, when subgroups contained four or more independent studies: dialysis type (peritoneal or haemodialysis); statin type; statin dose (equivalent to simvastatin); baseline cholesterol (< 230 mg/dL versus ≥ 230 mg/dL); age (≤ 55 years versus > 56 years); proportion with diabetes (> 20% versus < 20%); adequacy of allocation concealment. Insufficient numbers of studies reporting one or more events were available to explore for publication bias using visual inspection of an inverted funnel plot or formal statistical analysis.
Sensitivity analysis
Where a study's results differed considerably from other studies in a meta‐analysis, exclusion of the study was investigated to determine whether this altered the result of the meta‐analysis.
Results
Description of studies
Results of the search
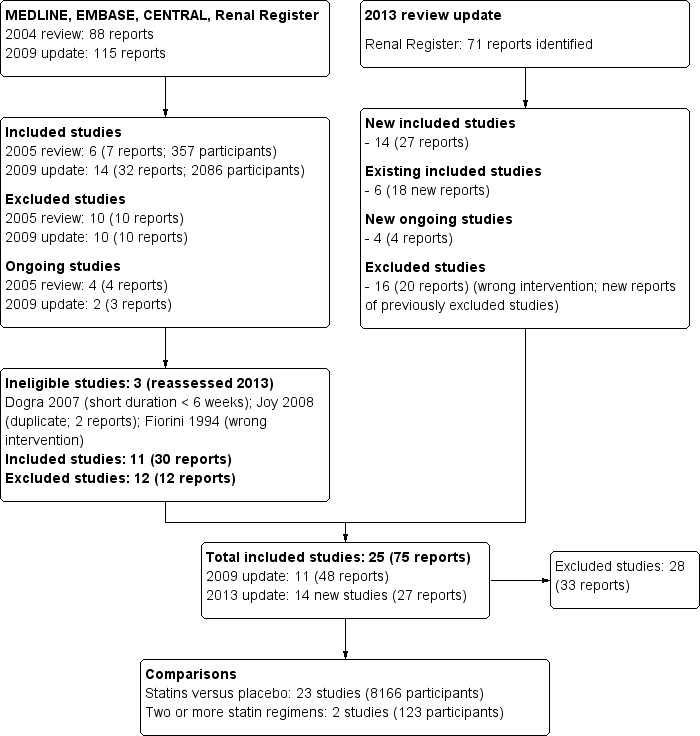
Initial review (2004) and first update (2009)
The searches identified 88 reports in 2004 and 115 reports in 2009. After title and abstract screening 67 (2004) and 97 (2009) reports were excluded. Full text assessment resulted in six studies (7 reports, 357 participants) included in our initial 2004 review and 14 studies (32 reports, 2086 participants) included in the 2009 update. Two studies reported as ongoing in 2004 and 2009 (AURORA Study 2005; SHARP Study 2010) have been included in our 2013 update.
2013 review update
Electronic searching to February 2012 identified 71 additional records. Of these, 33 were duplicate reports of existing studies and four were ongoing studies. After full‐text assessment, a study by Joy 2008 included in our 2009 review update was considered to be a part of Dornbrook‐Lavender 2005. We also removed Fiorini 1994 because it did not evaluate a statin versus another statin, placebo, or no treatment; and Dogra 2007, because treatment duration was only six weeks. This meant that 11 unique studies were retained from the 2009 published review (Navaneethan 2009a).
After detailed assessment of the remaining reports, 25 studies (14 new eligible studies) were identified. The flow chart for the review process is shown in Figure 1.
1.
Study selection flow diagram
Included studies
This review included 25 studies that involved 8289 participants. One study included relevant subsets of haemodialysis and peritoneal dialysis patient data and for purpose of the analyses have been identified as Saltissi HD 2002 and Saltissi PD 2002 respectively.
There were 14 new studies included in this update (Ahmadi 2005; Angel 2007; Arabul 2008; AURORA Study 2005; Burmeister 2006; Han 2011; SHARP Study 2010; Soliemani 2011; Tse 2008; van den Akker 2003; Vareesangthip 2005; Velickovic 1997; Vernaglione 2003; Yu 2007). Of these, two (AURORA Study 2005; SHARP Study 2010) were identified as ongoing studies in our 2009 review.
There were 23 studies (8166 participants) that compared statins with placebo or no treatment (4D Study 2004; Ahmadi 2005; Angel 2007; Arabul 2008; AURORA Study 2005; Burmeister 2006; Chang 2002; Diepeveen 2005; Dornbrook‐Lavender 2005; Han 2011; Harris 2002; Ichihara 2002; Lins 2004; PERFECT Study 1997; Saltissi HD 2002‐Saltissi PD 2002; SHARP Study 2010; Stegmayr 2005; Tse 2008; UK‐HARP‐I 2005; Vareesangthip 2005; Velickovic 1997; Vernaglione 2003;Yu 2007), and two (123 participants) directly compared two or more statins (Soliemani 2011; van den Akker 2003).
Study design
All included studies were RCTs; two were two‐by‐two factorial design with aspirin (UK‐HARP‐I 2005) and enalapril (PERFECT Study 1997).
Participants
All participants were undergoing dialysis.
Twelve studies only included participants undergoing haemodialysis (4D Study 2004; Ahmadi 2005; AURORA Study 2005; Burmeister 2006; Chang 2002; Dornbrook‐Lavender 2005; Ichihara 2002; Lins 2004; Soliemani 2011; UK‐HARP‐I 2005; Vareesangthip 2005; Vernaglione 2003);
Eight studies included participants treated with either haemodialysis or peritoneal dialysis (Arabul 2008; Diepeveen 2005; UK‐HARP‐I 2005; PERFECT Study 1997; Saltissi HD 2002‐Saltissi PD 2002; SHARP Study 2010; Stegmayr 2005; Yu 2007)
Five studies only included peritoneal dialysis patients (Angel 2007; Harris 2002; Han 2011; Tse 2008; Velickovic 1997).
Median baseline serum LDL cholesterol was 190 mg/dL (range 150 to 254 mg/dL).
Two studies only included participants with diabetes at baseline (4D Study 2004; Ichihara 2002).
Interventions
Five studies reported follow‐up of more than six months (4D Study 2004; AURORA Study 2005; SHARP Study 2010; Stegmayr 2005; UK‐HARP‐I 2005). Generally, studies were small (median 42 participants; range 13 to 3023 participants); three studies enrolled more than 1000 participants undergoing dialysis (4D Study 2004; AURORA Study 2005; SHARP Study 2010).
Doses of statin (equivalent to simvastatin) were generally 20 mg (5 to 80 mg) with a median follow‐up of six months (2 to 59 months) including studies reporting mortality and cardiovascular events. Non‐randomised co‐interventions included diet in three comparisons (Ichihara 2002; Saltissi HD 2002; Saltissi PD 2002).
Excluded studies
We excluded 28 studies: 13 were not randomised; seven did not include an appropriate intervention (other active treatment); one was a discontinued study; five were short durations (< 8 weeks); two were not conducted in dialysis populations (see Characteristics of excluded studies).
Risk of bias in included studies
Risk of bias in included studies is summarised in Figure 2 and Figure 3. The risk of bias was high in many of the included studies. Random sequence generation and allocation concealment was reported in three (12%) and four studies (16%), respectively. Participants and personnel were blinded in 13 studies (52%), and outcome assessors were blinded in five studies (20%). Complete outcome reporting occurred in nine studies (36%). Adverse events were only reported in nine studies (36%); 11 studies (44%) reported industry funding.The risk of bias was high in many included studies.
2.
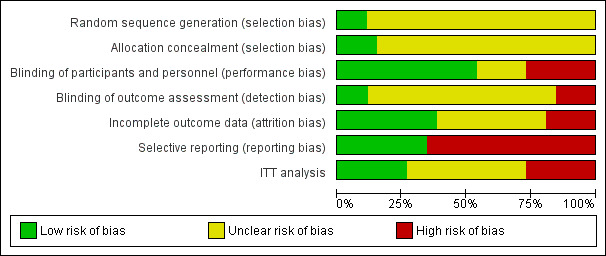
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
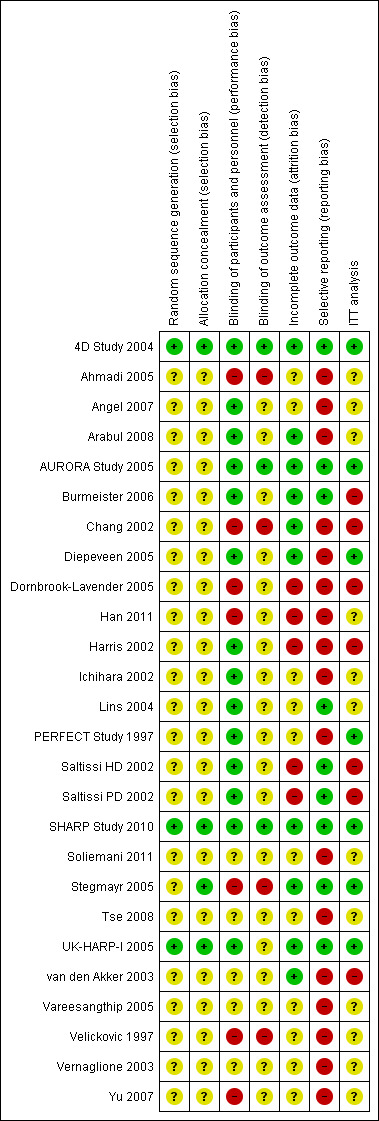
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
Random sequence generation was only reported in 3/25 studies (4D Study 2004; SHARP Study 2010; UK‐HARP‐I 2005).
Allocation concealment
Allocation to randomised groups was not reported adequately: only 4/25 included studies reported allocation methodology in detail (4D Study 2004; SHARP Study 2010; Stegmayr 2005; UK‐HARP‐I 2005).
Blinding
Blinding methodology was well reported: 13 provided adequate details (4D Study 2004; Angel 2007; Arabul 2008; AURORA Study 2005; Burmeister 2006; Diepeveen 2005; Harris 2002; Ichihara 2002; Lins 2004; PERFECT Study 1997; Saltissi HD 2002‐Saltissi PD 2002; SHARP Study 2010; UK‐HARP‐I 2005); six did not indicate blinding (Han 2011; SHARP Study 2010; Tse 2008; van den Akker 2003; Vareesangthip 2005; Vernaglione 2003); and six did not blind participants (Ahmadi 2005; Chang 2002; Dornbrook‐Lavender 2005; Stegmayr 2005; Velickovic 1997; Yu 2007).
Incomplete outcome data
Drop‐outs and losses to follow‐up ranged for 0% to 32%. Seven studies were judged to be at low risk of bias (4D Study 2004; Arabul 2008; AURORA Study 2005; Diepeveen 2005; SHARP Study 2010; Stegmayr 2005; UK‐HARP‐I 2005), six were at high risk (Burmeister 2006; Chang 2002; Dornbrook‐Lavender 2005; Harris 2002; Saltissi HD 2002‐Saltissi PD 2002; van den Akker 2003), and the remaining 12 studies were unclear.
