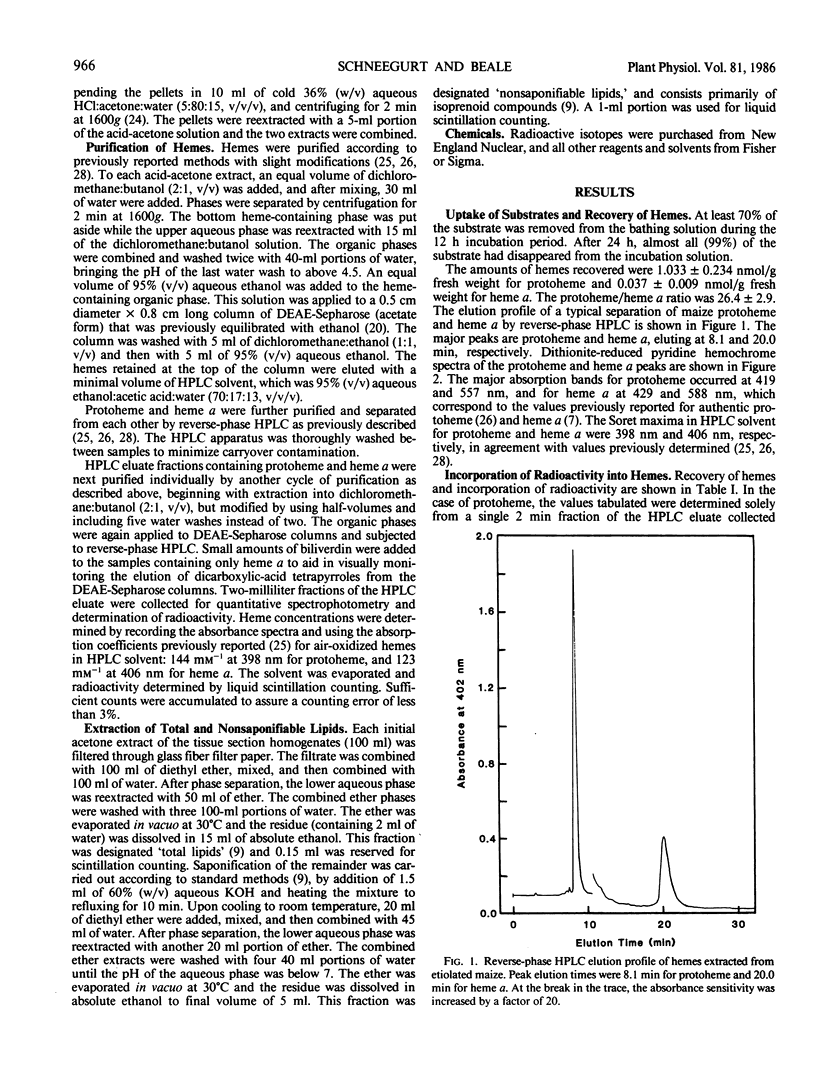
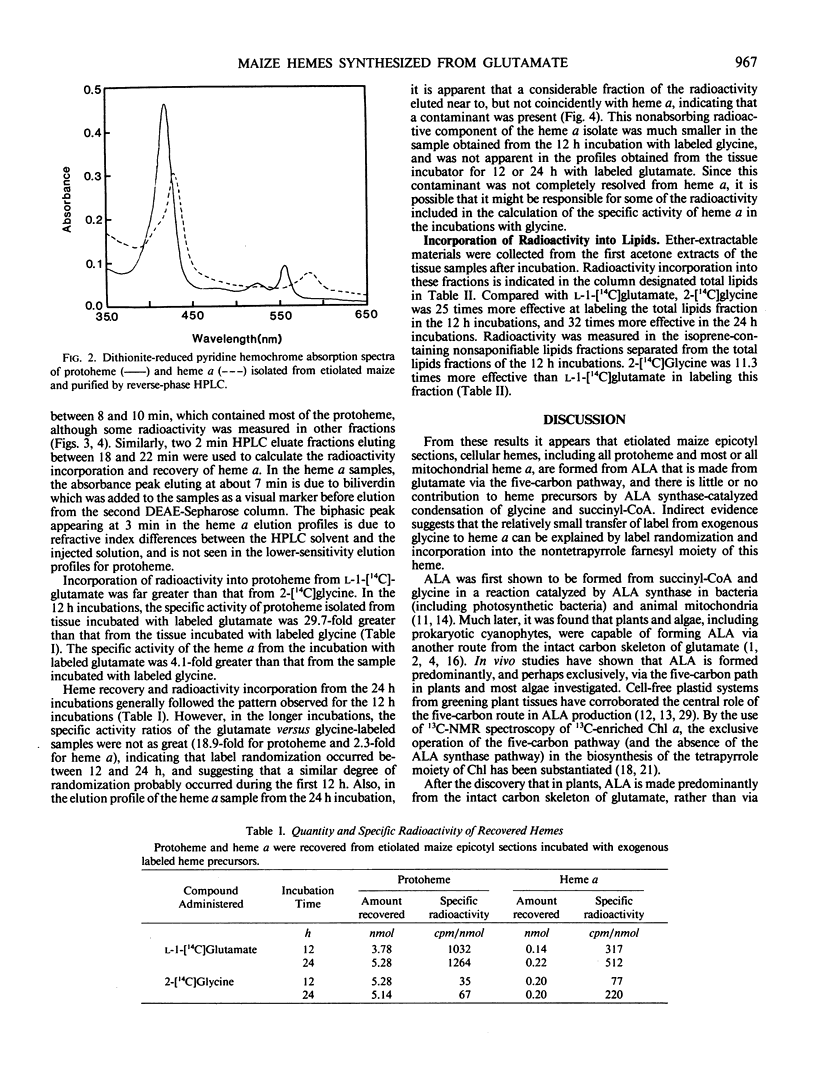
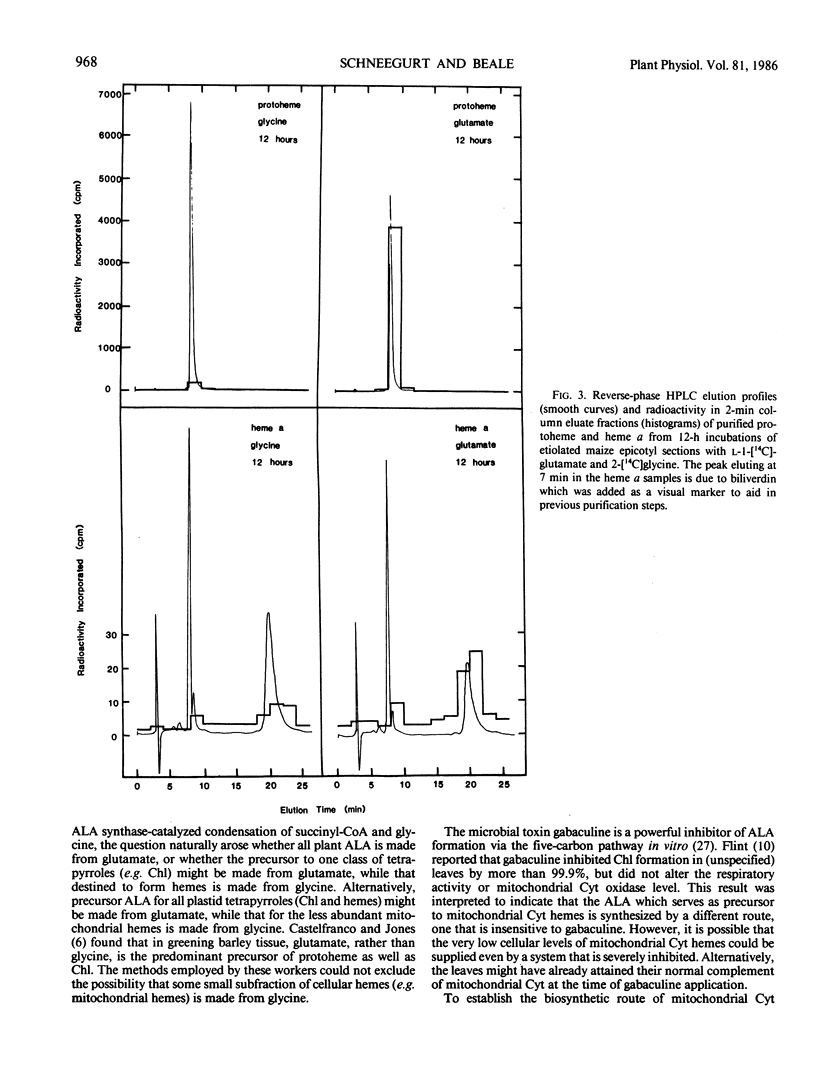
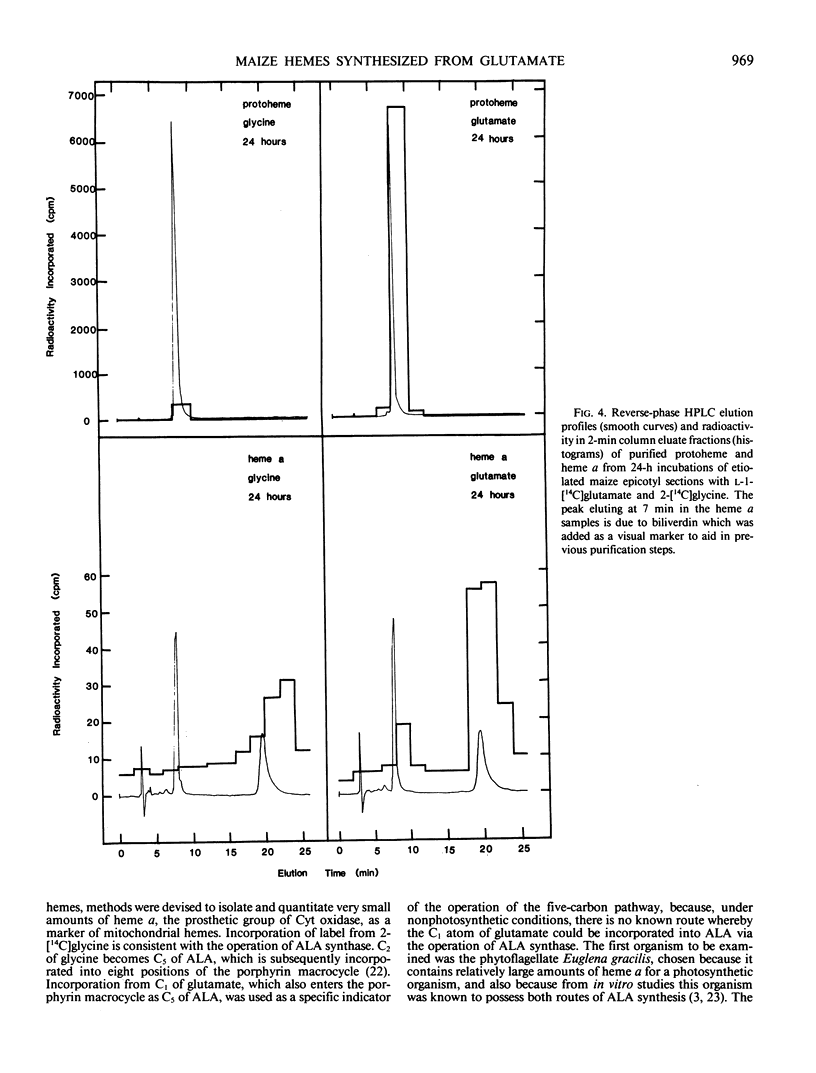
Abstract
The heme and chlorophyll precursor δ-aminolevulinic acid (ALA) can be formed by two biosynthetic routes: from the intact carbon skeleton of glutamate via a five-carbon pathway, which occurs in chloroplasts and bluegreen algae, and by ALA synthase-catalyzed condensation of succinyl-CoA and glycine, which occurs in bacteria and animal mitochondria. The biosynthetic route of plant mitochondrial heme a was determined by incubating terminal epicotyl sections of 8-day-old etiolated Zea mays seedlings in the dark with l-1-[14C]glutamate (which can be incorporated into ALA only via the five-carbon route) or 2-[14C]glycine (which would be incorporated via ALA synthase). Label incorporation was measured in highly purified protoheme and heme a. In 12-hour incubations, label uptake was greater than 70%. Total cellular protoheme was labeled 29.7 times more effectively by glutamate than glycine. Heme a was labeled 4.1 times more effectively by glutamate than by glycine. To assess the relative ability of the two amino acids to contribute label to the farnesyl moiety of heme a, label incorporation into total cellular nonsaponifiable lipids was measured. Glycine labeled this fraction 11.3 times more effectively than glutamate. Thus, a contribution by glycine to the farnesyl moiety may account for the small amount of label appearing in heme a. Our