Abstract
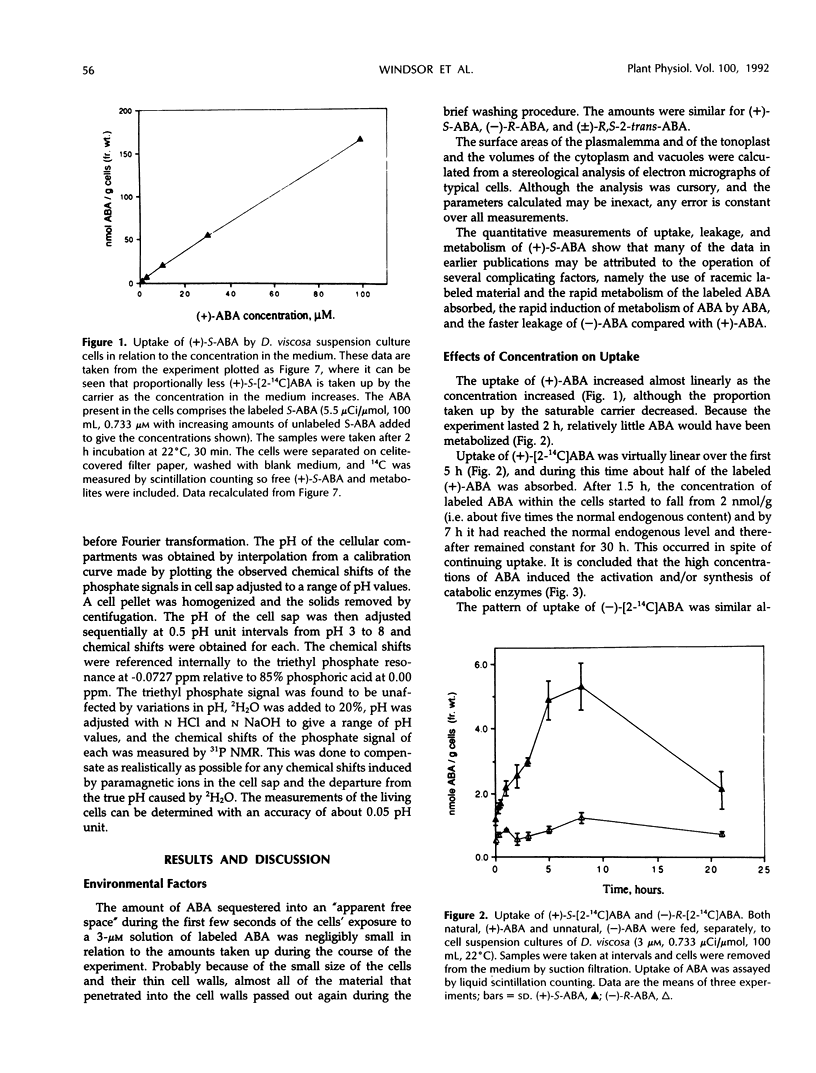
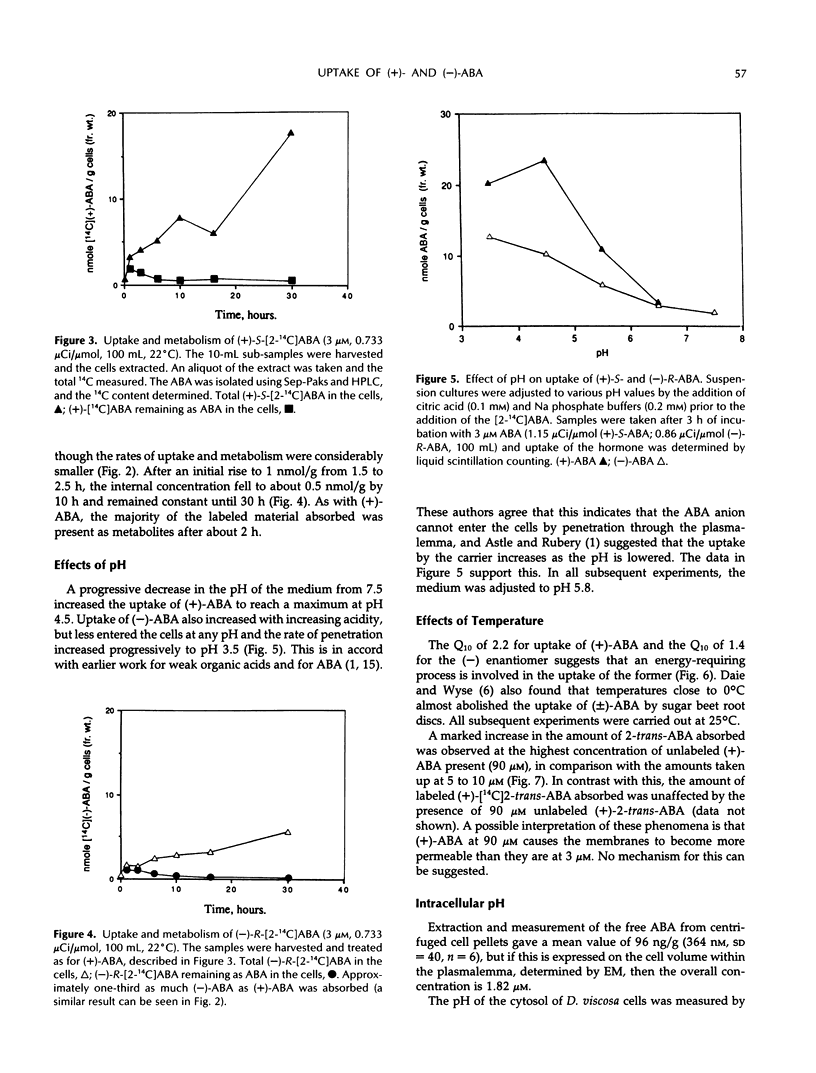
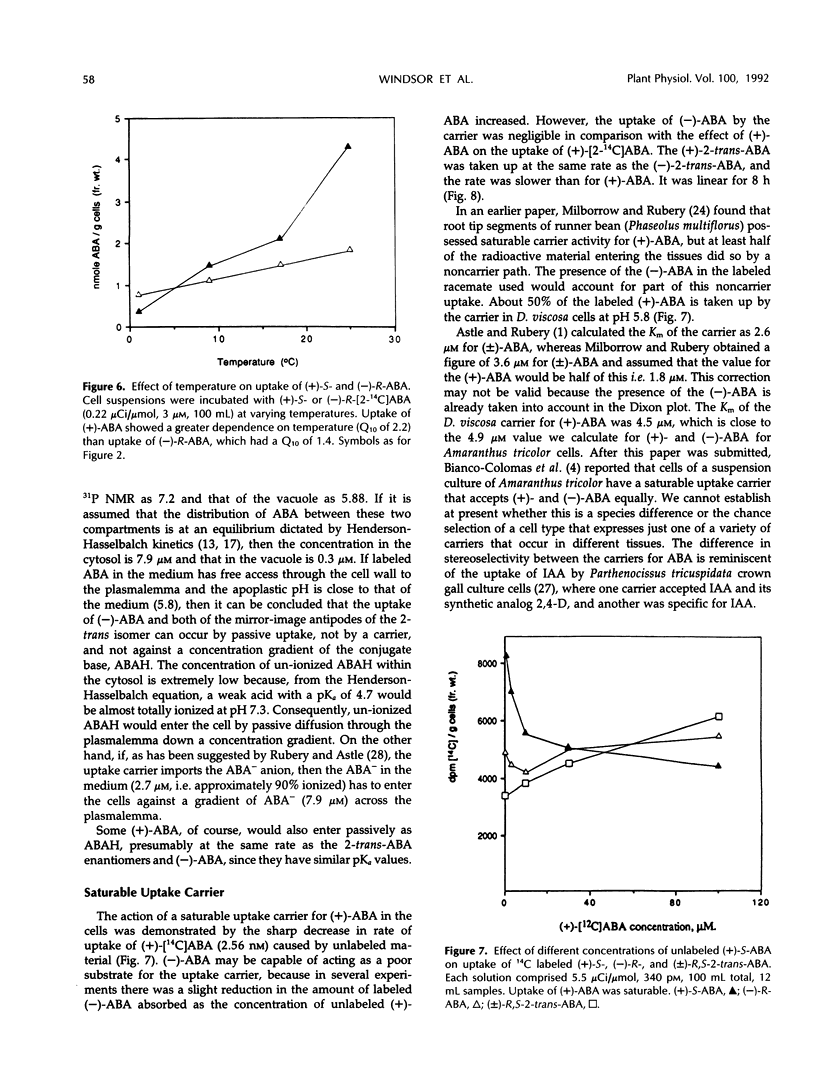
The uptake of (+)-S- and (−)-R-abscisic acid (ABA) by suspension culture cells of hopbush (Dodonaea viscosa L. Jacqu.) was followed over a range of temperatures, pH values, and time intervals. The natural (+)-S-ABA was taken up about five times faster than the unnatural (−)-R-ABA. Each 10°C rise in temperature from 1 to 31°C increased the rate of uptake (Q10) of (+)-S-ABA about 2.2-fold, whereas that of the (−)-R increased with a Q10 of 1.4. (+)-ABA was taken into the cells by a saturable carrier, but (−)-ABA and both enantiomers of 2-trans-ABA were not; they appeared to enter by passive diffusion. The uptake of (+)-ABA was linear over the first 8 hours but concentrations within the cells decreased after 2 hours to remain constant after 4 hours as rapid metabolism was induced. Electron microscopy of thin sections of the