Summary
Background
Accessible prebiotic foods hold strong potential to jointly target gut health and metabolic health in high-risk patients. The BE GONE trial targeted the gut microbiota of obese surveillance patients with a history of colorectal neoplasia through a straightforward bean intervention.
Methods
This low-risk, non-invasive dietary intervention trial was conducted at MD Anderson Cancer Center (Houston, TX, USA). Following a 4-week equilibration, patients were randomized to continue their usual diet without beans (control) or to add a daily cup of study beans to their usual diet (intervention) with immediate crossover at 8-weeks. Stool and fasting blood were collected every 4 weeks to assess the primary outcome of intra and inter-individual changes in the gut microbiome and in circulating markers and metabolites within 8 weeks. This study was registered on ClinicalTrials.gov as NCT02843425, recruitment is complete and long-term follow-up continues.
Findings
Of the 55 patients randomized by intervention sequence, 87% completed the 16-week trial, demonstrating an increase on-intervention in diversity [n = 48; linear mixed effect and 95% CI for inverse Simpson index: 0.16 (0.02, 0.30); p = 0.02] and shifts in multiple bacteria indicative of prebiotic efficacy, including increased Faecalibacterium, Eubacterium and Bifidobacterium (all p < 0.05). The circulating metabolome showed parallel shifts in nutrient and microbiome-derived metabolites, including increased pipecolic acid and decreased indole (all p < 0.002) that regressed upon returning to the usual diet. No significant changes were observed in circulating lipoproteins within 8 weeks; however, proteomic biomarkers of intestinal and systemic inflammatory response, fibroblast-growth factor-19 increased, and interleukin-10 receptor-α decreased (p = 0.01).
Interpretation
These findings underscore the prebiotic and potential therapeutic role of beans to enhance the gut microbiome and to regulate host markers associated with metabolic obesity and colorectal cancer, while further emphasizing the need for consistent and sustainable dietary adjustments in high-risk patients.
Funding
This study was funded by the American Cancer Society.
Keywords: Prebiotic, Dry beans, Gut microbiota, Metabolites, Proteomic biomarkers, Colorectal cancer, Obesity, Inflammation
Research in context.
Evidence before this study
-
•
The gut microbiome is increasingly implicated in patient outcomes from the management of obesity-related cardiometabolic conditions to cancer.
-
•
Prebiotic foods offer a safe and accessible strategy to shape the composition and activity of the gastrointestinal microbiota and to confer benefits to the host's health via inherent dietary nutrients selectively fermented by the large intestine.
Added value of this study
-
•
We conducted the Beans to Enrich the Gut microbiome vs. Obesity's Negative Effects (BE GONE) trial in obese surveillance patients with a history of colorectal cancer and/or polyps—a group that stands to benefit from an intervention that targets both gut health and metabolic health but may have unique challenges when it comes to making and tolerating changes to diet.
-
•
This highly accessible and adaptable prebiotic food intervention required patients to consume up to 1 cup per day of pressure-cooked, canned navy beans within the context of their usual diet (control); and was sufficient to enhance the diversity and composition the gut microbiome within 8 weeks with parallel shifts in host metabolites, immune and inflammatory biomarkers.
-
•
Notably, the effect of returning to the usual diet without beans was quite striking with reversals in favorable changes in bacteria and host metabolites within 4 weeks.
Implications of all the available evidence
-
•
Broad diet–microbiome interactions in clinical cohorts enforce a need for improved understanding of the effects of specific diet changes and predictable strategies to target and modulate the microbiome to ultimately improve outcomes and prolong survival.
-
•
Insights from BE GONE, reflecting practical real-world dietary changes, offer a robust foundation for the development of microbiome-targeted dietary strategies, leveraging measurable host biomarkers toward enhanced patient outcomes.
Introduction
The gut microbiome's role in dietary fiber and protein fermentation is increasingly implicated in patient outcomes from the management of obesity-related cardiometabolic conditions to cancer development, treatment, and survival.1, 2, 3 Prebiotic whole, plant foods offer a safe, scalable strategy to shape the composition and activity of the gastrointestinal microbiota and to confer benefits to the host's health via multiple inherent dietary nutrients selectively fermented by the large intestine.4 Dry beans (Phaseolus vulgaris) are rich in non-digestible oligosaccharides that reach the colon intact and have been extensively studied to selectively stimulate the growth of beneficial gut bacteria, while constraining pathogens, to modulate inflammation and immunity.5 The prebiotic properties of beans are further supported by the balance of microbiota-accessible carbohydrates, including resistant starch, insoluble and soluble fibers, as well as the high content of essential amino acids, polyphenols and other bioactive compounds. While inexpensive and accessible, beans are rarely consumed within the typical U.S. diet and frequently avoided due to mild or acute gastrointestinal side effects that can be mitigated through appropriate cooking or canning methods.6 The small, white navy bean is distinctively rich in lysine, iron, phosphatidylserine, apigenin, saponins, ferulic acid and p-coumaric acid compared to other legumes and prebiotic food sources, providing several unique properties of relevance to the microbiome, cardiometabolic risk and cancer.6,7 Preclinical studies demonstrate that navy bean supplementation within a high-fat diet is sufficient to improve intestinal health and mitigate the severity of the obese, inflammatory phenotype,6 a finding further supported by clinical and follow-up studies linking bean consumption to improvements in cardiovascular health8 and reduced risk of advanced colorectal adenoma recurrence.9 Previous clinical studies10 have not addressed whether simply increasing or adding beans to the usual diet is sufficient and effective in high-risk patients with known or expected challenges in cooperatively managing both gut and metabolic health.
We and others have observed perturbations or disturbances in the normal microbial balance of patients with obesity, poor diet and/or colorectal neoplasia1; and a joint effect of obesity and inflammation in colorectal cancer survival.11 Long after the cancer is treated or precancerous polyp is removed, entrenched dietary patterns continue to present significant hinderances to gut homeostasis and secondary prevention.12 Thus, microbiome modulation via diet changes presents a challenging but promising target for the management of high-risk patients. In a clinically characterized cohort of obese patients with a history of colorectal neoplasia, we conducted the Beans to Enrich the Gut microbiome vs. Obesity's Negative Effects (BE GONE) trial to test the effect of an 8-week increase in navy bean consumption within the usual diet on gut microbiota profiles from stool samples; and circulating marker and metabolite profiles from fasting blood samples.
Methods
Study design and ethical approval
BE GONE was a single-center, low-risk and minimally invasive dietary intervention study approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center and registered under the ClinicalTrials.gov identifier NCT02843425. Following initiation of a pilot protocol in July 2016, recruitment was expanded to patients with a history of colorectal cancer in May 2017. Shortly thereafter tropical storm Harvey hit the study site and recruitment remained open until January 2020.
Our design included consideration of (1) the baseline gut microbiota landscape that may be important when developing a prebiotic intervention delivering bean substrates for bacterial conversion to beneficial metabolites, as well as (2) the usual diet, a shaper of baseline status and substrates that influence the host and bacterial metabolome.13 Briefly, to establish the basal diet and microbiome and to track compliance with study procedures, patients entered a 4-week run-in and equilibration period prior to randomization. Following completion of this period where participants followed their usual diet without dry beans (control), patients were block randomized according to commonly prescribed medications for obesity-related conditions to one of two sequences–either continuing their usual diet without dry beans (control) or beginning the intervention of adding study beans to their usual diet. After the first 8-week cycle, patients immediately crossed over to the other sequence. No wash-out period was included because each participant, regardless of which sequence they were randomized to, began the intervention following a control period. This allowed for cross-arm comparisons during the on-intervention period; and facilitated the full assessment of temporal effects for future studies, i.e., how stable was the microbiome of these patients during the usual (control) diet period, how quickly would it change when beans were added and how long would the effect remain when they reverted to back to their usual (control) diet without beans.
