Abstract
The properties of potassium transport in carrot (Daucus carota L.) suspension culture cells and their isolated protoplasts were examined. Cells cultured in Murashige and Skoog (MS) medium (Plant Physiol 15: 473-497) were potassium saturated and, consequently, they exhibited little net potassium accumulation. Cells that transport and accumulate potassium were derived from the MS-grown cells by culturing them in a potassium-free modified medium. The transport properties of the modified medium cells included: (a) smooth nonsaturating kinetics with 80% of the maximum rates occurring at 0.1 millimolar KCl, (b) linear transport for at least 75 min, (c) alkaline pH optimum, (d) little accompanying anion uptake with increased malate concentrations balancing net increases in positive charge, and (3) little effect on transport by plasmolysis. Potassium transport activity appeared to be 50% lower in protoplasts isolated from the modified medium cells. Nevertheless, the protoplasts exhibited essentially the same kinetics, time course, pH response, and malate adjustment as the intact cells. We concluded from these results that the low potassium cells and their isolated protoplasts are ideally suited to investigating potassium transport at the cell level without the complications associated with multilayered and highly differentiated tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Glass A. D. Regulation of potassium absorption in barley roots: an allosteric model. Plant Physiol. 1976 Jul;58(1):33–37. doi: 10.1104/pp.58.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. The regulation of potassium absorption in barley roots. Plant Physiol. 1975 Sep;56(3):377–380. doi: 10.1104/pp.56.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwald J. W., Leonard R. T. Isolation and transport properties of protoplasts from cortical cells of corn roots. Plant Physiol. 1982 Nov;70(5):1391–1395. doi: 10.1104/pp.70.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy M., Reinhold L. Membrane transport of sugars and amino acids in isolated protoplasts. Plant Physiol. 1978 Apr;61(4):593–596. doi: 10.1104/pp.61.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland D. R., Broyer T. C. GENERAL NATURE OF THE PROCESS OF SALT ACCUMULATION BY ROOTS WITH DESCRIPTION OF EXPERIMENTAL METHODS. Plant Physiol. 1936 Jul;11(3):471–507. doi: 10.1104/pp.11.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Ordin L. Organic Acid Metabolism and Ion Absorption in Roots. Plant Physiol. 