Selective reporting
Overall, nine studies (36%) reported all expected outcomes (4D Study 2004; Arabul 2008; AURORA Study 2005; Burmeister 2006; Lins 2004; Saltissi HD 2002‐Saltissi PD 2002; SHARP Study 2010; Stegmayr 2005; UK‐HARP‐I 2005).
Other potential sources of bias
Eleven studies (44%) reported industry funding (4D Study 2004; AURORA Study 2005; Burmeister 2006; Chang 2002; Diepeveen 2005; Dornbrook‐Lavender 2005; Lins 2004; PERFECT Study 1997; Saltissi HD 2002‐Saltissi PD 2002; SHARP Study 2010; UK‐HARP‐I 2005)
Effects of interventions
See: Table 1
Statins versus placebo or no treatment
We found moderate‐to‐high quality evidence to indicate that statin therapy had little or no effect on risks of major cardiovascular events (Analysis 1.1 (4 studies, 7084 participants): RR 0.95, 95% CI 0.88 to 1.03), all‐cause mortality (Analysis 1.2 (13 studies, 4705 participants): RR 0.96, 95% CI 0.90 to 1.02) and cardiovascular mortality (Analysis 1.3 (13 studies, 4627 participants): RR 0.94, 95% CI 0.84 to 1.06) (Table 1). Statins had little or no effect on risks of fatal or non‐fatal myocardial infarction (Analysis 1.4 (3 studies, 4047 participants): RR 0.87, 95% CI 0.71 to 1.07) and had uncertain effects on fatal or non‐fatal stroke (Analysis 1.5 (2 studies, 4018 participants): RR 1.29, 95% CI 0.96 to 1.72). There was no evidence of heterogeneity in these analyses (I² = 0%).
1.1. Analysis.
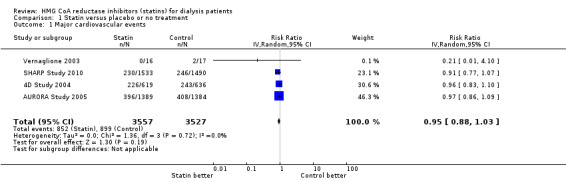
Comparison 1 Statin versus placebo or no treatment, Outcome 1 Major cardiovascular events.
1.2. Analysis.
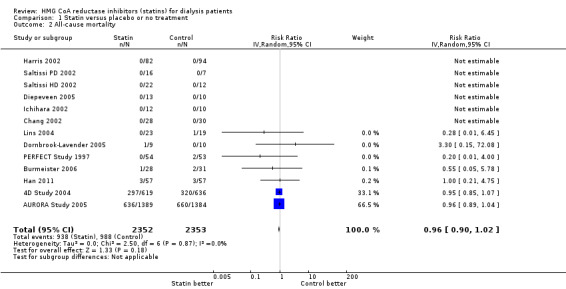
Comparison 1 Statin versus placebo or no treatment, Outcome 2 All‐cause mortality.
1.3. Analysis.
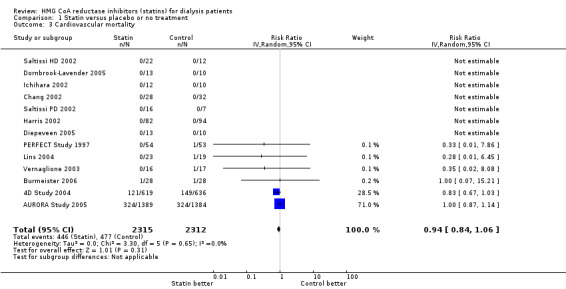
Comparison 1 Statin versus placebo or no treatment, Outcome 3 Cardiovascular mortality.
1.4. Analysis.
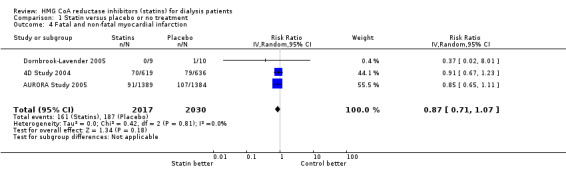
Comparison 1 Statin versus placebo or no treatment, Outcome 4 Fatal and non‐fatal myocardial infarction.
1.5. Analysis.
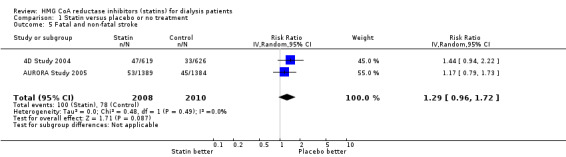
Comparison 1 Statin versus placebo or no treatment, Outcome 5 Fatal and non‐fatal stroke.
Statins had uncertain effects on adverse events, including elevation of creatine kinase (Analysis 1.6 (5 studies, 3067 participants): RR 1.25, 95% CI 0.55 to 2.83), elevated liver enzymes (Analysis 1.7 (4 studies, 3044 participants): RR 1.09, 95% CI 0.41 to 2.91), withdrawal due to adverse events (Analysis 1.8 (9 studies, 1832 participants): RR 1.04, 95% CI 0.87 to 1.25) and cancer (Analysis 1.9 (2 studies, 4012 participants): RR 0.90, 95% CI 0.72 to 1.11) (Table 1). There was no evidence of heterogeneity in these analyses (I² = 0%).
1.6. Analysis.
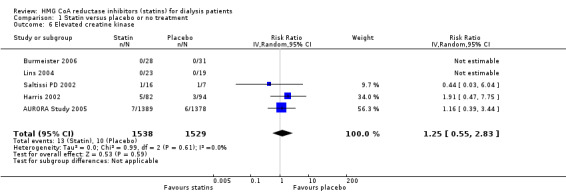
Comparison 1 Statin versus placebo or no treatment, Outcome 6 Elevated creatine kinase.
1.7. Analysis.
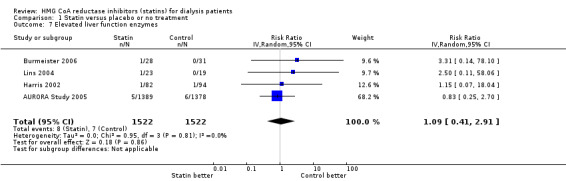
Comparison 1 Statin versus placebo or no treatment, Outcome 7 Elevated liver function enzymes.
1.8. Analysis.
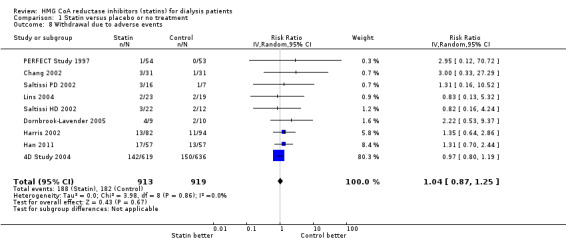
Comparison 1 Statin versus placebo or no treatment, Outcome 8 Withdrawal due to adverse events.
1.9. Analysis.
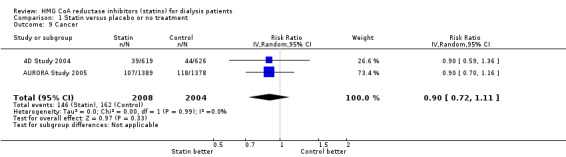
Comparison 1 Statin versus placebo or no treatment, Outcome 9 Cancer.
Statins significantly reduced total cholesterol (Analysis 1.10 (14 studies, 1803 participants): MD ‐44.86 mg/dL, 95% CI ‐55.19 to ‐34.53), LDL cholesterol (Analysis 1.11 (12 studies, 1747 participants): MD ‐39.99 mg/dL, 95% CI ‐52.46 to ‐27.52) and triglycerides (Analysis 1.12 (13 studies, 1692 participants): MD ‐18.02 mg/dL, 95% CI ‐33.00 to ‐3.04), but had uncertain effects on HDL cholesterol (Analysis 1.13 (13 studies, 1769 participants): MD 2.57 mg/dL, 95% CI ‐0.39 to 5.52).
1.10. Analysis.
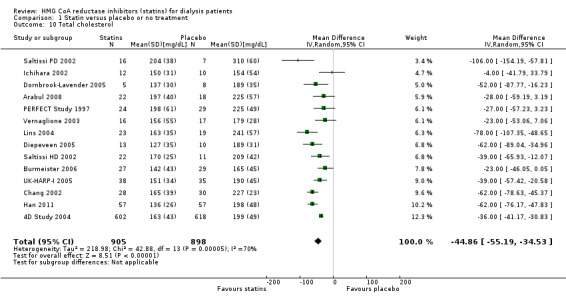
Comparison 1 Statin versus placebo or no treatment, Outcome 10 Total cholesterol.
1.11. Analysis.
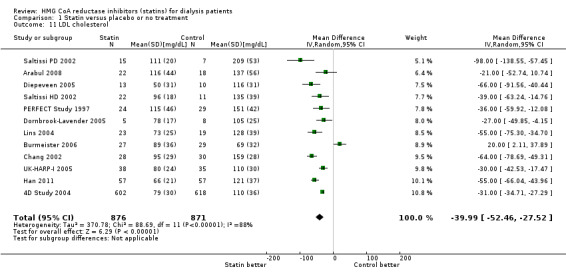
Comparison 1 Statin versus placebo or no treatment, Outcome 11 LDL cholesterol.
1.12. Analysis.
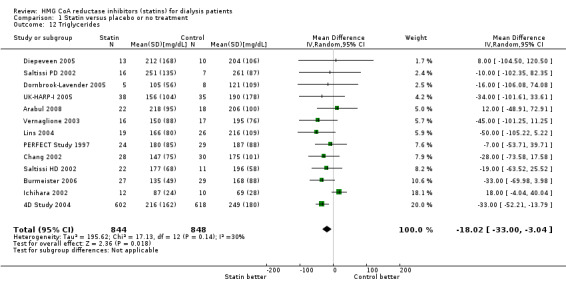
Comparison 1 Statin versus placebo or no treatment, Outcome 12 Triglycerides.
1.13. Analysis.
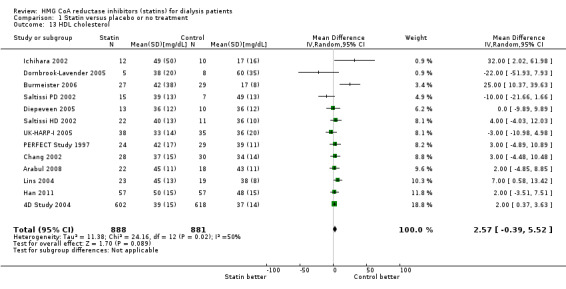
Comparison 1 Statin versus placebo or no treatment, Outcome 13 HDL cholesterol.
Analysis of heterogeneity
We did not identify any sources of heterogeneity in the analyses for total or LDL cholesterol using prespecified subgroup analyses (dialysis type or statin type or dose, age, proportion with diabetes, baseline serum cholesterol, risk of bias (allocation concealment)). The lack of specific populations with or without cardiovascular disease at baseline in the available studies prevented subgroup analysis for the effect of statins by the presence or absence of cardiovascular disease.
