Abstract
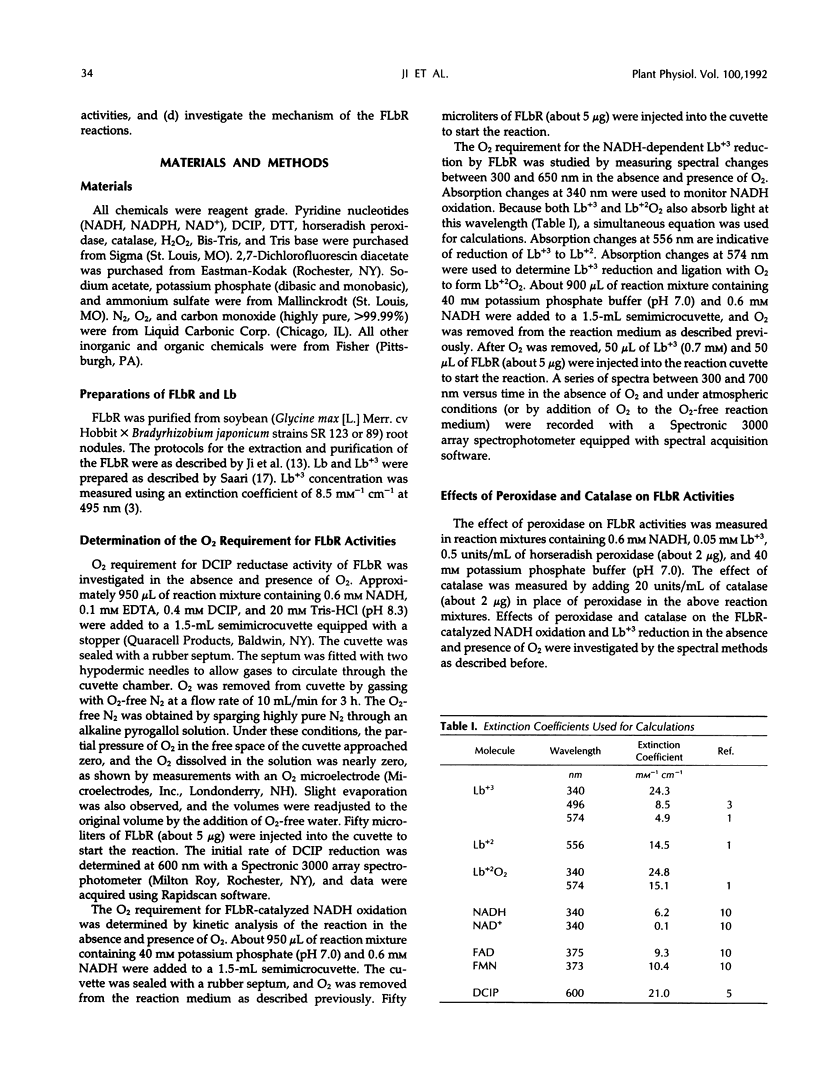
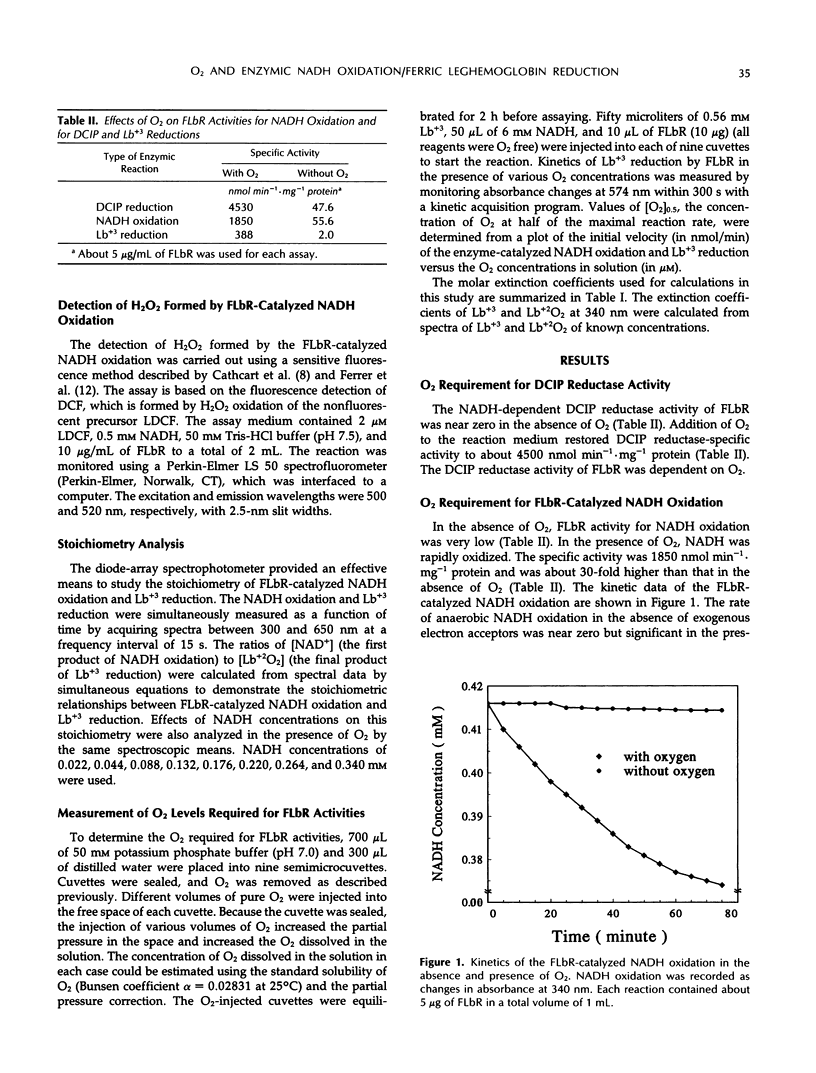
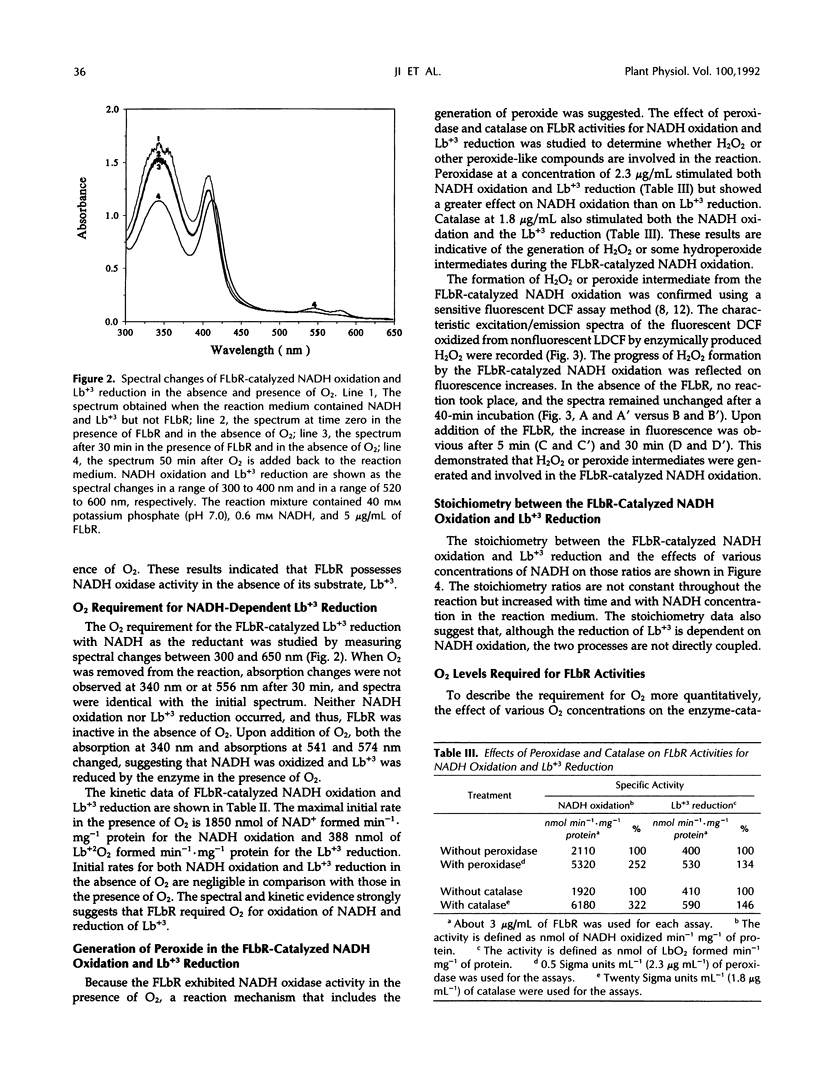
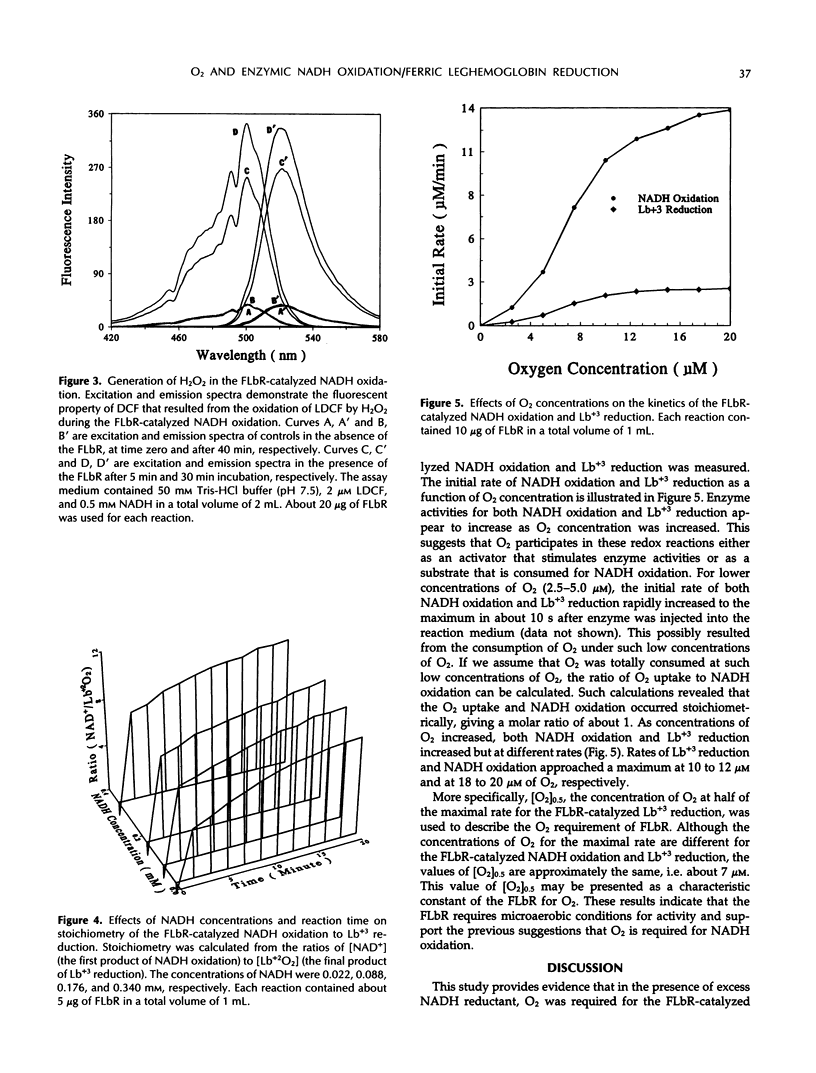
Ferric leghemoglobin reductase (FLbR) from soybean (Glycine max [L.] Merr) nodules catalyzed oxidation of NADH, reduction of ferric leghemoglobin (Lb+3), and reduction of dichloroindophenol (diaphorase activity). None of these reactions was detectable when O2 was removed from the reaction system, but all were restored upon readdition of O2. In the absence of exogenous electron carriers and in the presence of O2 and excess NADH, FLbR catalyzed NADH oxidation with the generation of H2O2 functioning as an NADH oxidase. The possible involvement of peroxide-like intermediates in the FLbR-catalyzed reactions was analyzed by measuring the effects of peroxidase and catalase on FLbR activities; both enzymes at low concentrations (about 2 μg/mL) stimulated the FLbR-catalyzed NADH oxidation and Lb+3 reduction. The formation of H2O2 during the FLbR-catalyzed NADH oxidation was confirmed using a sensitive assay based on the fluorescence emitted by dichlorofluorescin upon reaction with H2O2. The stoichiometry ratios between