The on-intervention period consisted of a short ramp-up to 1 cup/d of study beans (16 g dietary fiber; 14 g protein; 220 kcal). Pressure-cooked and canned organic navy beans stored in water with sea salt were purchased in two batches for the study and provided to participants every 4 weeks. Throughout the study, patients were free-living and able to choose and prepare their own meals with close follow-up and counseling support from the study dietitian. Participants provided stool and fasting blood samples to assess the primary outcome focused on shifts in the gut microbiome and parallel systemic changes in host metabolites and markers via longitudinal intra and inter-individual effects assessed every 4 weeks over the course of the 8-week intervention.
Full details of the design can be found in the published study protocol13 and supplemental materials.
Participants
Clinic-based recruitment was targeted to the major specialty centers at MD Anderson Cancer Center, including gastrointestinal medical oncology and gastroenterology/colonoscopy surveillance. Patients were also reached through the MD Anderson Cancer Registry14 and an adjacent primary referral clinic. Major inclusion criteria included men and women, 30 years or older, that met criteria for obesity via BMI or waist size; and who had either a (1) history of pathology-confirmed precancerous polyps of the colon or rectum or (2) were defined as a colorectal cancer survivor that had completed treatment with adequate maintenance of bowel length (eligible: hemicolectomy or low anterior resection; ineligible: total or near total colectomy, greater than 10 cm of small bowel resection) and reached normalized bowel habits. Major exclusion criteria included hereditary colorectal cancer syndromes, antibiotic use in the past month, current smoker or heavy drinker, exclusionary prescription medications (i.e., cytokines, immunosuppressive agents, chemopreventive drugs, bile acid sequestrants), major dietary restrictions relevant to the intervention and/or regularly taking anti-flatulence medications or relevant supplements (probiotics, prebiotics/fiber) that could not be safely discontinued.
Study procedures
Study procedures are described in detail within the published study protocol.13 Briefly, the randomization list was generated by an independent analyst and allocated by a research team member not directly interacting with participants. Block allocation considered no use vs. regular use of statins and/or metformin, as they were commonly prescribed in our target population. Although it was not possible to blind the intervention sequence, the dietitian and study staff administering the intervention were not involved in the assignment. Participants were asked to follow the control diet during the equilibration period and during one of two crossover sequences. During the intervention, participants were instructed to add study beans (only) to their usual diet. Randomized participants were expected to attend a total of 5 in-person visits every 4 weeks.
To establish baseline status and to monitor deviations in untargeted behaviors throughout the study period we measured anthropometrics and blood pressure, monitored medications and changes in health status, and carefully reviewed data collected via web-based assessments of dietary habits15,16 and physical activity17,18 at each in-clinic visit. When consuming the beans, participants were provided with special containers to measure servings and store the remainder of the can for the next day. They were instructed to keep week-by-week daily logs that included records of the date, time, amount and manner in which the beans were consumed (including other foods or ingredients added), as well as any side effects experienced. Between the in-clinic visits, the dietitian interacted with participants approximately twice per week by telephone and email. During these sessions they reviewed progress and completed dietary assessment (24-h recall15) interviews. Any side effects or events reported were escalated to the study physicians (SK and PCO) for immediate or standard review, as appropriate. Participants were similarly monitored with regard to both side effects and dietary intake during the usual (control) diet period when they were not consuming beans.
An in–home, fresh-frozen stool sample collection kit, similar to that used in the Human Microbiome Project and refined in our previous studies within this patient population19 was provided to participants with detailed instructions to collect a stool sample just before each in-clinic visit. Upon receipt, samples were transferred to −80 °C storage. During the morning in-clinic visits, fasting blood was drawn by trained phlebotomists at the MD Anderson Clinical and Translational Research Center and immediately processed.
Outcomes
Stool and fasting blood samples were collected every 4 weeks to assess the primary outcome of on-intervention intra and inter-individual changes in the gut microbiome and, in parallel, changes in circulating markers and metabolites within 8 weeks to further characterize function and host response (Fig. S1). Participants were considered adherent if they consumed ≥80% of the beans over the intervention period and followed the prescribed regimen on at least 5 days of the week.
Laboratory measurements
Details regarding the laboratory, computational and data processing methods for microbiome, metabolome and proteome biomarker profiling are provided in the supplemental materials. Briefly, bacterial genomic DNA was extracted and amplified with Illumina barcoded primers and analyzed on the Illumina MiSeq and NovaSeq 6000 platforms.20, 21, 22 Whole metagenomic shotgun sequencing was applied to a subset, allowing taxonomical and functional classification of sequencing reads.23,24 Untargeted metabolomics profiling was conducted using a Waters Acquity™ UPLC system with 2D column regeneration configuration (I-class and H-class) coupled to a Xevo G2-XS quadrupole time-of-flight mass spectrometer as previously described.25, 26, 27, 28 The serum lipid panel was processed within the same day as fasting blood collection in a standard manner by a CLIA-certified laboratory. Plasma specimens were also analyzed via the Olink® Target 96 Inflammation panel, a proximity extension immunoassay technology that uses a dual recognition DNA-coupled immunoassay for protein identification and relative quantification.29
Sample size and statistical analysis
The sample size calculation for the trial was reported in our published protocol.13 While we acknowledge that the proposed sample size was 60 in the original design, challenges associated with patient follow-up limited the intent-to-treat sample size to 48. However, the observed effect size for microbial alpha diversity is higher than what was assumed for the sample size calculation (20% increase in the original design vs. 32% increase in the actual data). This improvement in the effect size kept the power of the test above 80%.
The complete statistical analysis plan can be found in the supplemental materials with relevant annotation in the table footnotes and figure legends. To quantify microbiome changes during the study, we conducted a longitudinal analysis of alpha diversity and the relative abundance of individual taxa on the natural log scale (slope = 0 under the null hypothesis) across the trial sequence using generalized linear mixed models with random intercept. Similar methods were applied to circulating metabolites and markers. For all analyses in which the multiplicity of tests was an issue, we used the Benjamini-Hochberg FDR to report appropriately adjusted significance levels. We considered an FDR-adjusted p-value or q-value <0.20, two-sided, as statistically significant. We further explored correlation coefficients of intervention-responsive biomarkers estimated at baseline (usual diet) and 8-weeks post intervention using Fisher's Z-transformation of a Pearson correlation coefficient. Pairs with corresponding differences in correlation values of greater than 0.25 or less than −0.25 were pre-selected, estimating the p-value for the difference (under the null hypothesis of zero difference between the two time-points).
Role of the funding source
The funder of this peer-reviewed study had no role in the design, data collection, analysis, interpretation, the writing of the report, or the decision to publish the results. All authors had access to the data and shared the final responsibility to submit the study for publication.
Results
At total of 69 enrolled individuals began the 4-week equilibration/run-in. Following this, 55 individuals were randomized to one of two intervention sequences and 48 patients (87%) completed the full 16-week trial (Fig. 1). Of these, 75% and 25% had a history of colorectal cancer and precancerous polyps, respectively; and 44% used statins and/or metformin. No differences in baseline diet, microbiome or demographic factors were observed by randomization order or between the intent-to-treat group and those who withdrew pre- or post-randomization (Tables S1 and S2; Figs. S2 and S3). Of the 7 patients who withdrew post-randomization, all used statins and/or metformin, 6 began the study in the control arm and 5 were polyp patients (Table S2). Mild to moderate bloating/flatulence and/or change in bowel habits (constipation/loose stool) attributed to the intervention was reported in 33% and 10% of the patients who completed the study and in 0% who dropped out (Table S3). While adding study beans to the usual diet did appear to increase reports of gas in this population, similar profiles of unattributable bowel habit changes were also reported (12%) when patients were consuming their usual diet. Two participants who completed the trial did not provide sufficient information to accurately estimate adherence. Of those who did, 95% met the target for total amount of study beans consumed across the on-intervention period (28 cups) and 74% consumed the prescribed bean dose on all or most days within a week for each of the 8 weeks. Pipecolic acid, an established marker of dry bean consumption,7 was also considered as an objective measure of compliance (Fig. 2a).