cells, combined with a stereological analysis of their shape, showed that the vacuoles comprised 80% of the cell volume and the cytoplasm plus nucleus comprised 20%. There were no photosynthetically active plastids in the cells. Concentrations of the endogenous ABA in the cytoplasm (pH 7.32) and vacuoles (pH 5.88) were calculated by applying the Henderson-Hasselbalch equation (ABA pKa 4.7) so that, provided no active metabolic redistribution occurred, the concentration in the cytoplasm was 7.9 micromolar and that in the vacuole was 0.3 micromolar. In vivo pH was measured by 31P nuclear magnetic resonance spectroscopy.
Full text
PDF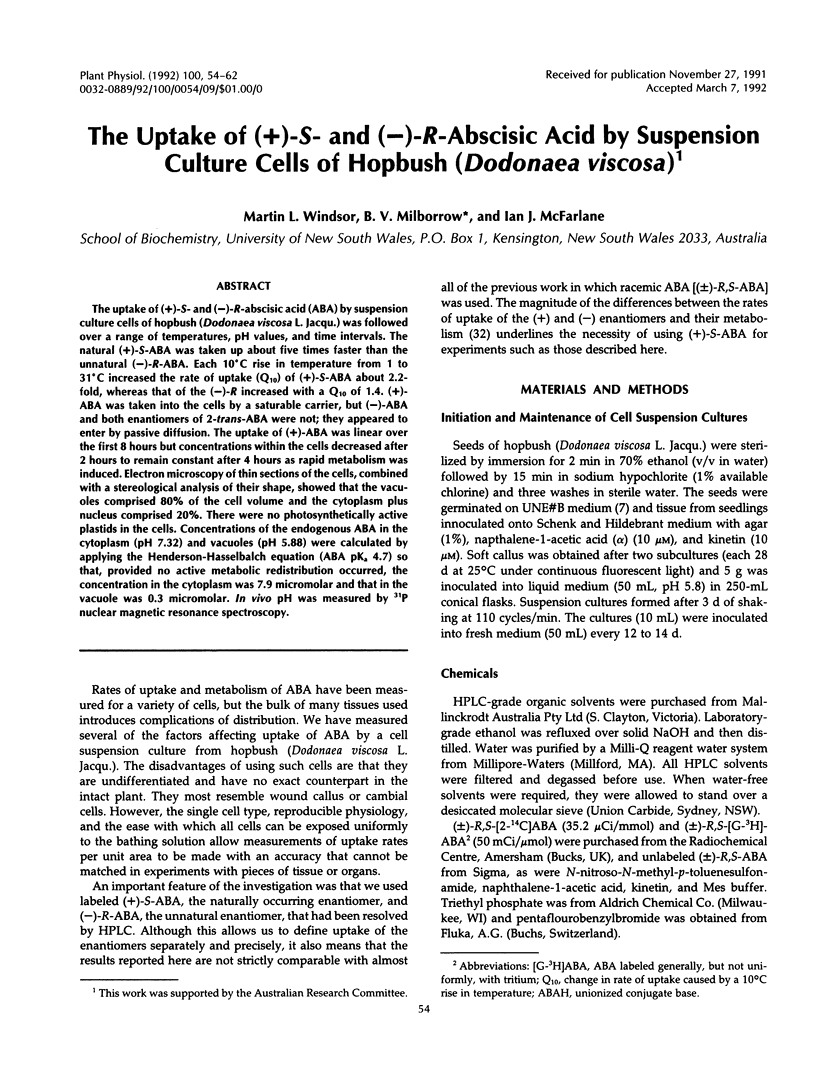




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco-Colomas J., Barthe P., Orlandini M., Le Page-Degivry M. T. Carrier-Mediated Uptake of Abscisic Acid by Suspension-Cultured Amaranthus tricolor Cells. Plant Physiol. 1991 Apr;95(4):990–996. doi: 10.1104/pp.95.