the FLbR-catalyzed NADH oxidation and Lb+3 reduction were not constant but changed with time and with concentrations of NADH and O2 in the reaction solution, indicating that the reactions were not directly coupled and electrons from NADH oxidation were transferred to Lb+3 by reaction intermediates. A study of the affinity of FLbR for O2 showed that the enzyme required at least micromolar levels of dissolved O2 for optimal activities. A mechanism for the FLbR-catalyzed reactions is proposed by analogy with related oxidoreductase systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A., Nicola N. A., Hurrell J. G., Leach S. J. Characterization and improved separation of soybean leghemoglobins. Biochemistry. 1975 Oct 7;14(20):4444–4450. doi: 10.1021/bi00691a016. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Properties of leghaemoglobin in vivo, and its isolation as ferrous oxyleghaemoglobin. Biochim Biophys Acta. 1969;188(2):222–229. doi: 10.1016/0005-2795(69)90069-5. [DOI] [PubMed] [Google Scholar]
- Aviram I., Wittenberg A., Wittenberg J. B. The reaction of ferrous leghemoglobin with hydrogen peroxide to form leghemoglobin(IV). J Biol Chem. 1978 Aug 25;253(16):5685–5689. [PubMed] [Google Scholar]
- Bando Y., Aki K. Mechanisms of generation of oxygen radicals and reductive mobilization of ferritin iron by lipoamide dehydrogenase. J Biochem. 1991 Mar;109(3):450–454. doi: 10.1093/oxfordjournals.jbchem.a123402. [DOI] [PubMed] [Google Scholar]
- Becana M., Klucas R. V. Enzymatic and nonenzymatic mechanisms for ferric leghemoglobin reduction in legume root nodules. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7295–7299. doi: 10.1073/pnas.87.18.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Ames B. N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983 Oct 1;134(1):111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Danson M. J. Dihydrolipoamide dehydrogenase: a 'new' function for an old enzyme? Biochem Soc Trans. 1988 Apr;16(2):87–89. doi: 10.1042/bst0160087. [DOI] [PubMed] [Google Scholar]
- Denison R. F., Layzell D. B. Measurement of legume nodule respiration and o(2) permeability by noninvasive spectrophotometry of leghemoglobin. Plant Physiol. 1991 May;96(1):137–143. doi: 10.1104/pp.96.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L., Wood S., Becana M., Klucas R. V. Purification and characterization of soybean root nodule ferric leghemoglobin reductase. Plant Physiol. 