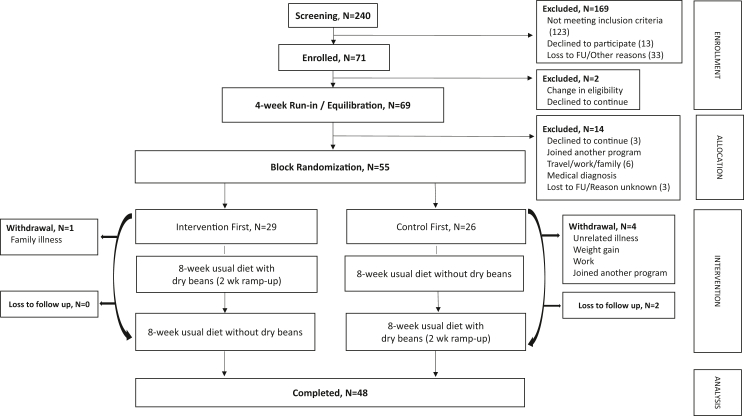
Fig. 1.
CONSORT. Of the 240 individuals initially contacted or approached for study recruitment, 71 provided informed consent. Of the 240, 123 did not meet inclusion criteria, 13 declined to participate, 25 were lost to follow-up and 8 withdrew for other stated reasons during the screening process. Immediately post-consent and before completing any study procedures, 1 individual experienced a change in eligibility and 1 decided not to continue; thus, 69 eligible patients began the run-in/equilibration period. Of these, 11 withdrew for various stated reasons and 3 individuals were lost to follow-up resulting in 80% (n = 55) of those initially eligible being randomized. Five participants withdrew post-randomization for various stated reasons, including 1 who withdrew in order to join another program. Two did not attend their final visit due to unrelated illness and were lost to follow-up, resulting in 48 patients (87%) who completed the study for intent-to-treat analysis.
Fig. 2.
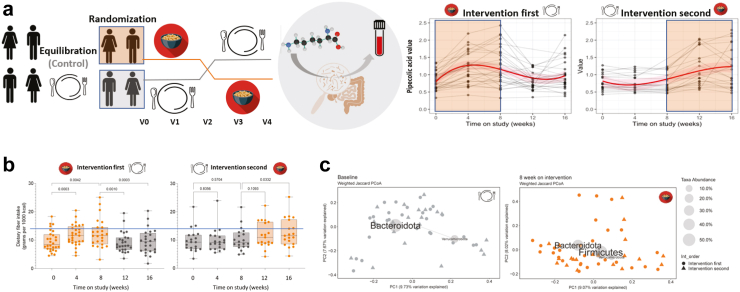
The BE GONE Trial tested the effect of cooked dry bean addition and depletion within the participant's usual diet. a. Study design, Left to Right: To establish the basal diet and microbiome and introduce study procedures prior to randomization, eligible and consented individuals began the equilibration/run-in. Participants who completed the run-in were randomized to begin the dry bean intervention or to continue the usual diet control for 8 weeks. The intervention diet consisted of adding ½ cup (1 serving) of cooked, canned navy beans to their usual diet over a 2-week ramp-up period followed by 1 cup (2 servings) per day for an additional 6 weeks. At week 8, participants who completed the intervention diet immediately crossed over to the control (usual diet without beans) and vice versa for a total of 5 in-person visits every 4 weeks with stool and fasting blood collection. The primary outcome focused on changes in stool 16S rRNA gene profiles and blood metabolites/markers. Trends in pipecolic acid, a specific marker of dry bean intake and microbiome-derived metabolite of lysine, paralleled the addition and the depletion of cooked navy beans within the usual diet, providing an objective measure of compliance. b. Total dietary fiber intake within the usual diet from multiple 24-h recalls collected across the intervention sequence showed that majority of participants remained below the adequate intake threshold for U.S. adults of 14 g per 1000 kcal [means, medians, interquartile range and outliers presented with p-values comparing intervention (orange) and control (gray) periods]. Within person change in total dietary fiber intake for the 8-week on-intervention period for both groups combined, linear mixed effect (LME) estimate and 95% CI: 0.22 (0.10, 0.35) interpreted as slope above 0 by generalized linear mixed models (proc glimmix, SAS 9.4) with random intercept in natural log scale. c. Beta diversity by weighted Jaccard similarity/distance across randomization groups visualized as PCoA biplots with the most abundant phylum-level taxa at baseline and 8 weeks post-intervention in the full trial cohort (n = 48).
Effect of the intervention on usual diet, circulating lipoproteins and other biometrics
Beyond the addition of study beans, participants were asked to not change their usual diet, physical activity and lifestyle habits during the study. Total dietary fiber intake increased on the intervention; however, most participants remained below the adequate intake threshold for U.S. adults of 14 g per 1000 kcal30 (Fig. 2b; Table S4). Compensatory shifts were observed in the proportion of total energy intake from carbohydrate vs. fat, while protein remained stable and we observed a modest increase in overall diet quality scores31 reflecting adherence to U.S. dietary recommendations that encourage legumes and plant proteins (Table S4). Total energy intake, BMI, blood pressure, waist circumference and other biometrics monitored for consistency throughout the study also remained stable (Fig S4; Table S4). Some prior dry bean and soluble fiber trials in the setting of obesity, diabetes and cardiovascular disease prevention reported reductions in circulating LDL cholesterol32; and we were curious if we could achieve the same in this population of patients with a history of bowel lesions through the addition of beans alone. However, without other lifestyle changes, circulating lipoprotein profiles remained largely unchanged within 8 weeks of consuming 1 daily cup of beans (p > 0.05; Table S5) nor did chronic disease medication use or basal diet appear to modulate the effect of the intervention on LDL (p for interaction >0.05; Table S6).
Effect of the intervention on the gut microbiome: diversity, composition and function
To evaluate the effect of the bean intervention on the gut microbiome, we assessed microbial alpha diversity, the relative abundance of individual taxa, and beta diversity distances from 16S rRNA gene profiles within each subject at each time point. As the equilibration and control was the usual diet, the primary outcome focused on changes during the on-intervention period in the full trial cohort [n = 48; intervention first V0–V2 (n = 28) combined with intervention second V2–V4 (n = 20); Fig. 2a]. We further characterized these findings across the crossover study sequence in each of the two randomization groups separately to assess the stability of these measures during the “return to control” [V2–V4 (n = 28)] and “remain on control” [V0–V2 (n = 20)] period; and visualized the group-level similarity of the microbial community at baseline and post-intervention (Fig. 2c; Fig. S3).