Statin versus other statin
van den Akker 2003 (28 participants) compared atorvastatin (10 to 40 mg/d) with simvastatin (10 to 40 mg/d), and Soliemani 2011 compared atorvastatin, simvastatin and lovastatin directly (95 participants). Compared to simvastatin, atorvastatin treatment had uncertain effects on elevation of liver enzymes (Analysis 2.1 (1 study, 28 participants): RR 5.71, 95% CI 0.30 to 109.22), withdrawal from treatment due to adverse events (Analysis 2.2 (1 study, 63 participants): RR 2.06, 95% CI 0.20 to 21.63), total cholesterol (Analysis 2.3 (1 study, 28 participants): MD 0.23 mg/dL, 95% CI ‐0.35 to 0.81), LDL cholesterol (Analysis 2.4 (1 study, 28 participants): MD 0.06 mg/dL, 95% CI ‐0.40 to 0.52), triglycerides (Analysis 2.5 ((1 study, 28 participants): MD ‐0.02 mg/dL, 95% CI ‐0.58 to 0.54), and HDL cholesterol (Analysis 2.6 (1 study, 28 participants): 0.10 mg/dL, 95% CI ‐0.13 to 0.33). Data for other outcomes were not available in extractable format.
2.1. Analysis.
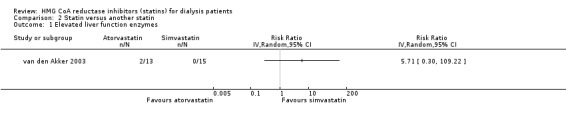
Comparison 2 Statin versus another statin, Outcome 1 Elevated liver function enzymes.
2.2. Analysis.
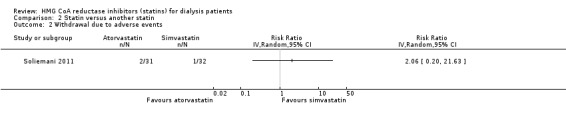
Comparison 2 Statin versus another statin, Outcome 2 Withdrawal due to adverse events.
2.3. Analysis.
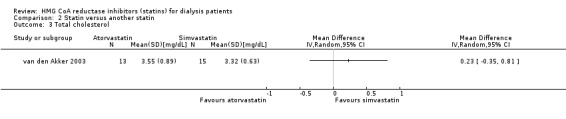
Comparison 2 Statin versus another statin, Outcome 3 Total cholesterol.
2.4. Analysis.
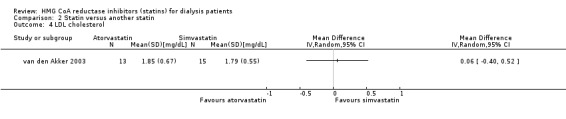
Comparison 2 Statin versus another statin, Outcome 4 LDL cholesterol.
2.5. Analysis.
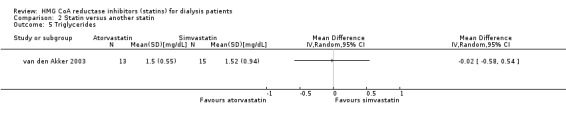
Comparison 2 Statin versus another statin, Outcome 5 Triglycerides.
2.6. Analysis.
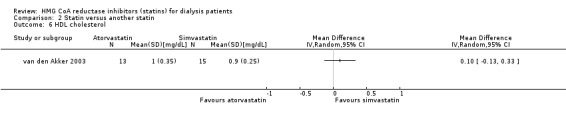
Comparison 2 Statin versus another statin, Outcome 6 HDL cholesterol.
Sensitivity analyses
When analyses were restricted to studies in which follow‐up data were provided for six months or more, the results were unchanged (major cardiovascular events, unchanged from primary result; all‐cause mortality (7 studies, 4328 participants): RR 0.96, 95% CI 0.90 to 1.02) (4D Study 2004; AURORA Study 2005; Ichihara 2002; Han 2011; PERFECT Study 1997; Saltissi HD 2002‐Saltissi PD 2002); cardiovascular mortality ((7 studies, 4247 participants): RR 0.94, 95% CI 0.84 to 1.06) (4D Study 2004; AURORA Study 2005; Ichihara 2002; Han 2011; PERFECT Study 1997; Saltissi HD 2002‐Saltissi PD 2002).
Discussion
Summary of main results
This review update on the benefits and harms of statins in people treated with dialysis found that data for mortality and cardiovascular events were generally moderate‐to‐high quality. Statin therapy (generally at doses equivalent to 20 mg of simvastatin) reduced total serum cholesterol levels by 46 mg/dL (1.2 mmol/L) in adult dialysis patients, but had little or no effect on major cardiovascular events or mortality. Statins were found to have little or no effect on myocardial infarction and uncertain effects on the risk of stroke. Statins were also found to have uncertain effects on risks of liver dysfunction, muscle damage or cancer in people on dialysis; and toxicity data were limited by a lack of systematic reporting in half the studies. Few data were available for people treated with peritoneal dialysis. Direct head‐to‐head studies of different statin agents were rare and estimated effects of atorvastatin versus simvastatin were imprecise.
Overall completeness and applicability of evidence
Three large and well‐conducted studies provided moderate‐to‐high quality data that showed consistent effects of statins on cardiovascular events in people treated with dialysis (4D Study 2004; AURORA Study 2005; SHARP Study 2010). Mortality data were assessed as moderate quality because information from SHARP Study 2010 could not be included as these were not reported in the published study separately for dialysis patients and could not be obtained from the authors on request.
The strengths of this review include consistent results for primary outcomes among studies (no evidence of heterogeneity), comprehensive systematic searching for eligible studies, rigid inclusion criteria for RCTs, and data extraction and analysis by two independent investigators. Furthermore, the possibility of publication bias was minimised by including both published and unpublished studies (such as abstracts from meetings), although we could not formally test for evidence of publication bias or small study effects due to the small numbers of available studies.
Despite comprehensive inclusion of available studies, the current evidence for statins in people treated with dialysis has some significant limitations. Studies were generally small (median number of participants was 42) and, with the exception of three large well‐conducted studies (4D Study 2004; AURORA Study 2005; SHARP Study 2010), were assessed to be at high risk of bias. Studies were also generally of short duration (six months) and may not have been sufficiently powered to identify the effects of statins on clinical end points such as mortality (Briel 2006) (although the larger studies that dominated analyses provided outcome data for three to five years of treatment).
Limited data were available for adverse events, which were not systematically captured in over half of the included studies, such that potential toxicities of statins in this population remain incompletely characterised. We were unable to determine whether treatment effects were different in people on peritoneal dialysis compared with those on haemodialysis. Eight studies enrolled both haemodialysis and peritoneal dialysis patients and only one presented separate outcome data for these two populations (Saltissi HD 2002‐Saltissi PD 2002). In addition, the small number of available studies meant that we were unable to explore other sources of heterogeneity in the treatment effects among studies on serum cholesterol levels, although this was a secondary (and surrogate) outcome. We could not identify whether treatment effects differed between men and women. Furthermore, we were unable to analyse the relative benefits of primary versus secondary prevention of cardiovascular events in people on dialysis, because there were too few studies specifically designed to address this question. SHARP Study 2010 evaluated a combination of simvastatin and ezetimibe, but it remains unclear whether there was an important difference in treatment effects compared with a statin alone, although it is unlikely because treatment effects were consistent among all studies for major cardiovascular events irrespective of the treatment used.
It was noteworthy that adverse mortality and cardiovascular events were not clearly prevented by statins in the dialysis population, despite clinically significant lowering of serum lipid levels. This finding is inconsistent with data from people with earlier stages of kidney disease not treated with dialysis, for which statins clearly reduce risks of death and major cardiovascular events (Palmer 2013a). It was possible that a lack of power in available studies for dialysis resulted in the small or no effects on all‐cause mortality and cardiovascular events, although the inclusion of nearly 2000 events in each analysis makes this unlikely. It has previously been suggested that the choice of endpoints for major cardiovascular events in AURORA Study 2005 and 4D Study 2004 (both showing no statistical effect on cardiovascular events) were a reason for negative studies of statins in dialysis, because definitions of endpoints included a smaller proportion of modifiable vascular events. While this is possible, even with the inclusion of SHARP Study 2010 (in which cardiovascular events were predominantly occlusive vascular outcomes including revascularisation procedures), statins had little or no effect on cardiovascular outcomes. Finally, data comparing a statin with another statin regimen (different drug or different dose) were sparse for people treated with dialysis.
Quality of the evidence
Overall, data evaluating the effects of statins on mortality and cardiovascular outcomes for dialysis patients is of moderate to high quality and suggests that additional studies are unlikely to change our confidence in the estimates of effect or our confidence in these results. The estimates of treatment effect for mortality, cardiovascular mortality and major cardiovascular events are derived from studies at generally low risks of bias, are consistent between studies, are precise, and are generalisable to dialysis populations outside the RCTs. Direct head‐to‐head data for different statin agents are sparse and inconclusive.
Potential biases in the review process
Although this review was conducted by two or more independent authors, used a comprehensive search of the literature designed by a specialist librarian that included grey literature, and examined all potentially relevant clinical outcomes, potential biases exist in the review process.
We were unable to include data for people treated with dialysis from SHARP Study 2010 or Stegmayr 2005 for all‐cause and cardiovascular mortality because reported data combined results for dialysis with earlier stages of kidney disease not treated with dialysis; separate unpublished data for dialysis populations were not available.
Many studies did not systematically report clinical outcomes: all but two either did not report or reported very few mortality events. Similarly, although meta‐analyses for mortality and cardiovascular events had no discernible heterogeneity, effects of statins on serum cholesterol levels were markedly different among studies. Subgroup analyses did not identify reasons for differences, including type of dialysis or baseline serum cholesterol.
Adverse events and stroke data were limited by wider confidence intervals and treatment effects were uncertain.
Agreements and disagreements with other studies or reviews
This review analysed current evidence on statin therapy in adults treated with dialysis, updating evidence from its previous two iterations in 2004 and 2009 (Navaneethan 2004; Navaneethan 2009a). This update included 23 studies of statins versus placebo or no treatment in 8166 participants treated with dialysis and two head‐to‐head studies comparing two different statins.
Data from AURORA Study 2005 were included in analyses for mortality and major cardiovascular events, and data from SHARP Study 2010 informed analyses of major cardiovascular events. The effect estimates for statins on mortality and adverse events in this review were largely similar to our 2009 review (Navaneethan 2009a), finding little or no effect from statins among people treated with dialysis. The possible benefit from statins on non‐fatal cardiovascular events in our 2009 review (which included one study of 1255 participants, 4D Study 2004) was not confirmed following inclusion of three additional studies and more than 5000 participants.