results indicate that in etiolated maize, noncovalently bound hemes, including mitochondrial heme a, are made mostly, and possibly entirely, from ALA synthesized via the five-carbon pathway. There is little or no contribution from ALA formed via ALA synthase, and no evidence was found for the operation of this enzyme in maize.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J. Biosynthesis of 5-aminolevulinate from glutamate in Anabaena variabilis. Biochim Biophys Acta. 1980;613(1):220–228. doi: 10.1016/0005-2744(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: II. Formation of C-delta-Aminolevulinic Acid from Labeled Precursors in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):297–303. doi: 10.1104/pp.53.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Foley T., Dzelzkalns V. delta-Aminolevulinic acid synthase from Euglena gracilis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1666–1669. doi: 10.1073/pnas.78.3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Gough S. P., Granick S. Biosynthesis of delta-aminolevulinic acid from the intact carbon skeleton of glutamic acid in greening barley. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2719–2723. doi: 10.1073/pnas.72.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEZY P. S., BARRETT J. The prosthetic group of cytochrome oxidase. 2. Chemistry of porphyrin alpha. Biochem J. 1961 Apr;78:798–806. doi: 10.1042/bj0780798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P. A., Jones O. T. Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol. 1975 Mar;55(3):485–490. doi: 10.1104/pp.55.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., LAVER W. G., NEUBERGER A. Initial stages in the biosynthesis of porphyrins. 2. The formation of delta-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes. Biochem J. 1958 Sep;70(1):71–81. doi: 10.1042/bj0700071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel E., Ne'eman E. Alternative Routes for the Synthesis of 5-Aminolevulinic Acid in Maize Leaves : II. Formation from Glutamate. Plant Physiol. 1983 Aug;72(4):1062–1067. doi: 10.1104/pp.72.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIKUCHI G., KUMAR A., TALMAGE P., SHEMIN D. The enzymatic synthesis of delta-aminolevulinic acid. J Biol Chem. 1958 Nov;233(5):1214–1219. [PubMed] [Google Scholar]
- Oh-Hama T., Seto H., Miyachi S. 13C nuclear magnetic resonance studies on bacteriochlorophyll a biosynthesis in Rhodopseudomonas spheroides S. Arch Biochem Biophys. 1985 Feb 15;237(1):72–79. doi: 10.1016/0003-9861(85)90255-3. [DOI] [PubMed] [Google Scholar]
- Oh-hama T., Seto H., Otake N., Miyachi S. 13c-NMR evidence for the pathway of chlorophyll biosynthesis in green algae. Biochem Biophys Res Commun. 1982 Mar 30;105(2):647–652. doi: 10.1016/0006-291x(82)91483-8. [DOI] [PubMed] [Google Scholar]
- Porra R. J., Klein O., Wright P. E. The proof by 13C-NMR spectroscopy of the predominance of the C5 pathway over the Shemin pathway in chlorophyll biosynthesis in higher plants and of the formation of the methyl ester group of chlorophyll from glycine. Eur J Biochem. 1983 Feb 15;130(3):509–516. doi: 10.1111/j.1432-1033.1983.tb07179.x. [DOI] [PubMed] [Google Scholar]