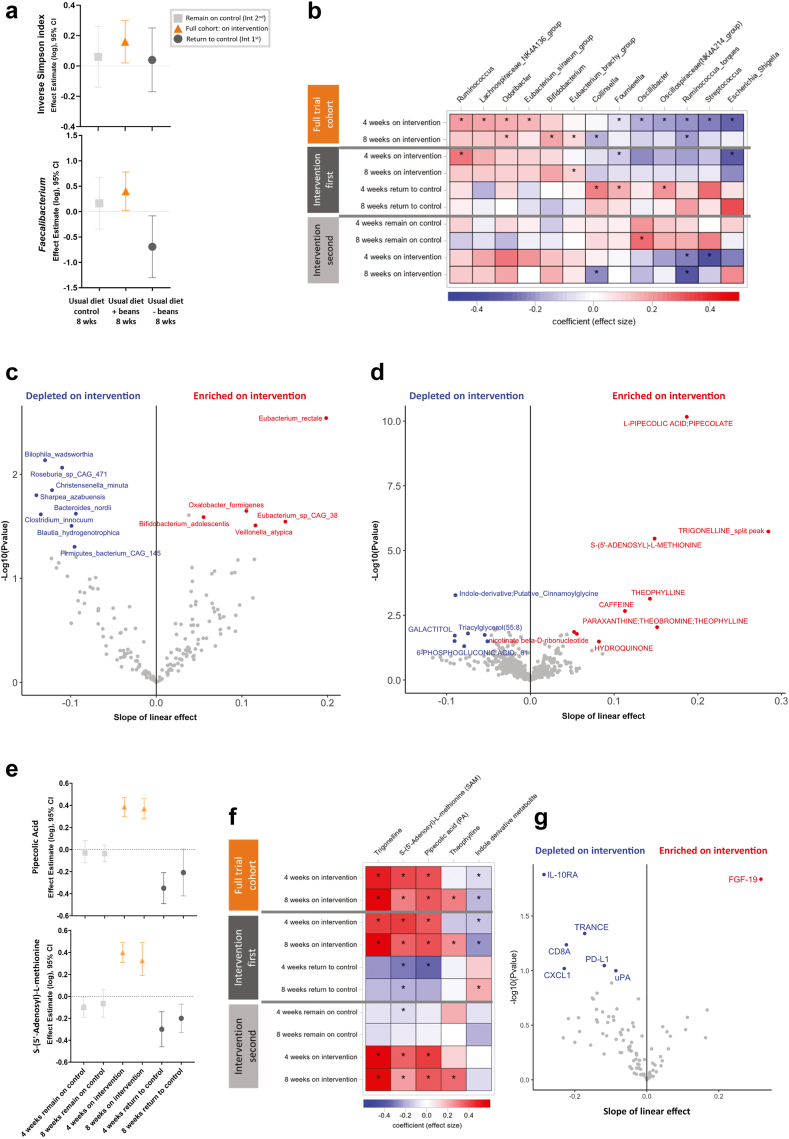
The inverse Simpson index, a measure of microbial alpha diversity that accounts for the number of distinct bacteria types within the sample, as well as the evenness of their relative abundance toward a more balanced distribution, increased on-intervention within 8 weeks [n = 48, linear mixed effect (LME) and 95% CI: 0.16 (0.02, 0.30), two-sided p = 0.02; Fig. 3a; Table S7], a finding consistent with a 4-week controlled feeding study providing a navy bean powder-enriched meal and snack daily to 10 colorectal cancer survivors.10 No change was observed during the control periods (Table S7); and results were consistent within major subgroups of patients. However, we noted that the small group who reported a change in bowel habits (constipation/loose stools) experienced a pronounced increase in diversity during the 8-week intervention (Table S6). To further assess how the prebiotic properties of beans influenced the relative abundance of common or highly prevalent bacteria within our population, we conducted analyses among genus-level bacteria carried by at least 80% of participants at randomization. In this analysis restricted to carriers only, we found that on-intervention the intra-patient relative abundance of Roseburia and Streptococcus [LME and 95% CI: −0.49 (−0.86, −0.13), two-sided p = 0.01, q = 0.16 and −0.48 (−0.92, −0.04), two-sided p = 0.03, q = 0.30), respectively] decreased at the 4-week mid-point, whereas the relative abundance of Faecalibacterium [0.40 (0.02, 0.78), two-sided p = 0.04, q = 0.65)] increased within 8 weeks (Fig. 3a; Table S8). When we examined the slope across all three on-intervention time points, the positive trend in the inverse Simpson index and Faecalibacterium remained consistent (Table S9). Among the patients randomized to the intervention first, Faecalibacterium dropped notably upon returning to their usual diet without beans [LME and 95% CI: −0.69 (−1.30, −0.08), two-sided p = 0.03, q = 0.24; Fig. 3a; Table S8).
Fig. 3.
Parallel effects of cooked dry beans on the gut microbiome, circulating metabolome and protein markers of immune and inflammatory response in obese surveillance patients with a history of colorectal neoplasia otherwise following their usual diet. a. Within 8 weeks, the bean intervention significantly increased the inverse Simpson index [LME and 95% CI: 0.16 (0.02, 0.30), two-sided p = 0.02; n = 48] and the relative abundance of Faecalibacterium [LME and 95% CI: 0.40 (0.02, 0.78), two-sided p = 0.04; n = 42 (88%) carried the genus at baseline]. Across the study sequence no change was observed among participants randomized to remain on the control for the first 8 weeks (n = 20), while among patients randomized to the intervention first, Faecalibacterium dropped significantly [LME and 95% CI: −0.69 (−1.30, −0.08), two-sided p = 0.03, n = 25] within 8 weeks of returning to their usual diet without beans. Forest plot of effect estimates (LME) and 95% confidence intervals by generalized linear mixed models (proc glimmix, SAS 9.4) with random intercept in the natural log scale. b. Heatmap of effects (β) observed by randomization group across the trial sequence by Multivariable Association Discovery in Population-scale Meta-omics Studies (MaAsLin2), a linear mixed model with random intercept of normalized relative abundance (natural log scale; half the minimum relative abundance as pseudo count) for use in sparse, compositional microbial communities. Analysis restricted to min prevalence = 0.1 (total n = 48; n = 28 among the intervention first; 20 among the intervention second). Selected taxa shown met an unadjusted p < 0.05 for the on-intervention effect at either 4- or 8-weeks across the full trial cohort (n = 48; Supplementary Table S10). ∗further indicates the effect within randomization group also met p < 0.05. c. Volcano plot visualizing the results of MaAsLin2 analysis applied to the metagenomic subset (140 stool samples in 48 patients) characterizing the slope across the three on-intervention time points. Maximum estimated false discovery rate when calling all p-values (one-sided) ≤ 0.05 is 0.30. d. Volcano plot visualizing the circulating metabolome results of LME analysis across the three on-intervention time points. Maximum estimated false discovery rate when calling all p-values (one-sided) ≤ 0.05 is 0.48. e. Plot of effect estimates demonstrating significant and consistent increases and decreases within 4 weeks in selected dry bean metabolites, PA and SAM. f. Heatmap of effects (β) observed by randomization group across the trial sequence for plasma metabolites meeting q < 0.2 in d. g. Volcano plot visualizing 8-week on-intervention change in plasma proteins associated with intestinal and systemic inflammatory and immune response (Olink). Maximum estimated false discovery rate when calling all p-values (one-sided) ≤ 0.05 is 0.47.
We then examined a larger pool of microbial taxa using a linear mixed model method that considers the sparsity of compositional data observed within microbial communities and over time, setting a lower minimum prevalence threshold (10%) and adding half the minimum relative abundance as a pseudo count (Fig. 3b; Tables S10–S14). These analyses further indicated that Streptococcus, Collinsella, Escherichia, Ruminoccocus torques, Fournierella, Oscillibacter and another member of the Oscillospiraceae family decreased post-intervention, while Odoribacter, Bifidobacterium, Eubacterium siraeum, Eubacterium brachy and other members of Ruminoccocus and Lachnospiraceae increased (all two-sided p < 0.05, q < 0.41; Fig. 3b; Table S10). As the 28 patients randomized to the bean intervention first returned to their usual diet without beans (control), Collinsella, Fournierella and a member of the Oscillospiraceae family subsequently increased (all two-sided p < 0.05, q < 0.30; Fig. 3b; Table S12). Further examination of the slope defined by all three on-intervention time points in the full trial cohort revealed consistent findings for the decrease in Collinsella and Ruminococcus torques; and the increase in Eubacterium brachy, Odoribacter, and Bifidobacterium (Table S15 and Fig. S5). Consistent with the modest increase in alpha diversity and shifts in specific taxa on the intervention diet, we observed similar patterns for beta-diversity. On-intervention (n = 48), community-level shifts were apparent at the 4-week mid-point but did not appear to differ from what was achieved across the full 8 weeks (Fig. S6) with more pronounced shifts observed in older participants (Table S6). Among participants randomized to the intervention first (n = 28), the on-bean effects again appeared to be attenuated upon returning to their usual diet without beans (Fig. S6).
In a subset of 140 on-intervention samples from 48 patients that underwent shotgun metagenomic sequencing to further characterize the microbiome and its metagenome, we observed consistent results for alpha diversity (Table S9) and similar patterns of compositional shifts (Tables S16 and S17). The prebiotic dry bean intervention increased the relative abundance of Bifidobacterium adolescentis, Eubacterium rectale, Eubacterium sp. CAG:38, Oxalobacter formigenes and Veillonella atypica. Conversely, Bilophila wadsworthia, Blautia hydrogenotrophica, Christensenella minuta, Clostridium innocuum, Roseburia sp. CAG:471 and Sharpea azabuensis decreased on-intervention (Fig. 3c; Table S17). The main species within the Faecalibacterium genus, F. prausnitzii was not a key finding in the metagenomic subset; however, overall results remained consistent with those shown in the 16S analysis. We also found that the intervention shifted the relative abundance of microbial gene content pathways associated with branched amino acid biosynthesis, l-lysine biosynthesis, pyruvate fermentation to isobutanol, and thiazole biosynthesis (Tables S18 and S19; Fig. S7).