The finding that statins had little or no effect on mortality and cardiovascular outcomes in people treated with dialysis contrasts with a similar systematic review and meta‐analysis of studies in people with earlier stages of CKD (Palmer 2013a) and a prospective meta‐analysis of data of more general populations Baigent 2005. Statin therapy in people with less severe kidney disease proportionally reduced major cardiovascular events by 25% (RR 0.72, 95% CI 0.66 to 0.79) and all‐cause mortality by 20% (RR 0.79, 95% CI 0.69 to 0.91) (Palmer 2013a), and similarly reduced vascular events (RR 0.79, 95% CI 0.77 to 0.81) and all‐cause mortality (RR 0.88, 95% CI 0.84 to 0.91) in people with or at risk of cardiovascular disease in the general population (Baigent 2005).
In a recent analysis using the current data we showed that treatment effects of statins on mortality and cardiovascular events differ significantly based on stage of kidney disease (data not shown; Palmer 2012). Although it is unclear why, despite equivalent lowering of serum cholesterol, statins have less effect in people treated with dialysis, reasons may relate to the competing causes of cardiovascular morbidity (known and unknown) in people treated with dialysis that cannot be modified significantly by the lipid‐lowering or other pleiotropic effects of statins.
The smaller risk reductions from statins on death and cardiovascular disease in people treated with dialysis may reflect the competing mechanisms of cardiovascular disease in dialysis patients for whom vascular disease is dominated by vascular calcification, cardiomyopathy, hyperkalaemia, and sudden death, which might be modified to a lesser extent by statin therapy (ANZDATA 2009). We note that reductions in mortality were small in this meta‐analysis despite end of treatment LDL cholesterol lowering by 41 mg/dL (1.1 mmol/L) on average. This small relative effect of lipid‐lowering contrasts with a 12% risk reduction (95% CI 9% to 16%) for each 1 mmol/L reduction in LDL cholesterol in a meta‐analysis of studies in the general population (Baigent 2005). However, because few studies in the current meta‐analysis provided data for both all‐cause mortality and end of treatment lipid levels, we could not be certain if larger reductions in cholesterol levels might reduce mortality to a greater extent in the dialysis population or whether more aggressive lipid‐lowering approaches can be safely achieved with statin therapy.
Authors' conclusions
Implications for practice.
Statins have little or no effect on mortality or major cardiovascular outcomes in adults treated with dialysis and cannot be routinely recommended to prevent cardiovascular events in this population. The body of included evidence did not address whether statin treatment should be stopped when a person commences dialysis, although the benefits associated with continued treatment are likely to be small. Risks of adverse events for statins on muscle and liver dysfunction and cancer with statin treatment remain uncertain. Insufficient data are available to understand whether treatment effects differ in the clinical setting of haemodialysis compared to peritoneal dialysis or the effect of statin therapy in patients with established vascular disease or recent vascular event.
Implications for research.
Statin therapy consistently provides little or no benefit for people treated with dialysis. Despite some limitations, the evidence is generally moderate to high quality according to GRADE recommendations (Guyatt 2008), indicating further large studies may have an important impact on our confidence in the estimate of effect. Additional data for people treated with peritoneal dialysis would improve our confidence in the effects of therapy in this clinical setting. Well‐designed RCTs of other interventions to reduce cardiovascular morbidity and death in people on dialysis are now required.
What's new
| Date | Event | Description |
|---|---|---|
| 7 May 2014 | Amended | Minor copy edit made to study names |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 30 July 2013 | New citation required and conclusions have changed | 14 new studies have been included |
| 11 May 2012 | Amended | Author added: Jorgen Hegbrandt; Suetonia Palmer |
| 1 March 2012 | New search has been performed | Updated search to February 2012. Ten new trials added including AURORA and SHARP. Results and conclusions updated. Conclusions generally unchanged. |
| 21 January 2009 | New citation required and conclusions have changed | New studies included, additional outcomes now available. New authors for this update. |
| 1 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We acknowledge the contribution of study authors (Drs Wanner, Baigent, Diepeveen, Harris) who provided data about their studies upon request. We also thank Narelle Willis, for her help in coordinating and editing this review and Ruth Mitchell and Gail Higgins for assistance in the development of search strategies. We also acknowledge Dr Rakesh Srivastava who contributed to a previous version of this review.
We wish to thank the referees for their comments and feedback during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Statin versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major cardiovascular events | 4 | 7084 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.88, 1.03] |
| 2 All‐cause mortality | 13 | 4705 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.90, 1.02] |
| 3 Cardiovascular mortality | 13 | 4627 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.84, 1.06] |
| 4 Fatal and non‐fatal myocardial infarction | 3 | 4047 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.71, 1.07] |
| 5 Fatal and non‐fatal stroke | 2 | 4018 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.96, 1.72] |
| 6 Elevated creatine kinase | 5 | 3067 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.55, 2.83] |
| 7 Elevated liver function enzymes | 4 | 3044 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.41, 2.91] |
| 8 Withdrawal due to adverse events | 9 | 1832 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.87, 1.25] |
| 9 Cancer | 2 | 4012 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.72, 1.11] |
| 10 Total cholesterol | 14 | 1803 | Mean Difference (IV, Random, 95% CI) | ‐44.86 [‐55.19, ‐34.53] |
| 11 LDL cholesterol | 12 | 1747 | Mean Difference (IV, Random, 95% CI) | ‐39.99 [‐52.46, ‐27.52] |
| 12 Triglycerides | 13 | 1692 | Mean Difference (IV, Random, 95% CI) | ‐18.02 [‐31.00, ‐3.04] |
| 13 HDL cholesterol | 13 | 1769 | Mean Difference (IV, Random, 95% CI) | 2.57 [‐0.39, 5.52] |
Comparison 2. Statin versus another statin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Elevated liver function enzymes | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2 Withdrawal due to adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3 Total cholesterol | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 LDL cholesterol | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Triglycerides | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 HDL cholesterol | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
4D Study 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Industry funding received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Randomisation code prepared by a central unit that was independent of local study personnel |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All analyses of primary and secondary endpoints were based on the classification by the endpoint committee that was agreed by consensus or majority vote. All committee members were blinded to treatment assignments until 13 August 2004 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in ITT analysis |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| ITT analysis | Low risk | ITT |
Ahmadi 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NR |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Unclear risk | NR |
Angel 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Abstract only publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NR |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Unclear risk | NR |
Arabul 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Unclear risk | NR |
AURORA Study 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Industry funding received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All MIs, strokes, and deaths were reviewed and adjudicated by a clinical endpoint committee whose members were unaware of the randomised treatment assignments to ensure consistency of the event diagnosis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| ITT analysis | Low risk | Conducted |
Burmeister 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Industry funding received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/59 (5%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| ITT analysis | High risk | Not conducted |
Chang 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Industry funding received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/62 (6.5%) patients did not complete study |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | High risk | Not conducted |
Diepeveen 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Treatment duration: 3 months |
|
| Outcomes |
|
|
| Notes | Study included 4 arms and we compared treatment group 1 and the control group (see interventions) Industry funding received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Low risk | Conducted |
Dornbrook‐Lavender 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Industry funding received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/19 (32%) did not complete study |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | High risk | Not conducted |
Han 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 36/114 patients (32%) withdrawn |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Unclear risk | NR |
Harris 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 130/153 (85%) completed the study |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | High risk | Not conducted |
Ichihara 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NR |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Unclear risk | NR |
Lins 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Industry funding received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NR |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| ITT analysis | Unclear risk | NR |
PERFECT Study 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group (B)
Control group (D)
|
|
| Outcomes |
|
|
| Notes | Study had four arms
Industry funding received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | "...the code identifying the treatment received by individual patients was maintained by a person remote from the investigators." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NR |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Low risk | Conducted |
Saltissi HD 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Lipid parameters (TC, LDL, HDL, TG, Lp (a), Apo A1) | |
| Notes | This is the same study as Saltissi PD 2002 Industry funding received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 42/57 patients (74%) completed study |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| ITT analysis | High risk | Not conducted |
Saltissi PD 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Lipid parameters (TC, LDL, HDL, TG, Lp (a), Apo A1) | |
| Notes | This is the same study as Saltissi HD 2002 Industry funding received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 42/57 patients (74%) completed study |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| ITT analysis | High risk | Not conducted |
SHARP Study 2010.
| Methods |
|
|
| Participants |
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Only dialysis patients data from the SHARP Study 2010 trial have been included in this review Industry funding received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated by local study laptop computer with minimised randomisation |
| Allocation concealment (selection bias) | Low risk | Local laptop computer that was synchronised regularly with central database and double‐dummy treatment to ensure blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy 2 x 2 factorial design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Central adjudication by trained clinicians who were masked to study treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analyses |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| ITT analysis | Low risk | Conducted |
Soliemani 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Unclear risk | Unclear |
Stegmayr 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Low risk | Randomisation by means of a telephone call to the study data centre where sealed envelopes were drawn |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients analysed |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| ITT analysis | Low risk | Conducted |
Tse 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NR |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Unclear risk | NR |
UK‐HARP‐I 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimised randomisation used to balance the treatment groups; 2 x 2 factorial design |
| Allocation concealment (selection bias) | Low risk | Randomisation was by telephone to the Clinical Trial Service Unit |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | All events were coded centrally according to a standard protocol. Otherwise unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 442/448 (98.7%) patients completed follow‐up |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| ITT analysis | Low risk | Conducted |
van den Akker 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/28 patients (7%) discontinued therapy |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | High risk | Not conducted |
Vareesangthip 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NR |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Unclear risk | NR |
Velickovic 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NR |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Unclear risk | NR |
Vernaglione 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NR |
| Selective reporting (reporting bias) | High risk | Published reports did not include all expected outcomes |
| ITT analysis | Unclear risk | NR |
Yu 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes | Abstract publication only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | High risk | Publication reports did not provide all expected outcomes |
| ITT analysis | Unclear risk | NR |
ALT ‐ alanine aminotransferase; APD ‐ automated peritoneal dialysis; Apo ‐ apoprotein; AST ‐ aspartate aminotransferase; AMI ‐ acute myocardial infarction; BP ‐ blood pressure; CABG ‐ coronary artery bypass graft; CAPD ‐ continuous ambulatory peritoneal dialysis; CHF ‐ chronic heart failure; CK ‐ creatine kinase; CKD ‐ chronic kidney disease; CRP ‐ C‐reactive protein; CVA ‐ cerebrovascular accident; CVD ‐ cardiovascular disease; DM ‐ diabetes mellitus; ESKD ‐ end‐stage kidney disease; ESR ‐ erythrocyte sedimentation rate; HD ‐ haemodialysis; HDL ‐ high‐density lipoprotein; HRT ‐ hormone replacement therapy; hs‐CRP ‐ highly‐sensitive CRP; IL‐6 ‐ interleukin 6; ITT ‐ intention‐to‐treat; LDL ‐ low‐density lipoprotein; LFT ‐ liver function test; MI ‐ myocardial infarction; NR ‐ not reported; NSAIDs ‐ non‐steroidal anti‐inflammatory drugs; PD ‐ peritoneal dialysis; PTCA ‐ percutaneous transluminal coronary angioplasty; SCr ‐ serum creatinine; TC ‐ total cholesterol; TG ‐ triglycerides; ULN ‐ upper limit of normal
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akcicek 1996 | Not RCT (prospective cohort study) |
| Bunio 2004 | Active comparator (not statin) |
| Cappelli 2000 | Active comparator (not statin) |
| Cheng 1995 | Active comparator (not statin) |
| CHORUS Study 2001 | Study discontinued |
| Dogra 2007 | Short duration |
| Fiorini 1992 | Not RCT |
| Fiorini 1994 | Active comparator (not statin) |
| Hufnagel 2000 | Not RCT (prospective cohort study) |
| Khajehdehi 2000 | Not appropriate intervention |
| Kim 2009 | Not dialysis |
| Kishimoto 2010 | Not RCT |
| Li 1993 | Study duration < 8 weeks |
| Lins 2003 | Study duration 2 weeks |
| Lynoe 2004 | Not appropriate intervention |
| Malyszko 2002 | Not RCT (prospective cohort study) |
| Nishikawa 1999 | Not RCT (prospective cohort study) |
| Nishizawa 1995 | Not RCT (prospective cohort study) |
| Rincon 1995 | Not RCT |
| Samuelsson 2002 | Not dialysis |
| Sezer 2004 | Duration 1 month |
| Singh 2002 | Duration 4 weeks |
| Tani 1998 | Not RCT (prospective cohort study) |
| UK‐HARP‐II 2006 | Not appropriate intervention |
| Wanner 1991 | Not RCT (prospective cohort study) |
| Wanner 1992 | Not RCT (prospective cohort study) |
| Yigit 2004 | Not RCT |
| Zhu 2000 | Not RCT (prospective cohort study) |
Characteristics of ongoing studies [ordered by study ID]
Nardi 2005.