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daddow L. Y. A double lead stain method for enhancing contrast of ultrathin sections in electron microscopy: a modified multiple staining technique. J Microsc. 1983 Feb;129(Pt 2):147–153. doi: 10.1111/j.1365-2818.1983.tb04169.x. [DOI] [PubMed] [Google Scholar]
- Daie J., Wyse R. ABA Uptake in Source and Sink Tissues of Sugar Beet. Plant Physiol. 1983 Jun;72(2):430–433. doi: 10.1104/pp.72.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z. Induced changes in permeability of plant cell membranes to water. Plant Physiol. 1972 Apr;49(4):602–606. doi: 10.1104/pp.49.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber M., Norris M. D., Kavallaris M., Bell D. R., Davey R. A., White L., Stewart B. W. Atypical multidrug resistance in a therapy-induced drug-resistant human leukemia cell line (LALW-2): resistance to Vinca alkaloids independent of P-glycoprotein. Cancer Res. 1989 Oct 1;49(19):5281–5287. [PubMed] [Google Scholar]
- Harris M. J., Outlaw W. H. Rapid adjustment of guard-cell abscisic Acid levels to current leaf-water status. Plant Physiol. 1991 Jan;95(1):171–173. doi: 10.1104/pp.95.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung W., Radin J. W., Hendrix D. L. Abscisic Acid Movement into the Apoplastic solution of Water-Stressed Cotton Leaves: Role of Apoplastic pH. Plant Physiol. 1988 Mar;86(3):908–913. doi: 10.1104/pp.86.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Hartung W. Uptake and Release of Abscisic Acid by Isolated Photoautotrophic Mesophyll Cells, Depending on pH Gradients. Plant Physiol. 1981 Jul;68(1):202–206. doi: 10.1104/pp.68.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Sondheimer E., Galson E. C., Chang Y. P., Walton D. C. Asymmetry, its importance to the action and metabolism of abscisic Acid. Science. 1971 Nov 19;174(4011):829–831. doi: 10.1126/science.174.4011.829. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]