Effect of the intervention on the host metabolome
Given that the gut microbiome has profound influence on host metabolism,4 we performed a global metabolomic analysis to identify changes in circulating metabolites associated with the intervention and changes in microbial composition (Fig. 2a). Shifts in circulating metabolites over the study sequence paralleled what we observed in the gut microbiota, implicating microbial markers of nutrient metabolism with five key metabolites meeting q < 0.20 across the three on-intervention time points (Fig. 3d; Table S20). On-intervention (n = 48), pipecolic acid (PA), a metabolite previously linked to dry bean intake and microbial lysine metabolism7 increased within 8 weeks [LME and 95% CI: 0.37 (0.28, 0.46), two-sided p < 0.001, q < 0.001], as did S-(5′-Adenosyl)-l-methionine (SAM) [0.30 (0.19, 0.40), two-sided p < 0.001, q < 0.001], a methyl group donor33 (Fig. 3e and f; Table S21). Each of these metabolites decreased as the 28 individuals randomized to the intervention first returned to their usual diet without dry beans (V2 to V4) but remained stable during the extended control period (V0 to V2) in the 20 individuals randomized to the intervention second (Fig. 3e and f; Table S21). On-intervention (n = 48), we also observed a decrease in an indole-derivative [LME and 95% CI: −0.18 (−0.29, −0.07), two-sided p = 0.002, q = 0.19], a class of metabolites associated with the degradation of tryptophan by microbes,34 with levels returning to baseline upon discontinuation of dry beans (Fig. 3f; Tables S17 and S21). Trigonelline, a microbial-derived catabolite of niacin35 and marker of legume intake,36 also increased on-intervention [LME and 95% CI: 0.57 (0.33, 0.82), two-sided p < 0.001, q = 0.004; Fig. 3f). Theophylline, a methylxanthine, increased in concordance with trigonelline, but unlike trigonelline, theophylline and other metabolites (in Fig. 3d) did not decrease or change when participants crossed over to the control.
Effect of the intervention on protein markers of host immune and inflammatory response
In vitro and in vivo data support that multiple bioactive compounds present in beans and other legumes have the potential to modulate colonic inflammation and carcinogenesis through systemic immunity and inflammation.37 In pre and post 8-week intervention blood samples, we detected 75 immunologic proteins measured by proximity extension assay (PEA) using the Olink 96 inflammation panel. We found an increase in fibroblast growth factor (FGF)-19, while interleukin 10 receptor alpha (IL10ra) and other immuno-oncology cytokines, including tumor necrosis factor-related activation-induced cytokine (TRANCE), T-cell surface glycoprotein CD8 alpha chain (CD8A), programmed cell death 1 ligand 1 (PD-L1), growth-regulated alpha protein C-X-C motif chemokine 1 (CXCL1) and urokinase plasminogen activator (uPA) decreased in response to 8-week bean intervention (Fig. 3g and Table S22).
Shifting interactions among responsive biomarkers
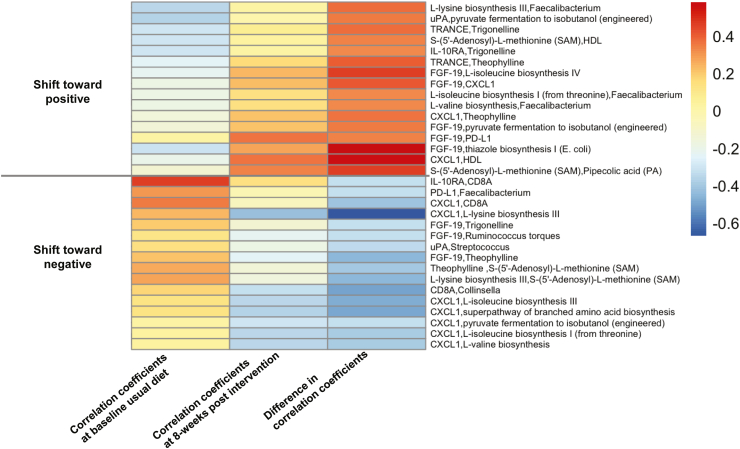
Differential correlations among bean-responsive biomarkers and key study variables visualized in Fig. 4 reflect a move or shift in co-expression or co-exclusion between paired features pre vs. post 8-week intervention. Shifts reflected interactions of microbial pathways and metabolites with other host markers that changed in direction, were uncoupled, or newly appeared over the course of the intervention. To highlight a few, this included shifts in interactions among microbial thiazole biosynthesis and other bean–responsive pathways with FGF-19; microbial l-lysine biosynthesis with Faecalibacterium abundance and CXCL1; circulating metabolite trigonelline with IL-10ra and TRANCE; and CXCL1 with microbial branched amino acid biosynthesis and HDL.
Fig. 4.
Differential co-expression of the gut microbiome, circulating metabolome and protein markers of immune and inflammatory response induced by the 8-week intervention. Differentially correlated module pairs between baseline usual diet (left column) and 8-weeks post intervention (middle column). Heatmaps depict Fisher's z-transformation of Pearson correlation coefficients between paired features that significantly increased or decreased pre-and post-intervention (FDR adjusted p-value <0.25).
Discussion
The BE GONE trial supports that adding navy beans to the usual diet of high-risk patients provides a viable prebiotic food source to target and shift both the gut microbiome and host metabolome. Taken overall, the gut microbiota findings suggest that changes in the relative abundance of individual bacteria reflect early or transient shifts in dominant dietary fiber sources and amino acids within the usual diet (e.g., on-intervention decreases in Oscillibacter and Roseburia with increases in Odoribacter and Eubacterium38, 39, 40) and in several potentially pathogenic or opportunistic bacteria that threaten the integrity of the gut barrier and colorectal cancer prevention (e.g., decreases in Streptococcus, Collinsella, and Ruminococcus torques).1,41,42 Increased alpha diversity and potential markers of a healthy or homeostatic gut microbiome (e.g., Faecalibacterium and Bifidobacterium) with therapeutic potential in inflammatory bowel disease, colon and other cancers3,43 were more readily apparent within 8 weeks post-intervention. Intervention-responsive nutrient and microbiome-derived circulating biomarkers and metabolites, including pipecolic acid, an indole derivative, FGF-19 and Il-10ra, highlight potential mechanisms whereby the simple addition of this prebiotic food modulates both gut and host health.
Metagenomic sequencing and functional profiling of the gut microbiome was generally consistent with this pattern of findings and shed light on specific species that increased over time during the dry bean intervention (e.g., Eubacterium rectale and Bifidobacterium adolescentis). Interestingly, some of the bacteria that shifted on intervention are sulfur-metabolizing species with established links to diet and colorectal cancer (e.g., Bilophila wadsworthia).44 While shifts in functional profiles did support an increase in the bacterial degradation of the sugar sulfoquinovose (SQ, 6-deoxy-6-sulfoglucose) produced by plants and decrease in the superpathway of sulfur amino acid biosynthesis and transsulfuration, we observed stronger signals for downregulated expression of microbial pathways involved in branched chain amino acid biosynthesis. A growing body of evidence suggests that dysbiosis of gut microbiota plays an important role in the pathogenesis of metabolic dysregulation among obese populations through production of branched chain amino acids, increased gut permeability and low-grade endotoxemia.45 Notably, these changes may be targeted by diet and exercise interventions that shift gate-keeping bacteria, such as Faecalibacterium, Bifidobacterium and Akkermansia.46 Microbial lysine and thiazole (thiamine) biosynthesis also decreased with the bean intervention, perhaps reflecting a reduced priority or need for the microbiota to produce these nutrients when they could be directly obtained by the host from study beans. We expect that individual microbes are members of an ecology that carry complementary functions with the host, which is reflected in overlapping microbial pathways that respond to the bean intervention. For example, the microbial superpathway of branched chain amino acid biosynthesis includes the subpathways of l-leucine biosynthesis, l-isoleucine biosynthesis I (from threonine), and l-valine biosynthesis. A shared intermediate, 3-methyl-2-oxopentanoate, is involved in pyruvate fermentation to isobutanol, l-isoleucine biosynthesis IV from propionate, and l-isoleucine biosynthesis III from glutamate. Components of the l-lysine biosynthesis pathway (from aspartate), where gram-positive bacteria uniquely convert tetrahydrodipicolinate to meso-diaminopimelate in a single enzymatic step, supports homoserine biosynthesis which feeds into the SAM superpathway.