| Trial name or title | Prévention par la pravastatine de la dégradation de l’état nutritionnel des patients hémodialysés présentant un état inflammatoire chronique |
| Methods | Randomised trial, multicentre, double‐blinded, placebo‐controlled |
| Participants | Haemodialysis > 3 months, aged > 18 years and < 80 years, serum albumin < 40 g/L, and existence of chronic inflammation defined as CRP 10 to 50 mg/L on two separate occasions, without an identifiable cause |
| Interventions | Pravastatin 20 to 40 mg/d or placebo for 12 months |
| Outcomes | Inflammation |
| Starting date | 2005 |
| Contact information | CHU de Bordeaux, hôpital Pellegrin, Département de Néphrologie–Hémodialyse, 1, place Amélie‐Raba‐Léon, 33076 Bordeaux Cedex, France |
| Notes |
NCT00291863.
| Trial name or title | Simvastatin effect on end stage renal failure patients treated by peritoneal dialysis |
| Methods | Randomised, parallel group, double‐blind |
| Participants | 18 to 80 years, ESKD, LDL cholesterol > 100 mg/dL |
| Interventions | Simvastatin |
| Outcomes | Endothelial venodilation, inflammatory markers, lipoproteins, oxidative stress |
| Starting date | February 2006 |
| Contact information | Maristela Bohlke |
| Notes | Recruitment status of this study is unknown because the information has not been verified recently |
NCT00858637.
| Trial name or title | Efficacy and safety study of MCI‐196 versus simvastatin for dyslipidaemia in chronic kidney disease (CKD) subjects on dialysis |
| Methods | Phase III, multicentre, double‐blind, double‐dummy, randomised, flexible‐dose, comparative study of MCI‐196 versus simvastatin |
| Participants | > 18 years, male or female, stable dialysis, negative pregnancy test and appropriate contraception |
| Interventions | Simvastatin, MCl‐196, or placebo |
| Outcomes | Change in LDL cholesterol, change in total cholesterol, HDL cholesterol, triglycerides and additional lipid parameters, change in phosphorus (P), Calcium (Ca), calcium‐phosphorus ion product (PxCa) and parathyroid hormone (PTH), vital signs, adverse events, and laboratory values |
| Starting date | March 2009 |
| Contact information | Mitsubishi Tanabe Pharma Corporation |
| Notes | Unclear contact information |
NCT00999453.
| Trial name or title | The effects of lowering low‐density lipoprotein cholesterol levels to new targets on cardiovascular complications in peritoneal dialysis patients |
| Methods | Randomised, parallel group, open label |
| Participants | 20 to 70 years, treated with peritoneal dialysis for 3 or more months, LDL cholesterol 100 mg/dL or higher within 3 months and total cholesterol level 220 mg/dL or higher |
| Interventions | Either aggressive targets of LDL cholesterol of 70 mg/dL or current standard targets of LDL cholesterol of 100 mg/dL |
| Outcomes | Cardiovascular complication including acute coronary syndrome, cerebrovascular infarction and cardiovascular death |
| Starting date | October 2009 |
| Contact information | Shin‐Wook Kang |
| Notes | This study is currently recruiting participants |
LDL ‐ low density lipoprotein; HDL ‐ high density lipoprotein
Differences between protocol and review
The duration of treatment in included studies was reduced from 12 weeks to 8 weeks.
Differences between original review and review update
The study by Joy 2008 (included in the 2009 update of this review) is now considered to be a report of Dornbrook‐Lavender 2005 and has been incorporated with the references for that study. Fiorini 1994 has now been excluded from the review because the study did not compare a statin with either another statin, placebo, or no treatment. Dogra 2007 has now been excluded because the treatment duration was only six weeks.
Contributions of authors
Sankar D Navaneethan: concept and design of the review, data extraction, analysis and interpretation of data, writing the final manuscript, final approval of version to be published
Suetonia Palmer: data extraction, analysis and interpretation of data, drafting the final manuscript, final approval of version to be published
Vlado Perkovic: critical revision for intellectual content, interpretation of data, assistance with writing of the final manuscript, final approval of the manuscript to be submitted for publication
Sagar Nigwekar: data extraction, analysis and interpretation of data, writing the final manuscript
David W Johnson: data extraction, analysis and interpretation of data, writing the final manuscript, final approval of version to be published
Jonathan Craig: concept and design, analysis and interpretation of data, writing the final manuscript, final approval of version to be published
Giovanni FM Strippoli: concept and design of the review, data extraction, analysis and interpretation of data, writing the final manuscript, final approval of version to be published
Sources of support
Internal sources
No sources of support supplied
External sources
SP receives a Fellowship from the Consorzio Mario Negri Sud from an unrestricted grant from Amgen Dompe, Italy.
Declarations of interest
Vlado Perkovic is supported by a fellowship from the Heart Foundation of Australia and various grants from the Australian National Health and Medical Research Council. He has received speaker fees from Roche, Servier and Astra Zeneca, funding for a clinical trial from Baxter, and serves on Steering Committees for trials funded by Johnson and Johnson, Boehringer Ingelheim, Vitae and Abbott. His employer conducts clinical trials funded by Servier, Johnson and Johnson, Roche and Merck.
David Johnson is a consultant for Baxter Healthcare Pty Ltd and has previously received research funds from this company. He has also received speakers’ honoraria and research grants from Fresenius Medical Care and is a current recipient of a Queensland Government Health Research Fellowship. He has also received speakers' honoraria and consultancy fees from Amgen, Janssen‐Cilag, Shire, Lilley, Boehringer‐Ingelheim and Merck Sharpe & Dohme. He has received a research grant from Pfizer.
Suetonia Palmer received a fellowship administered by the Consorzio Mario Negri Sud from Amgen Dompe for assistance with travel for collaboration and supervision.
Edited (no change to conclusions)
References
References to studies included in this review
4D Study 2004 {published and unpublished data}
- Drechsler C, Krane V, Grootendorst DC, Ritz E, Winkler K, Marz W, et al. The association between parathyroid hormone and mortality in dialysis patients is modified by wasting. Nephrology Dialysis Transplantation 2009;24(10):3151‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler C, Krane V, Ritz E, Marz W, Wanner C. Glycemic control and cardiovascular events in diabetic hemodialysis patients. Circulation 2009;120(24):2421‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Drechsler C, Krane V, Winkler K, Dekker FW, Wanner C. Increasing adiponectin is associated with cardiovascular events and mortality in diabetic patients on hemodialysis [abstract]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):727A. [Google Scholar]
- Krane V, Berger M, Lilienthal J, Winkler K, Schambeck C, Wanner C, et al. Antibodies to platelet factor 4‐heparin complex and outcome in hemodialysis patients with diabetes. Clinical Journal of The American Society of Nephrology: CJASN 2010;5(5):874‐81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane V, Heinrich F, Meesmann M, Olschewski M, Lilienthal J, Angermann C, et al. Electrocardiography and outcome in patients with diabetes mellitus on maintenance hemodialysis. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(2):394‐400. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane V, Krieter DH, Olschewski M, Maerz W, Mann JFE, Ritz E, et al. Impact of dialyzer membrane characteristics on outcome in type 2 diabetic patients on maintenance hemodialysis: results of the 4D study [abstract]. Journal of the American Society of Nephrology 2006;17(Abstracts):22A. [Google Scholar]
- Krane V, Maerz W, Ruf G, Ritz E, Wanner C. Atorvastatin in Type 2 diabetic patients on hemodialysis (4D‐study): baseline characteristics and 1st interim analysis [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):496A. [Google Scholar]
- Krane V, Winkler K, Drechsler C, Lilienthal J, Marz W, Wanner C, et al. Association of LDL cholesterol and inflammation with cardiovascular events and mortality in hemodialysis patients with type 2 diabetes mellitus. American Journal of Kidney Diseases 2009;54(5):902‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Krane V, Winkler K, Drechsler C, Lilienthal J, Marz W, Wanner C, et al. Effect of atorvastatin on inflammation and outcome in patients with type 2 diabetes mellitus on hemodialysis. Kidney International 2008;74(11):1461‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Marz W, Genser B, Drechsler C, Krane V, Grammer TB, Ritz E, et al. Atorvastatin and low‐density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(6):1316‐25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner C, Krane V, Marz W, Olschewski M, Asmus HG, Kramer W, et al. Randomized controlled trial on the efficacy and safety of atorvastatin in patients with type 2 diabetes on hemodialysis (4D study): demographic and baseline characteristics. Kidney & Blood Pressure Research 2004;27(4):259‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. New England Journal of Medicine 2005;353(3):238‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wanner C, Krane V, Ruf G, Marz W, Ritz E. Rationale and design of a trial improving outcome of type 2 diabetics on hemodialysis. Die Deutsche Diabetes Dialyse Studie Investigators. Kidney International ‐ Supplement 1999;56(Suppl 71):S222‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ahmadi 2005 {published data only}
- Ahmadi FI, Eslami K, Maziar S, Lessan‐Pezeshki M, Khatami MR, Mahdavi‐mazdeh M, et al. Effect of lovastatin on high‐sensitivity C‐reactive protein and hemoglobin in hemodialysis patients [abstract]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv229. [Google Scholar]
- Ahmadi FL, Eslami K, Maziar S, Lessan‐Pezeshki M, Reza‐Khatami M, Mahdavi‐mazdeh M, et al. Effect of lovastatin on C‐reactive protein and hemoglobin in hemodialysis patients [abstract]. Nephrology 2005;10(Suppl):A283‐4. [Google Scholar]
Angel 2007 {published data only}
- Angel JR, Rojas E, Campo FM, Nava D, Cueto‐Manzano A. Effect of pravastatin on inflammatory status of CAPD patients: a randomized, double‐blinded, controlled and cross‐over clinical trial [abstract]. Journal of the American Society of Nephrology 2007;18(Abstracts):270A. [Google Scholar]
- Zuniga J, Rojas‐Campos E, Campo FM, Nava D, Cueto‐Manzano A. Effect of pravastatin on the inflammatory status of CAPD patients: a randomized, double‐blinded, controlled and cross‐over clinical trial [abstract]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi301. [Google Scholar]
Arabul 2008 {published data only}
- Arabul M, Gullulu M, Yilmaz Y, Akdag I, Kahvecioglu S, Eren MA, et al. Effect of fluvastatin on serum prohepcidin levels in patients with end‐stage renal disease. Clinical Biochemistry 2008;41(13):1055‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
AURORA Study 2005 {published data only}
- Fellstrom B, Holdaas H, Jardine AG, Rose H, Schmieder R, Wilpshaar W, et al. Effect of rosuvastatin on outcomes in chronic haemodialysis patients: baseline data from the AURORA study. Kidney & Blood Pressure Research 2007;30(5):314‐22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellstrom B, Holdaas H, Jardine AG, Schmieder RE, Holme I, Zannad F. Normalisation of CRP and LDL‐C levels is related to reduction of cardiovascular morbidity and mortality in haemodialysis patients on rosuvastatin treatment ‐ a posthoc analysis of the AURORA trial [abstract]. Journal of the American Society of Nephrology 2010;21:218a. [Google Scholar]
- Fellstrom B, Jardine AG, Holdaas H, Schmieder R, Gottlow M, Johnsson E, et al. Effect of rosuvastatin versus placebo on cardiovascular outcomes in patients with end‐stage renal disease on hemodialysis ‐ results of the Aurora Study [abstract]. World Congress of Nephrology; 2009 May 22‐26; Milan (Italy). 2009.