1954 Jan;29(1):70–75. doi: 10.1104/pp.29.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Lucas W. J. Potassium Transport in Corn Roots : II. The Significance of the Root Periphery. Plant Physiol. 1983 Oct;73(2):208–215. doi: 10.1104/pp.73.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Lucas W. J. Potassium transport in corn roots : I. Resolution of kinetics into a saturable and linear component. Plant Physiol. 1982 Dec;70(6):1723–1731. doi: 10.1104/pp.70.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin W. Corn Root Protoplasts: ISOLATION AND GENERAL CHARACTERIZATION OF ION TRANSPORT . Plant Physiol. 1980 Oct;66(4):550–554. doi: 10.1104/pp.66.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
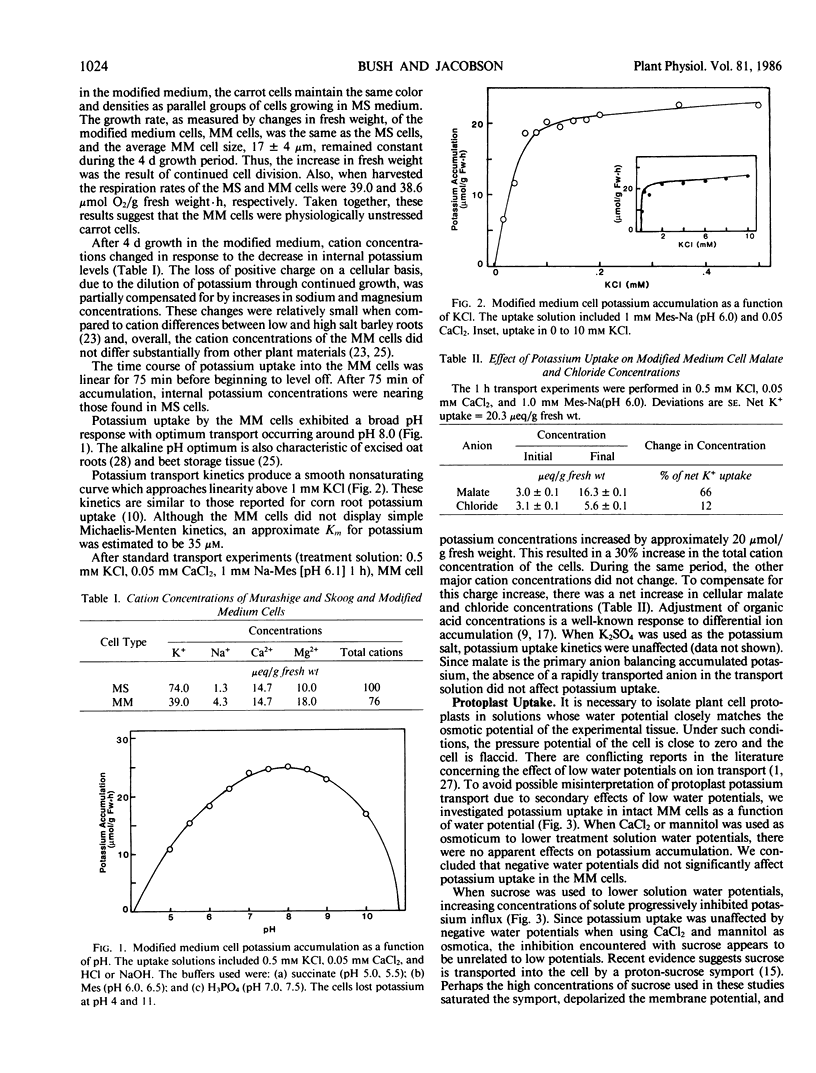
- Lin W. Energetics of sucrose transport into protoplasts from developing soybean cotyledons. Plant Physiol. 1985 May;78(1):41–45. doi: 10.1104/pp.78.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Inhibition of anion transport in corn root protoplasts. Plant Physiol. 1981 Aug;68(2):435–438. doi: 10.1104/pp.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Ion Transport in Isolated Protoplasts from Tobacco Suspension Cells: II. Selectivity and Kinetics. Plant Physiol. 1979 Jan;63(1):191–194. doi: 10.1104/pp.63.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Ion transport in isolated protoplasts from tobacco suspension cells: I. General characteristics. Plant Physiol. 1979 Jan;63(1):183–190. doi: 10.1104/pp.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley L. D., Hopkins J. W. Rubidium (potassium) uptake by Arabidopsis: a comparison of uptake by cells in suspension culture and by roots of intact seedlings. Plant Physiol. 1979 Sep;64(3):374–378. doi: 10.1104/pp.64.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Z. R. Turbidimetric measurement of plant cell culture growth. Plant Physiol. 1976 Mar;57(3):460–462. doi: 10.1104/pp.57.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
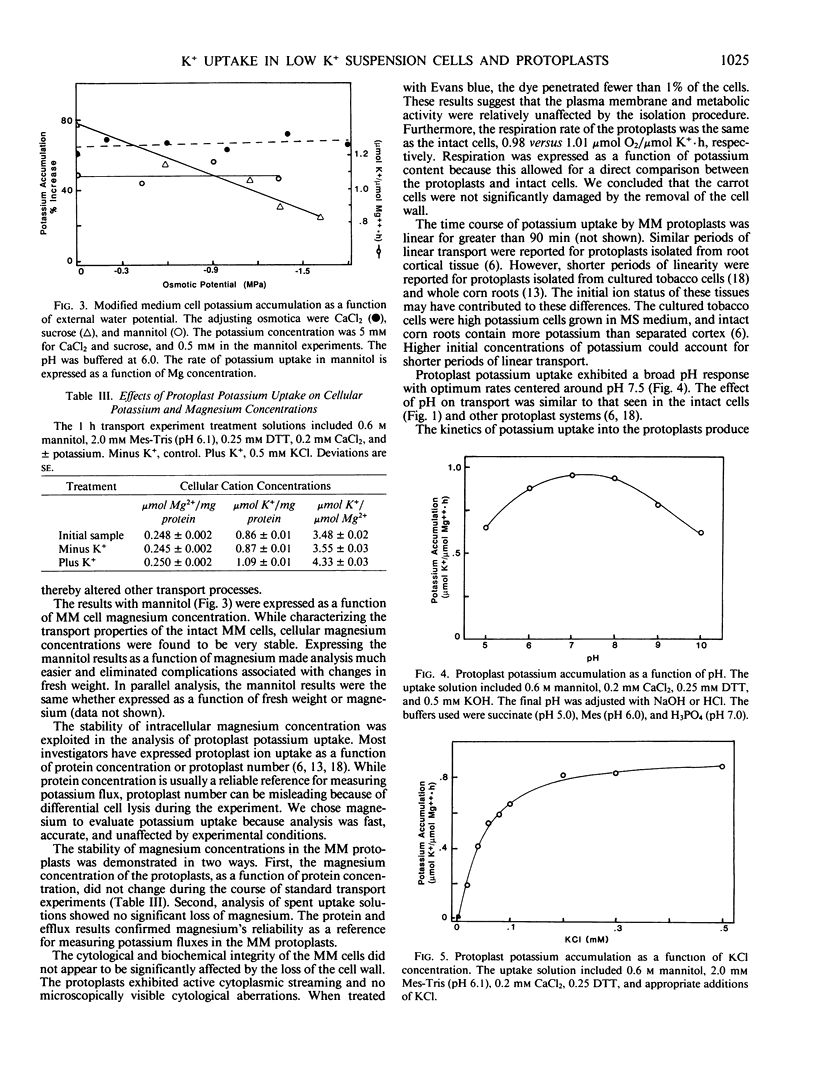
- Sze H., Hodges T. K. Selectivity of alkali cation influx across the plasma membrane of oat roots: cation specificity of the plasma membrane ATPase. Plant Physiol. 1977 Apr;59(4):641–646. doi: 10.1104/pp.59.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimiya H., Murashige T. Evaluation of parameters in the isolation of viable protoplasts from cultured tobacco cells. Plant Physiol. 1974 Dec;54(6):936–944. doi: 10.1104/pp.54.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]