Parallel shifts in circulating PA, SAM, trigonelline and an indole-derivative further support the role of microbial metabolism in converting dietary components to bioactive metabolites with potential therapeutic relevance to this patient population. Nutritional profiles of navy beans provide a complete source of plant-based protein, including lysine, methionine and tryptophan, as well as B vitamins and several mineral co-factors. The findings for PA, a microbial metabolite of the essential amino acid lysine with anti-inflammatory, antitumor and antibiotic properties, is consistent with those from the Polyp Prevention Trial cohort, as well as preclinical and clinical studies specifically testing the effect of navy bean extracts.7,47 Trends over the study sequence in this dry bean biomarker support the relative efficacy of this 8-week “add a cup of beans” intervention to achieve measurable effects without broader or controlled changes in the usual diet. Trigonelline, a nutritional metabolite often linked to its most commonly consumed non-leguminous source (coffee), is formed through the microbial metabolism and methylation of vitamin B3 (niacin) and accumulates in the seeds of legumes.36 In preclinical and clinical studies, trigonelline has been shown to demonstrate inhibitory effects on obesity-related conditions, including diabetes and cancer.35 Similarly, SAM has been tested in the prevention and treatment of multiple conditions, including liver disease, colorectal and other cancers.48 Humans and microbes have methionine adenosyltransferase enzymes that catalyze the biosynthesis of SAM from methionine. Other host and microbiota metabolites, including trigonelline, act as substrates and cofactors in reactions where SAM donates an activated methyl group for many fundamental cellular processes, including the methylation of DNA, RNA and histones that modulates autophagy through epigenetic effects.48 Conversely, this process can be derailed and exploited by cancer cells, such that dietary methionine restriction demonstrates therapeutic potential in preclinical models. However, dietary methionine has also been shown to support a healthy gut microbiome and host immune response suggesting that the timing, quantity and source of other nutritional co-factors may be critical to understand in this context. Interestingly, the navy bean intervention also decreased an indole-derivative (putatively cinnamoylglycine), which may be linked to an immunologically relevant class of metabolites associated with the degradation of tryptophan by microbes,33,49 that subsequently increased when patients returned to their usual diet without dry beans. Theophylline increased in concordance with trigonelline on-intervention, but unlike trigonelline, SAM and PA, theophylline did not decrease or change when participants crossed over to the control; and thus, could be related to other dietary or drug interactions.
Intervention-responsive immune and inflammation-modulating biomarkers, FGF-19 and Il-10ra, shed further light on the role of specific prebiotic foods in the dynamic process of maintaining intestinal homeostasis whereby acute immune responses toward foods and microbes are balanced by the control of these responses in order to prevent chronic, systemic inflammation that promotes cancer and other chronic diseases.50 The increase in FGF-19 may be particularly relevant to high-risk patients with a history of both gut and metabolic health issues, as diminished FGF-19 is linked to inflammatory bowel disease, type 2 diabetes and non-alcoholic fatty liver disease.51,52 As a gut hormone that regulates bile acid synthesis, glucose and lipid metabolism, FGF-19 serves as both a metabolic coordinator between the gut microbiota and liver and an inhibitor of intestinal inflammation through modulation of immune responses. Similarly, downregulation of IL10ra, a mediator of the immunosuppressive signals of IL-10 and target in the treatment of colorectal cancer, may also reflect shifting immune responses or tolerance in the intestinal mucosa and the liver.53 This is also consistent with the pattern of suppression in several other pro-inflammatory and immune markers, including TRANCE and CD8a that regulate T-cell dependent immune responses against both external and internal stimuli and CXCL1 that is expressed at higher levels during inflammatory responses.54,55 Although there is clear biological overlap between inflammatory processes, FGF19, bile acid and cholesterol metabolism,56,57 the duration of bean consumption might not have been long or robust enough to observe any improvement in lipoprotein profiles. However, concurrent shifts in microbes, metabolites, cytokine profiles and immune proteins hint at well-established mechanisms driven by diet. Beans contain plant oligosaccharides and multiple other microbiota-accessible fibers, amino acids, and vitamin co-factors involved in the formation of bioactive metabolites involved in favorable (i.e., anti-cancer) immune and intracellular processes among host cells.58 For example, galacto-oligosaccharides (GOS) in beans, selectively promote the growth of beneficial bacteria, such as Faecalibacterium and Bifidobacterium spp., that play specialized roles in the fermentation of dietary fiber to short chain fatty acids (SCFA), the main energy source for intestinal epithelial cells, ensuring mucosal barrier integrity and preventing bacterial translocation.41 Beans are also a readily available source of lysine, an essential amino acid precursor for microbial-derived SCFA and another suspected host-microbiota metabolic pathway linked to beans’ preventive effects. The effects of prebiotic foods are further supported through cross-feeding, symbiotic relationships between keystone or gate-keeping bacteria that help maintain the overall gut ecosystem by producing metabolites that selectively stimulate the growth of other beneficial bacteria.59 This phenomenon may be particularly important to address the natural variability in microbiome communities between patients and over time.
The crossover design revealed patterns across the study sequence that provide important context to the key findings. Acknowledging the expected variability in individual responses to a dietary intervention conducted within usual eating habits and natural living conditions, as well as the restrictions on statistical significance given multiple comparisons, the effects of “coming off” the intervention were quite striking and have not been reported in prior dry bean or legume trials with reversals in favorable changes in bacteria and host metabolites within 4–8 weeks. Our findings spanning the effects of bean addition and depletion track with real-life diet modifications and mirror the findings of a recent study50 testing a 5 day cycle of diets containing >40 g/d followed by <5 g/day of dietary fiber whereby rapid depletion in beneficial microbes and host immune responses created a “window” for opportunistic pathogens. Taken together, our results suggest that more modest and extended diet modification (i.e., beans alone) could have similar consequences. This 8-week intervention exceeded the length of previous studies10,60 and in post-trial follow-up within a year of completion, we observed a modest pre-vs. post-study increase in the reported frequency of consuming dry beans or dry bean-containing mixed dishes [LME and 95% CI for change in monthly frequency: 2.1 (−1.2, 5.4), p = 0.19; data not shown]); however, some patients also reported new diagnoses and health issues that limited their overall intake.
Our collective findings for beneficial microbes, PA, SAM and trigonelline and FGF-19 demonstrate that the simple diet change and the bean “dose” was sufficient to influence host metabolites and biomarkers with potential therapeutic relevance. Although broader changes in diet and lifestyle in conjunction with increased bean intake are likely to be more effective in improving LDL and overall metabolic health,2 simple and accessible dietary strategies targeting the composition and metabolic function of the microbiome are of increasing interest to scientists, patients, and providers in multiple settings. Adding one cup of navy beans to the diet on all or most days of the week was a safe, scalable dietary strategy to modulate the gut microbiome of high-risk patients, who may be unwilling or unable to sustain more dramatic changes to their usual dietary pattern without substantial support. Given the tolerable side effect profile for a population with a history of bowel lesions, beans should not be intentionally avoided. Future research is needed to continue to address whether beans (and other prebiotic foods patients may not be consuming in sufficient amounts) should be a prominent component of prescriptive or adjunct dietary regimens delivered as part of the usual care and management plan in other clinical settings relevant to this population (e.g., inflammatory bowel disease, type 2 diabetes, or cancer immunotherapy). Advancing our understanding of the metabolites and biological markers paralleling interactions of the microbiota with prebiotic foods is critical to effectively design and monitor extended trials with clinically relevant outcomes.