- RADIN N. S., RITTENBERG D., SHEMIN D. The rôle of glycine in the biosynthesis of heme. J Biol Chem. 1950 Jun;184(2):745–753. [PubMed] [Google Scholar]
- Stillman L. C., Gassman M. L. Protoheme extraction from plant tissue. Anal Biochem. 1978 Nov;91(1):166–172. doi: 10.1016/0003-2697(78)90827-8. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Biosynthesis of Protoheme and Heme a Precursors Solely from Glutamate in the Unicellular Red Alga Cyanidium caldarium. Plant Physiol. 1984 Jan;74(1):146–151. doi: 10.1104/pp.74.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys. 1985 Mar;237(2):454–464. doi: 10.1016/0003-9861(85)90299-1. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J Biol Chem. 1983 Jun 10;258(11):6799–6807. [PubMed] [Google Scholar]
- Weinstein J. D., Branchaud R., Beale S. I., Bement W. J., Sinclair P. R. Biosynthesis of the farnesyl moiety of heme a from exogenous mevalonic acid by cultured chick liver cells. Arch Biochem Biophys. 1986 Feb 15;245(1):44–50. doi: 10.1016/0003-9861(86)90188-8. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Castelfranco P. A. Mg-protoporphyrin-IX and delta-aminolevulinic acid synthesis from glutamate in isolated greening chloroplasts. delta-Aminolevulinic acid sysnthesis. Arch Biochem Biophys. 1978 Mar;186(2):376–382. doi: 10.1016/0003-9861(78)90448-4. [DOI] [PubMed] [Google Scholar]