- Fellstrom B, Zannad F, Schmieder R, Holdaas H, Jardine A, Armstrong J, et al. A study to evaluate the use of rosuvastatin in subjects on regular haemodialysis: an assessment of survival and cardiovascular events ‐ the AURORA study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):713. [Google Scholar]
- Fellstrom B, Zannad F, Schmieder R, Holdaas H, Jardine A, Rose H, et al. Effect of rosuvastatin on outcomes in chronic haemodialysis patients ‐ Design and rationale of the AURORA study. Current Controlled Trials in Cardiovascular Medicine 2005;6:9. [EMBASE: 2005487466] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. New England Journal of Medicine 2009;360(14):1395‐407. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holdaas H, Fellstrom B, Holme I, Schmieder RE, Zannad F, Jardine AG. Rosuvastatin and LDL cholesterol in diabetic patients receiving hemodialysis ‐ a posthoc analysis of the AURORA trial [abstract]. Journal of the American Society of Nephrology 2010;21:12A. [Google Scholar]
- Holdaas H, Holme I, Schmieder RE, Jardine AG, Zannad F, Norby GE, et al. Rosuvastatin in diabetic hemodialysis patients. Journal of the American Society of Nephrology 2011;22(7):1335‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaas H, Jardine AG, Schmieder R, Gottlow M, Johnsson E, Zannad F, et al. Effect of rosuvastatin on cardiovascular outcomes in diabetic patients receiving hemodialysis ‐ results from the Aurora Study [abstract]. World Congress of Nephrology; 2009 May 22‐26; Milan (Italy). 2009.
- Jardine AG, Fellstrom B, Holdaas H, Gottlow M, Johnsson E, Zannad F, et al. Risk factors for cardiovascular events in patients receiving haemodialysis ‐ post hoc analyses of the AURORA study [abstract]. World Congress of Nephrology; 2009 May 22‐26; Milan (Italy). 2009.
Burmeister 2006 {published data only}
- Burmeister JE, Miltersteiner DR, Campos BM. Rosuvastatin in hemodialysis: short‐term effects on lipids and C‐reactive protein. Journal of Nephrology 2009;22(1):83‐89. [MEDLINE: ] [PubMed] [Google Scholar]
- Burmeister JE, Miltersteiner DR, Costa MG, Campos BM. Short‐term results of rosuvastatin therapy over lipid profile and hs‐C‐reactive protein in patients on regular hemodialysis [abstract]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv472. [Google Scholar]
Chang 2002 {published data only}
- Chang JW, Lee MS, Kin SB, Yang WS, Park SK, Lee SK, et al. Simvastatin decreased high‐sensitive c‐reactive protein (hs‐CRP) and increased serum albumin in hemodialysis (HD) patients [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):353A. [Google Scholar]
- Chang JW, Yang WS, Min WK, Lee SK, Park JS, Kim SB. Effects of simvastatin on high‐sensitivity C‐reactive protein and serum albumin in hemodialysis patients. American Journal of Kidney Diseases 2002;39(6):1213‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diepeveen 2005 {published data only}
- Bilo HJ, Diepeveen SJ, Verhoeven GH, Palen J, Dikkeschei BD, et al. Vitaestat 1: alpha‐tocopherol and atorvastatin in HD‐ and PD‐ patients: a short‐term, prospective randomised placebo‐controlled intervention trial [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):191A. [Google Scholar]
- Diepeveen SH, Verhoeven GH, Palen J, Dikkeschei LD, Denmacker PN, Kolsters G, et al. Effects of atorvastatin and vitamin E on lipoproteins and oxidative stress in dialysis patients: a randomised‐controlled trial. Journal of Internal Medicine 2005;257(5):438‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Diepeveen SH, Verhoeven GH, Palen J, Dikkeschei LD, Denmacker PN, Kolsters G, et al. Vitaestat 1: a prospective randomised placebo controlled trial with a‐tocoferol and atorvastatin in patients in hemo‐ and peritoneal dialysis [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A87. [Google Scholar]
Dornbrook‐Lavender 2005 {published data only}
- Dornbrook‐Lavender KA, Joy MS, Denu‐Ciocca CJ, Chin H, Hogan SL, Pieper JA. Effects of atorvastatin on low‐density lipoprotein cholesterol phenotype and C‐reactive protein levels in patients undergoing long‐term dialysis. Pharmacotherapy 2005;25(3):335‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Joy MS, Dornbrook‐Lavender KA, Chin H, Hogan SL, Denu‐Ciocca C. Effects of atorvastatin on Lp(a) and lipoprotein profiles in hemodialysis patients. Annals of Pharmacotherapy 2008;42(1):9‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Joy MS, Dornbrook‐Lavender KA, Chin H, Hogan SL, Denu‐Ciocca CJ. Atorvastatin can safely and effectively improve cholesterol and contemporary measures of cardiovascular risks in hemodialysis patients [abstract]. Journal of the American Society of Nephrology 2004;15(Oct):615A. [Google Scholar]
Han 2011 {published data only}
- Han SH, Kang EW, Yoon SJ, Lee HC, Yoo TH, Choi KH, et al. Combined vascular effects of HMG‐CoA reductase inhibitor and angiotensin receptor blocker in non‐diabetic patients undergoing peritoneal dialysis. Nephrology Dialysis Transplantation 2011;26(11):3722‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harris 2002 {published data only}
- Harris KP, Wheeler DC, Chong CC, Atorvastatin in CAPD Study Investigators. A placebo controlled trial examining atorvastatin in dyslipidemic patients undergoing CAPD. Kidney International 2002;61(4):1469‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wheeler D, Harris KP, UK CAPD Investigators. A placebo‐controlled trial examining the efficacy and safety of atorvastatin in dyslipidaemic patients undergoing CAPD [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A196. [Google Scholar]
Ichihara 2002 {published data only}
- Ichihara A, Hayashi M, Ryuzaki M, Handa M, Furukawa T, Saruta T. Effects of fluvastatin on leg arterial stiffness in hemodialysis patients with type 2 diabetes mellitus [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):148A. [Google Scholar]
- Ichihara A, Hayashi M, Ryuzaki M, Handa M, Furukawa T, Saruta T. Fluvastatin prevents development of arterial stiffness in haemodialysis patients with type 2 diabetes mellitus. Nephrology Dialysis Transplantation 2002;17(8):1513‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lins 2004 {published data only}
- Lins RL, Carpentier YA, Clinical Investigators Study Group. Plasma lipids and liporoteins during atorvastatin (ATVS) up‐titration in hemodialysis patients with hyperlipidemia: a placebo‐controlled study [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):124. [Google Scholar]
- Lins RL, Matthys KE, Billiouw JM, Dratwa M, Dupont P, Lameire NH, et al. Lipid and apoprotein changes during atorvastatin up‐titration in hemodialysis patients with hypercholesterolemia: a placebo‐controlled study. Clinical Nephrology 2004;62(4):287‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PERFECT Study 1997 {published data only}
- Collins JF, Robson R, MacMahon S, Bailey RR, PERFECT Study Group. Safety and efficacy of enalapril and simvastatin in dialysis patients [abstract]. 6th Asian Pacific Congress of Nephrology; 1995 Dec 5‐9; Hong Kong. 1995:144.
- Robson R, Collins J, Johnson R, Kitching R, Searle M, Walker R, et al. Effects of simvastatin and enalapril on serum lipoprotein concentrations and left ventricular mass in patients on dialysis. Journal of Nephrology 1997;10(1):33‐40. [MEDLINE: ] [PubMed] [Google Scholar]
- Robson R, Collins J, Kitchings R, Searle M, Walker R, Sharpe N, et al. A randomized controlled trial of simvastatin and enalapril in dialysis patients: effects on serum lipoproteins and left ventricular mass [abstract]. Journal of the American Society of Nephrology 1994;5(3):526. [Google Scholar]
- Robson RA, Collins J, Walker RJ, MacMahon S. A randomised controlled trial of simvastatin and enalapril in dialysis patients: effects on serum lipoproteins and left ventricular mass [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid (Spain). 1995:530.
- Walker RJ, Sutherland WH, Walker H. Simvastatin and cholesteryl ester transfer (CET) activity in renal failure (RF) [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid (Spain). 1995:529.
- Walker RJ, Sutherland WH, Walker H, Robson RA, MacMahon SA. The effects of simvastatin and enalapril on plasma cholesteryl ester transfer (CET) activity in renal failure (RF) [abstract]. 6th Asian Pacific Congress of Nephrology; 1995 Dec 5‐9; Hong Kong. 1995:28.