Contributors
Conceptualization: CRD.
Development of the study design, objectives or methodology: JWL, KBE, PCO, SK, SMH, NJA, KLH, JFP, PS.
Data acquisition or provision of study resources: GJB, WS, JBD, JV, RW, EM, JLM, JAW, SMH, KLH, NJA, JFP, PCO, SK.
Formal data analysis and data visualization: XZ, EI, KLH, FL.
Development of the statistical analysis design and pipelines: XZ, EI, KLH, NJA, PNL, KAD, JPL, PS; Interpretation of data or results: CRD, XZ, EI, KLH, JFF, PNL, JPL, KAD, NJA, YDS, JLM, JAW, JWL, KBE, SMH, PS, SK, PCO.
Drafted the manuscript: XZ, CRD, EI, KLH, JFF.
Funding acquisition: CRD.
All of the authors have critically reviewed and approved the submitted version.
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon request. The raw microbial sequencing reads will be posted in a secure NCBI database. Processed microbiome and metabolomics data will be available upon reasonable requests. Limitations apply to metadata variables that may compromise participant privacy or consent.
Declaration of interests
Study beans were independently purchased with funds from the Dry Bean Health Research Program, a peer-reviewed incentive award (to CRD) created by the Northarvest Bean Growers Association, Communique Inc. to identify and encourage researchers that apply for NIH-funding to support studies on beans and human health.
Acknowledgements
The BE GONE trial was funded by the American Cancer Society (RSG-17-049-01-NEC to CRD) and the University of Texas MD Anderson Cancer Center (Institutional Research Grant to CRD). Additional sources of relevant funding supporting the study team: The MD Anderson NCI Cancer Center Support Grant P30 CA016672 (CRD, PS, KB, SMH, SK), Andrew Sabin Family Fellows Program (CRD, JLM, JW), Cancer Prevention and Research Institute of Texas Research Training Program RP160097 (XZ), and the generous philanthropic contributions to The University of Texas MD Anderson Cancer Center Moon Shots Program™ (JFF, SMH, JBD, NJA, SK, JAW, JLM, CRD). We wish to further acknowledge: Dr. Roberd M. Bostick, MD, MPH (Professor Emeritus, Emory University) for his expertise, encouragement and mentorship to CRD; the Kelsey-Seybold Research Foundation for assistance with trial recruitment; and the following MD Anderson shared resources, centers, platforms and programs: Assessment, Intervention and Measurement Core, Bionutrition Research Core, Clinical and Translational Research Center, Population Science Laboratory, Center for Energy Balance in Cancer Prevention and Survivorship, Platform for Innovative Microbiome and Translational Research and the Moon Shots Program. The BE GONE Trial would not be possible without the substantial time and effort contributions from our dedicated participants and patients, as well as the families and friends who support them.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104873.
Appendix A. Supplementary data
References
- 1.Clay S.L., Fonseca-Pereira D., Garrett W.S. Colorectal cancer: the facts in the case of the microbiota. J Clin Invest. 2022;132(4) doi: 10.1172/JCI155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller N.T., Zhang M., Juraschek S.P., Miller E.R., Appel L.J. Effects of high-fiber diets enriched with carbohydrate, protein, or unsaturated fat on circulating short chain fatty acids: results from the OmniHeart randomized trial. Am J Clin Nutr. 2020;111(3):545–554. doi: 10.1093/ajcn/nqz322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer Christine N., McQuade Jennifer L., Gopalakrishnan V., et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliphant K., Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mavrogeni M.E., Asadpoor M., Henricks P.A.J., Keshavarzian A., Folkerts G., Braber S. Direct action of non-digestible oligosaccharides against a leaky gut. Nutrients. 2022;14(21):4699. doi: 10.3390/nu14214699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monk J.M., Wu W., Lepp D., Pauls K.P., Robinson L.E., Power K.A. Navy bean supplementation in established high-fat diet-induced obesity attenuates the severity of the obese inflammatory phenotype. Nutrients. 2021;13(3):757. doi: 10.3390/nu13030757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perera T., Young M.R., Zhang Z., et al. Identification and monitoring of metabolite markers of dry bean consumption in parallel human and mouse studies. Mol Nutr Food Res. 2015;59(4):795–806. doi: 10.1002/mnfr.201400847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira N.M., Peghinelli V.V., Monte M.G., et al. Beans comsumption can contribute to the prevention of cardiovascular disease. Clin Nutr ESPEN. 2023;54:73–80. doi: 10.1016/j.clnesp.2023.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Lanza E., Hartman T.J., Albert P.S., et al. High dry bean intake and reduced risk of advanced colorectal adenoma recurrence among participants in the polyp prevention trial. J Nutr. 2006;136(7):1896–1903. doi: 10.1093/jn/136.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheflin A.M., Borresen E.C., Kirkwood J.S., et al. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol Nutr Food Res. 2017;61(1) doi: 10.1002/mnfr.201500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah M.S., Fogelman D.R., Raghav K.P., et al. Joint prognostic effect of obesity and chronic systemic inflammation in patients with metastatic colorectal cancer. Cancer. 2015;121(17):2968–2975. doi: 10.1002/cncr.29440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner H., Chen C. The colorectal cancer epidemic: challenges and opportunities for primary, secondary and tertiary prevention. Br J Cancer. 2018;119(7):785–792. doi: 10.1038/s41416-018-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X., Browman G., Siu W., et al. The BE GONE trial study protocol: a randomized crossover dietary intervention of dry beans targeting the gut microbiome of overweight and obese patients with a history of colorectal polyps or cancer. BMC Cancer. 2019;19(1):1233. doi: 10.1186/s12885-019-6400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merriman K.W., Broome R.G., De Las Pozas G., Landvogt L.D., Qi Y., Keating J. Evolution of the cancer registrar in the era of informatics. JCO Clin Cancer Inform. 2021;5:272–278. doi: 10.1200/CCI.20.00123. [DOI] [PubMed] [Google Scholar]
- 15.Subar A.F., Kirkpatrick S.I., Mittl B., et al. The automated self-administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the national cancer institute. J Acad Nutr Diet. 2012;112(8):1134–1137. doi: 10.1016/j.jand.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millen A.E., Midthune D., Thompson F.E., Kipnis V., Subar A.F. The National Cancer Institute diet history questionnaire: validation of pyramid food servings. Am J Epidemiol. 2006;163(3):279–288. doi: 10.1093/aje/kwj031. [DOI] [PubMed] [Google Scholar]
- 17.Hagströmer M., Oja P., Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755–762. doi: 10.1079/phn2005898. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg D.E., Bull F.C., Marshall A.L., Sallis J.F., Bauman A.E. Assessment of sedentary behavior with the international physical activity questionnaire. J Phys Act Health. 2008;5(Suppl 1):S30–S44. doi: 10.1123/jpah.5.s1.s30. [DOI] [PubMed] [Google Scholar]