1991 May;96(1):32–37. doi: 10.1104/pp.96.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keevil T., Mason H. S. Molecular oxygen in biological oxidations--an overview. Methods Enzymol. 1978;52:3–40. doi: 10.1016/s0076-6879(78)52003-x. [DOI] [PubMed] [Google Scholar]
- MASSEY V., VEEGER C. Studies on the reaction mechanism of lipoyl dehydrogenase. Biochim Biophys Acta. 1961 Mar 18;48:33–47. doi: 10.1016/0006-3002(61)90512-1. [DOI] [PubMed] [Google Scholar]
- Massey V., Schopfer L. M., Anderson R. F. Structural determinants of the oxygen reactivity of different classes of flavoproteins. Prog Clin Biol Res. 1988;274:147–166. [PubMed] [Google Scholar]
- Saari L. L., Klucas R. V. Ferric leghemoglobin reductase from soybean root nodules. Arch Biochem Biophys. 1984 May 15;231(1):102–113. doi: 10.1016/0003-9861(84)90367-9. [DOI] [PubMed] [Google Scholar]
- Sanchez Ferrer A., Santema J. S., Hilhorst R., Visser A. J. Fluorescence detection of enzymatically formed hydrogen peroxide in aqueous solution and in reversed micelles. Anal Biochem. 1990 May 15;187(1):129–132. doi: 10.1016/0003-2697(90)90429-d. [DOI] [PubMed] [Google Scholar]