- Walker RJ, Sutherland WH, Walker HL, MacMahon S, Robson RA. Effect of treatment with simvastatin on serum cholesteryl ester transfer in patients on dialysis. PERFECT Study Collaborative Group. Nephrology Dialysis Transplantation 1997;12(1):87‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saltissi HD 2002 {published data only}
- Saltissi D, Morgan C, Rigby RJ, Westhuyzen J. Safety and efficacy of simvastatin in hypercholesterolemic patients undergoing chronic renal dialysis. American Journal of Kidney Diseases 2002;39(2):283‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Westhuyzen J, Saltissi C, Morgan C, Rigby RJ. Safety and efficacy of simvastatin in hypercholesterolaemic patients undergoing chronic renal dialysis [abstract]. Nephrology 2002;7(Suppl 3):A38. [DOI] [PubMed] [Google Scholar]
Saltissi PD 2002 {published data only}
- Saltissi D, Morgan C, Rigby RJ, Westhuyzen J. Safety and efficacy of simvastatin in hypercholesterolemic patients undergoing chronic renal dialysis. American Journal of Kidney Diseases 2002;39(2):283‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Westhuyzen J, Saltissi C, Morgan C, Rigby RJ. Safety and efficacy of simvastatin in hypercholesterolaemic patients undergoing chronic renal dialysis [abstract]. Nephrology 2002;7(Suppl 3):A38. [DOI] [PubMed] [Google Scholar]
SHARP Study 2010 {published data only}
- Study of Heart and Renal Protection (SHARP). Final protocol (Version 5: 12th July 2005). http://www.ctsu.ox.ac.uk/˜sharp/download_protocol_en_v5.pdf (accessed 23 July 2013).
- Baigent C, Landry M. Study of Heart and Renal Protection (SHARP). Kidney International ‐ Supplement 2003;63(Suppl 84):S207‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baigent C, Reith C, Emberson J, Wheeler DC, Tomson CR, Wanner C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet 2011;377(9784):2181‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP Collaborative Group. SHARP: an international randomized placebo‐controlled trial of lipid‐lowering in chronic kidney disease. 1‐year safety and efficacy experience [abstract no: SU‐PO1044]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):817A. [Google Scholar]
- Sharp Collaborative Group. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low‐density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. American Heart Journal 2010;160(5):785‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Soliemani 2011 {published data only}
- Soliemani A, Nikoueinejad H, Tabatabaizade M, Mianehsaz E, Tamadon M. Effect of hydroxymethylglutaryl‐CoA reductase inhibitors on low‐density lipoprotein cholesterol, interleukin‐6, and high‐sensitivity C‐reactive protein in end‐stage renal disease. Iranian journal of Kidney Diseases 2011;5(1):29‐33. [MEDLINE: ] [PubMed] [Google Scholar]
Stegmayr 2005 {published data only}
- Holmberg B, Brannstrom M, Bucht B, Crougneau V, Dimeny E, Ekspong A, et al. Safety and efficacy of atorvastatin in patients with severe renal dysfunction. Scandinavian Journal of Urology & Nephrology 2005;39(6):503‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stegmayr BG, Brannstrom M, Bucht S, Dimeny E, Ekspong A, Granroth B, et al. Safety and efficacy of atorvastatin in patients with impaired renal function [abstract]. 38th Congress. European Renal Association. European Dialysis and Transplantation Association; 2001 Jun 24‐27; Vienna (Austria). 2001:134.
- Stegmayr BG, Brännström M, Bucht S, Crougneau V, Dimeny E, Ekspong A, et al. Low‐dose atorvastatin in severe chronic kidney disease patients: a randomized, controlled endpoint study. Scandinavian Journal of Urology & Nephrology 2005;39(6):489‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stegmayr BG, Nasstrom BG, Brannstrom M, Bucht S, Dimeny E, Gosch J, et al. Safety and efficacy of atorvastatin in patients with severe renal dysfunction [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):161A. [Google Scholar]
- Stegmayr BG, Nasstrom BG, Brannstrom M, Bucht S, Dimeny E, Granroth B, et al. Safety and efficacy of atorvastatin in patients with severe renal dysfunction [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):268A. [Google Scholar]
Tse 2008 {published data only}
- Tse KC, Yung S, Tang CS, Tam S, Lai KN, Chan TM. Atorvastatin at conventional dose did not reduce C‐reactive protein in patients on peritoneal dialysis. Journal of Nephrology 2008;21(3):283. [MEDLINE: ] [PubMed] [Google Scholar]
UK‐HARP‐I 2005 {published data only}
- Baigent C, Landray M, Leaper C, Altmann P, Armitage J, Baxter A, et al. First United Kingdom Heart and Renal Protection (UK‐HARP‐I) study: biochemical efficacy and safety of simvastatin and safety of low‐dose aspirin in chronic kidney disease. American Journal of Kidney Diseases 2005;45(3):473‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baigent C, UK‐HARP Steering Committee. Efficacy and safety of simvastatin and safety of low‐dose aspirin among patients with chronic kidney disease: final results of the first UK‐heart and renal protection (UK‐HARP‐I) study [abstract]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):437A. [Google Scholar]
van den Akker 2003 {published data only}
- Akker JM, Bredie SJ, Diepenveen SH, Tits LJ, Stalenhoef AF, Leusen R. Atorvastatin and simvastatin in patients on hemodialysis: effects on lipoproteins, C‐reactive protein and in vivo oxidized LDL. Journal of Nephrology 2003;16(2):238‐44. [MEDLINE: ] [PubMed] [Google Scholar]
Vareesangthip 2005 {published data only}
- Vareesangthip K, Laouthaiwattana P, Hanlakorn P, Suwannaton L, Larpkitkachorn R, Chuawattana D, et al. Effects of simvastatin on the kinetics of erythrocyte sodium lithium countertransport and C‐reactive protein in hemodialysis patients [abstract]. Journal of the American Society of Nephrology 2005;16:276A. [Google Scholar]
Velickovic 1997 {published data only}
- Velickovic‐Radovanovic R, Avramovic M, Malobabic Z, Djordjevic V, Kostic S, Mitic B. Therapeutic effects of simvastatin on hyperlipidemia in uraemic patients on CAPD [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A187. [Google Scholar]
Vernaglione 2003 {published data only}
- Vernaglione L, Cristofano C, Muscogiuri P, Chimienti S. Does atorvastatin influence serum C‐reactive protein levels in patients on long‐term hemodialysis?. American Journal of Kidney Diseases 2004;43(3):471‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vernaglione L, Cristofano C, Muscogiuri P, Pennacchiotti F, Chimienti S. Analysis of the impact of atorvastatin (ATO) on c‐reactive protein (CRP) levels in haemodialysis patients (HDP) [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):41A. [Google Scholar]
- Vernaglione L, Cristofano C, Pennacchiotti F, Muscogiuri P, Chimienti S. Effects of atorvastatin (ATO) on C‐reactive protein (CRP) levels in haemodialysis patients (HDP) : a prospective study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):712‐3. [Google Scholar]
Yu 2007 {published data only}
- Yu MH, Lee JH, Min WK, Chi HS, Chang JW, Yang WS, et al. Effects of ezetimibe and ezetimibe plus simvastatin (Vytorin®) on markers for inflammation and thrombogenesis in end‐stage renal disease (ESRD) patients [abstract]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi322. [Google Scholar]
References to studies excluded from this review
Akcicek 1996 {published data only}
- Akcicek F, Ok E, Duman S, Kursad S, Unsal A, Alev M, et al. Lipid‐lowering effects of simvastatin and gemfibrozil in CAPD patients: a prospective cross‐over study. Advances in Peritoneal Dialysis 1996;12:261‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Bunio 2004 {published data only}
- Bunio A, Kwiecinski R, Steciwko A, Migas A. Effect of lipid lowering treatment on direct markers of bone metabolism in hemodialysis patients [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon (Portugal). 2004:320‐1.
Cappelli 2000 {published data only}
- Cappelli P, Liberato L, Rosso G, Bonomini M. Lipid‐lowering therapy in patients with chronic renal failure [abstract]. 37th Congress. European Renal Association. European Dialysis and Transplantation Association. European Kidney Research Organisation; 2000 Sept 17‐20; Nice (France). 2000:181.
Cheng 1995 {published data only}
- Cheng IKP, Li PWC, Janus ED, Tang CSO, Chan TM, Lo CY. Comparative efficacy of gemfibrozil (G) and simvastatin (S) in the treatment of hyperlipidaemia in renal transplant recipients (RT) ‐ a randomised prospective study [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid (Spain). 1995:383.
CHORUS Study 2001 {published data only}
- Keane WF, Brenner BM, Mazzu A, Agro A. The CHORUS (Cerivastatin in Heart Outcomes in Renal Disease: Understanding Survival) protocol: a double‐blind, placebo‐controlled trial in patients with ESRD. American Journal of Kidney Diseases 2001;37(1 Suppl 2):S48‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dogra 2007 {published data only}
- Dogra G, Irish A, Chan D, Watts G. A randomized trial of the effect of statin and fibrate therapy on arterial function in CKD. American Journal of Kidney Diseases 2007;49(6):776‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dogra S, Kanganas C, Chan D, Irish A, Watts G. Effect of statin and fibrate therapy on vascular function in chronic kidney disease (CKD) [abstract]. Nephrology 2005;10(Suppl 3):A422. [Google Scholar]
Fiorini 1992 {published data only}
- Fiorini F, Patrone E, Ardu F, Castelluccio A. Efficacy and safety of simvastatin in the treatment of hyperlipidemia in uremic patients undergoing hemodialysis treatment. Minerva Urologica e Nefrologica 1992;44(2):165‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Fiorini 1994 {published data only}
- Fiorini F, Patrone E, Castelluccio A. Clinical investigation on the hypolipidemic effect of simvastatin versus probucol in hemodialysis patients. Clinica Terapeutica 1994;145(9):213‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Hufnagel 2000 {published data only}
- Hufnagel G, Michel C, Vrtovsnik F, Queffeulou G, Kossari N, Mignon F. Effects of atorvastatin on dyslipidaemia in uraemic patients on peritoneal dialysis. Nephrology Dialysis Transplantation 2000;15(5):684‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khajehdehi 2000 {published data only}
- Khajehdehi A, Khajehdehi P. Lipid lowering effect of polyunsaturated fatty acids in hemodialysis (HD) patients [abstract]. 37th Congress. European Renal Association. European Dialysis and Transplantation Association. European Kidney Research Organisation; 2000 Sept 17‐20; Nice (France). 2000:200.
Kim 2009 {published data only}
- Kim YJ, Lee HK, Jeong JU, Kim SB, Park JS, Moon KH, et al. Effects of rosuvastatin and gemfibrozil on small dense LDL‐cholesterol in chronic hemodialysis (CHD) patients [abstract]. World Congress of Nephrology 2009; May 22‐26; Milan (Italy). 2009.