- 19.Zheng J., Hoffman K.L., Chen J.S., et al. Dietary inflammatory potential in relation to the gut microbiome: results from a cross-sectional study. Br J Nutr. 2020;124(9):931–942. doi: 10.1017/S0007114520001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha R., Abu-Ali G., Vogtmann E., et al. Assessment of variation in microbial community amplicon sequencing by the Microbiome Quality Control (MBQC) project consortium. Nat Biotechnol. 2017;35(11):1077–1086. doi: 10.1038/nbt.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart C.J., Ajami N.J., O'Brien J.L., et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562(7728):583–588. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Methé B.A., Nelson K.E., Pop M., et al. A framework for human microbiome research. Nature. 2012;486(7402):215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segata N., Waldron L., Ballarini A., Narasimhan V., Jousson O., Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat Methods. 2012;9(8):811–814. doi: 10.1038/nmeth.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franzosa E.A., McIver L.J., Rahnavard G., et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods. 2018;15(11):962–968. doi: 10.1038/s41592-018-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahrmann J.F., Bantis L.E., Capello M., et al. A plasma-derived protein-metabolite multiplexed panel for early-stage pancreatic cancer. J Natl Cancer Inst. 2019;111(4):372–379. doi: 10.1093/jnci/djy126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahrmann J.F., Irajizad E., Kobayashi M., et al. A MYC-driven plasma polyamine signature for early detection of ovarian cancer. Cancers. 2021;13(4):913. doi: 10.3390/cancers13040913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fahrmann J.F., Vykoukal J., Fleury A., et al. Association between plasma diacetylspermine and tumor spermine synthase with outcome in triple-negative breast cancer. J Natl Cancer Inst. 2020;112(6):607–616. doi: 10.1093/jnci/djz182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vykoukal J., Fahrmann J.F., Gregg J.R., et al. Caveolin-1-mediated sphingolipid oncometabolism underlies a metabolic vulnerability of prostate cancer. Nat Commun. 2020;11(1):4279. doi: 10.1038/s41467-020-17645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assarsson E., Lundberg M., Holmquist G., et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahl W.J., Stewart M.L. Position of the academy of nutrition and dietetics: health implications of dietary fiber. J Acad Nutr Diet. 2015;115(11):1861–1870. doi: 10.1016/j.jand.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Krebs-Smith S.M., Pannucci T.E., Subar A.F., et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–1602. doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doma K.M., Dolinar K.F., Dan Ramdath D., Wolever T.M.S., Duncan A.M. Canned beans decrease serum total and LDL cholesterol in adults with elevated LDL cholesterol in a 4-wk multicenter, randomized, crossover study. J Nutr. 2021;151(12):3701–3709. doi: 10.1093/jn/nxab323. [DOI] [PubMed] [Google Scholar]
- 33.Benjdia A., Martens E.C., Gordon J.I., Berteau O. Sulfatases and a radical S-adenosyl-L-methionine (AdoMet) enzyme are key for mucosal foraging and fitness of the prominent human gut symbiont, Bacteroides thetaiotaomicron. J Biol Chem. 2011;286(29):25973–25982. doi: 10.1074/jbc.M111.228841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roager H.M., Licht T.R. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palau-Rodriguez M., Tulipani S., Isabel Queipo-Ortuño M., Urpi-Sarda M., Tinahones F.J., Andres-Lacueva C. Metabolomic insights into the intricate gut microbial-host interaction in the development of obesity and type 2 diabetes. Front Microbiol. 2015;6:1151. doi: 10.3389/fmicb.2015.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madrid-Gambin F., Brunius C., Garcia-Aloy M., Estruel-Amades S., Landberg R., Andres-Lacueva C. Untargeted (1)H NMR-based metabolomics analysis of urine and serum profiles after consumption of lentils, chickpeas, and beans: an extended meal study to discover dietary biomarkers of pulses. J Agric Food Chem. 2018;66(27):6997–7005. doi: 10.1021/acs.jafc.8b00047. [DOI] [PubMed] [Google Scholar]
- 37.Juárez-Chairez M.F., Meza-Márquez O.G., Márquez-Flores Y.K., Jiménez-Martínez C. Potential anti-inflammatory effects of legumes: a review. Br J Nutr. 2022;128(11):2158–2169. doi: 10.1017/S0007114522000137. [DOI] [PubMed] [Google Scholar]
- 38.Oliver A., Chase A.B., Weihe C., et al. High-fiber, whole-food dietary intervention alters the human gut microbiome but not fecal short-chain fatty acids. mSystems. 2021;6(2):e00115–e00121. doi: 10.1128/mSystems.00115-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asnicar F., Berry S.E., Valdes A.M., et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. 2021;27(2):321–332. doi: 10.1038/s41591-020-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X., Ning Z., Mayne J., et al. Widespread protein lysine acetylation in gut microbiome and its alterations in patients with Crohn's disease. Nat Commun. 2020;11(1):4120. doi: 10.1038/s41467-020-17916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai M.S., Seekatz A.M., Koropatkin N.M., et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez-Arango L.F., Barrett H.L., Wilkinson S.A., et al. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microb. 2018;9(3):189–201. doi: 10.1080/19490976.2017.1406584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobhani I., Bergsten E., Couffin S., et al. Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures. Proc Natl Acad Sci U S A. 2019;116(48):24285–24295. doi: 10.1073/pnas.1912129116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf P.G., Cowley E.S., Breister A., et al. Diversity and distribution of sulfur metabolic genes in the human gut microbiome and their association with colorectal cancer. Microbiome. 2022;10(1):64. doi: 10.1186/s40168-022-01242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheithauer T.P.M., Rampanelli E., Nieuwdorp M., et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.571731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y., Wang Y., Ni Y., et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metabol. 2020;31(1):77–91.e5. doi: 10.1016/j.cmet.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Zarei I., Baxter B.A., Oppel R.C., Borresen E.C., Brown R.J., Ryan E.P. Plasma and urine metabolite profiles impacted by increased dietary navy bean intake in colorectal cancer survivors: a randomized-controlled trial. Cancer Prev Res. 2021;14(4):497–508. doi: 10.1158/1940-6207.CAPR-20-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanderson S.M., Gao X., Dai Z., Locasale J.W. Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat Rev Cancer. 2019;19(11):625–637. doi: 10.1038/s41568-019-0187-8. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L.S., Davies S.S. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 2016;8(1):46. doi: 10.1186/s13073-016-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siracusa F., Schaltenberg N., Kumar Y., et al. Short-term dietary changes can result in mucosal and systemic immune depression. Nat Immunol. 2023;24(9):1473–1486. doi: 10.1038/s41590-023-01587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gadaleta R.M., Moschetta A. Metabolic Messengers: fibroblast growth factor 15/19. Nat Metab. 2019;1(6):588–594. doi: 10.1038/s42255-019-0074-3. [DOI] [PubMed] [Google Scholar]
- 52.Gadaleta R.M., Garcia-Irigoyen O., Cariello M., et al. Fibroblast growth factor 19 modulates intestinal microbiota and inflammation in presence of Farnesoid X Receptor. eBioMedicine. 2020;54 doi: 10.1016/j.ebiom.2020.102719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan K.M., Jiang X., Guha P., et al. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut. 2023;72(2):325–337. doi: 10.1136/gutjnl-2021-325808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ochi T., Feng Y., Kitamoto S., et al. Diet-dependent, microbiota-independent regulation of IL-10-producing lamina propria macrophages in the small intestine. Sci Rep. 2016;6(1) doi: 10.1038/srep27634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yogev N., Bedke T., Kobayashi Y., et al. CD4(+) T-cell-derived IL-10 promotes CNS inflammation in mice by sustaining effector T cell survival. Cell Rep. 2022;38(13) doi: 10.1016/j.celrep.2022.110565. [DOI] [PubMed] [Google Scholar]
- 56.Kliewer S.A., Mangelsdorf D.J. Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis. 2015;33(3):327–331. doi: 10.1159/000371670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Degirolamo C., Sabbà C., Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15(1):51–69. doi: 10.1038/nrd.2015.9. [DOI] [PubMed] [Google Scholar]
- 58.Baruch E.N., Wang J., Wargo J.A. Gut microbiota and antitumor immunity: potential mechanisms for clinical effect. Cancer Immunol Res. 2021;9(4):365–370. doi: 10.1158/2326-6066.CIR-20-0877. [DOI] [PubMed] [Google Scholar]
- 59.Rios-Covian D., Gueimonde M., Duncan S.H., Flint H.J., de Los Reyes-Gavilan C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol Lett. 2015;362(21):fnv176. doi: 10.1093/femsle/fnv176. [DOI] [PubMed] [Google Scholar]
- 60.Baxter B.A., Oppel R.C., Ryan E.P. Navy beans impact the stool metabolome and metabolic pathways for colon health in cancer survivors. Nutrients. 2018;11(1):28. doi: 10.3390/nu11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.