Kishimoto 2010 {published data only}
- Kishimoto N, Hayashi T, Sakuma I, Kano‐Hayashi H, Tsunekawa T, Osawa M, et al. A hydroxymethylglutaryl coenzyme A reductase inhibitor improves endothelial function within 7 days in patients with chronic hemodialysis. International Journal of Cardiology 2010;145(1):21‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 1993 {published data only}
- Li PK, Mak TW, Chiu K, Mak GY, Leung CB, Lui SF, et al. Effect of lovastatin on serum lipid profile in the treatment of dyslipoproteinaemia in uraemic patients on continuous ambulatory peritoneal dialysis. Australian & New Zealand Journal of Medicine 1993;23(3):252‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Li PK, Mak TW, Lam CW, Lai KN. Lovastatin treatment of dyslipoproteinemia in patients on continuous ambulatory peritoneal dialysis. Peritoneal Dialysis International 1993;13 Suppl 2:S428‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Lins 2003 {published data only}
- Lins RL, Matthys KE, Verpooten GA, Peeters PC, Dratwa M, Stolear JC, et al. Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephrology Dialysis Transplantation 2003;18(5):967‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lynoe 2004 {published data only}
- Lynoe N, Nasstrom B, Sandlund M. Study of the quality of information given to patients participating in a clinical trial regarding chronic hemodialysis. Scandinavian Journal of Urology & Nephrology 2004;38(6):517‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Malyszko 2002 {published data only}
- Malyszko J, Malyszko JS, Hryszko T, Brzosko S, Mysliwiec M. Simvastatin and markers of endothelial function in patients undergoing continuous ambulatory peritoneal dialysis. International Journal of Tissue Reactions 2002;24(3):111‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Nishikawa 1999 {published data only}
- Nishikawa O, Mune M, Miyano M, Nishide T, Nishide I, Maeda A, et al. Effect of simvastatin on the lipid profile of hemodialysis patients. Kidney International ‐ Supplement 1999;71:219‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nishizawa 1995 {published data only}
- Nishizawa Y, Shoji T, Emoto M, Kawaskai K, Konishi T, Tabata T, et al. Reduction of intermediate density lipoprotein by pravastatin in hemo‐ and peritoneal dialysis patients. Clinical Nephrology 1995;43(4):268‐77. [MEDLINE: ] [PubMed] [Google Scholar]
Rincon 1995 {published data only}
- Rincon B, Tornero F, Uson J, Garcia‐Carzon A, Lozano L. Efficacy and side effects of HMG‐CoA reductase inhibitors in the treatment of hyperlipemia in hemodialysis patients [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid (Spain). 1995:531.
Samuelsson 2002 {published data only}
- Alaupovic P, Attman PO, Knight‐Gibson C, Mulec H, Weiss L, Samuelsson O. Effect of fluvastatin on apolipoprotein‐defined lipoprotein subclasses in patients with chronic renal insufficiency. Kidney International 2006;69(10):1865‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Samuelsson O, Attman PO, Knight‐Gibson C, Mulec H, Weiss L, Alaupovic P. Fluvastatin improves lipid abnormalities in patients with moderate to advanced chronic renal insufficiency. American Journal of Kidney Diseases 2002;39(1):67‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sezer 2004 {published data only}
- Sezer M, Katirci S, Adana S, Erturk J, Kaya S. Simvastatin decreases tumor necrosis factor alpha in patients on peritoneal dialysis [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon (Portugal). 2004:181.
- Sezer MT, Katirci S, Demir M, Erturk J, Adana S, Kaya S. Short‐term effect of simvastatin treatment on inflammatory parameters in peritoneal dialysis patients. Scandinavian Journal of Urology & Nephrology 2007;41(5):436‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singh 2002 {published data only}
- Singh B, Srinivasan B, Narsipur SS. Effect of simvastatin use on autonomic function in patients with end stage renal disease [abstract]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):217A. [DOI] [PubMed] [Google Scholar]
Tani 1998 {published data only}
- Tani M, Inagaki M, Oguchi K. Favorable effects of long‐term low‐dose pravastatin administration on lipid metabolism in chronic hemodialysis patients with hypercholesterolemia. Journal of the Showa Medical Association 1999;59(1):22‐7. [EMBASE: 1999206546] [Google Scholar]
UK‐HARP‐II 2006 {published data only}
- Landray M, Baigent C, Leaper C, Adu D, Altmann P, Armitage J, et al. The second United Kingdom Heart and Renal Protection (UK‐HARP‐II) Study: a randomized controlled study of the biochemical safety and efficacy of adding ezetimibe to simvastatin as initial therapy among patients with CKD. American Journal of Kidney Diseases 2006;47(3):385‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Landray M, Baigent C, Leaper C, the UK‐HARP Steering Committee. Biochemical safety and efficacy of co‐administration of Ezetimibe and Simvastatin among patients with chronic kidney disease: the second UK‐heart and renal protection (UK‐HARP‐II) study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):119‐20. [Google Scholar]
Wanner 1991 {published data only}
- Wanner C, Horl WH, Luley CH, Wieland H. Effects of HMG‐CoA reductase inhibitors in hypercholesterolemic patients on hemodialysis. Kidney International 1991;39(4):754‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wanner 1992 {published data only}
- Wanner C, Lubrich‐Birkner I, Summ O, Weiland H, Schollmeyer P. Effect of simvastatin on qualitative and quantitative changes of lipoprotein metabolism in CAPD patients. Nephron 1992;62(1):40‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yigit 2004 {published data only}
- Yigit F, Muderrisoglu H, Guz G, Bozbas H, Korkmaz ME, Ozin MB, et al. Comparison of intermittent with continuous simvastatin treatment in hypercholesterolemic patients with end stage renal failure. Japanese Heart Journal 2004;45(6):959‐68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhu 2000 {published data only}
- Zhu XP, Li J, Liu FY, Liu YH. Effects of Simvastatin in continuous ambulatory peritoneal dialysis patients with hyperlipidemia. Bulletin of Hunan Medical University 2000;25(2):154‐6. [MEDLINE: ] [PubMed] [Google Scholar]
References to ongoing studies
Nardi 2005 {published data only}
- Nardi H. Clinical protocol. Pravastatin for the prevention of the destruction of the nutritional status of hemodialysis patients exhibiting chronic inflammation. Nephrologie et Therapeutique 2005;1(1):75‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00291863 {published data only}
- NCT00291863. Simvastatin effect on endothelium dependent venodilation in chronic renal failure patients treated by peritoneal dialysis. http://www.clinicaltrials.gov/ct2/show/NCT00291863 (accessed 23 July 2013).
NCT00858637 {published data only}
- NCT00858637. Efficacy and safety study of MCI‐196 versus simvastatin for dyslipidaemia in chronic kidney disease (CKD) subjects on dialysis. http://www.clinicaltrials.gov/ct2/show/NCT00858637 (accessed 23 July 2013).
NCT00999453 {published data only}
- Kang SW. The effects of lowering low‐density lipoprotein cholesterol levels to new targets on cardiovascular complications in peritoneal dialysis patients. http://www.clinicaltrials.gov/ct2/show/NCT00999453 (accessed 23 July 2013).
Additional references
ANZDATA 2009
- Australia, New Zealand Transplant Registry (ANZDATA). The 32nd Annual Report 2009 Report ‐ Data to 2008. http://www.anzdata.org.au/v1/report_2009.html (accessed 24 July 2013).
Baigent 2005
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Briel 2006
- Briel M, Schwartz GG, Thompson PL, Lemos JA, Blazing MA, Es GA, et al. Effects of early treatment with statins on short‐term clinical outcomes in acute coronary syndromes: a meta‐analysis of randomized controlled trials. JAMA 2006;295(17):2046‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
FDA 2011
- Smith JP. Clinical Briefing Document. Endocrinologic and Metabolic Drugs Advisory Committee Meeting. November 2, 2011. New Drug Application 21‐687/S‐039:Vytorin (ezetimibe/simvastatin). New Drug Application 21‐445/S‐033:Zetia (ezetimibe). http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM277649.pdf (accessed 24 July 2013).
Foley 2007
- Foley RN, Collins AJ. End‐stage renal disease in the United States: an update from the United States Renal Data System. Journal of the American Society of Nephrology 2007;18(10):2644‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ganesh 2001
- Ganesh SK, Stack AG, Levin NW, Hulbert‐Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. Journal of the American Society of Nephrology 2001;12(10):2131‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herzog 1998
- Herzog C A, Ma J Z, Collins A J. Poor long‐term survival after acute myocardial infarction among patients on long‐term dialysis. New England Journal of Medicine 1998;339(12):799‐805. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herzog 2011
- Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney International 2011;80(6):572‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jungers 1997
- Jungers P, Massy ZA, Khoa TN, Fumeron C, Labrunie M, Lacour B, et al. Incidence and risk factors of atherosclerotic cardiovascular accidents in predialysis chronic renal failure patients: a prospective study. Nephrology Dialysis Transplantation 1997;12(12):2597‐602. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Law 1994
- Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease?. BMJ 1994;308(6925):367‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mallamaci 2002
- Mallamaci F, Zoccali C, Tripepi G, Fermo I, Benedetto FA, Cataliotti A, et al. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney International 2002;61(2):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Navaneethan 2009b
- Navaneethan SD, Pansini F, Perkovic V, Manno C, Pellegrini F, Johnson DW, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007784] [DOI] [PubMed] [Google Scholar]
Navaneethan 2009c
- Navaneethan SD, Perkovic V, Johnson DW, Nigwekar SU, Craig JC, Strippoli GF. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD005019.pub3] [DOI] [PubMed] [Google Scholar]
Palmer 2012
- Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta‐analysis. Annals of Internal Medicine 2012;157(4):263‐75. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2013a
- Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Hegbrant J, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database of Systematic Reviews 2013, Issue in press. [DOI] [PubMed] [Google Scholar]
Palmer 2013b
- Palmer SC, Navaneethan SD, Craig JC, Perkovic V, Johnson DW, Nigwekar SU, et al. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database of Systematic Reviews 2013, Issue in press. [Google Scholar]
Rossouw 1990
- Rossouw JE, Lewis B, Rifkind BM. The value of lowering cholesterol after myocardial infarction. New England Journal of Medicine 1990;323(16):1112‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Trivedi 2009
- Trivedi H, Xiang Q, Klein J P. Risk factors for non‐fatal myocardial infarction and cardiac death in incident dialysis patients. Nephrology Dialysis Transplantation 2009;24(1):258‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USRDS 2011
- United States Renal Data System. USRDS 2011 Annual Data Report. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. http://www.usrds.org/2011/pdf/v2_ch04_11.pdf (accessed 24 July 2013).
Weiner 2006
- Weiner D E, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith J, et al. Cardiovascular outcomes and all‐cause mortality: exploring the interaction between CKD and cardiovascular disease. American Journal of Kidney Diseases 2006;48(3):392‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wetmore 2009
- Wetmore JB, Shireman TI. The ABCs of cardioprotection in dialysis patients: a systematic review. American Journal of Kidney Diseases 2009;53(3):457‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Navaneethan 2003
- Navaneethan SD, Shrivastava R. HGM CoA reductase inhibitors (statins) for lowering cholesterol in dialysis patients. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004289] [DOI] [PubMed] [Google Scholar]
Navaneethan 2004
- Navaneethan SD, Shrivastava R. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004289.pub2] [DOI] [PubMed] [Google Scholar]
Navaneethan 2009a
- Navaneethan SD, Nigwekar SU, Perkovic V, Johnson DW, Craig JC, Strippoli GF. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD004289.pub4] [DOI] [PubMed] [Google Scholar]


