Abstract
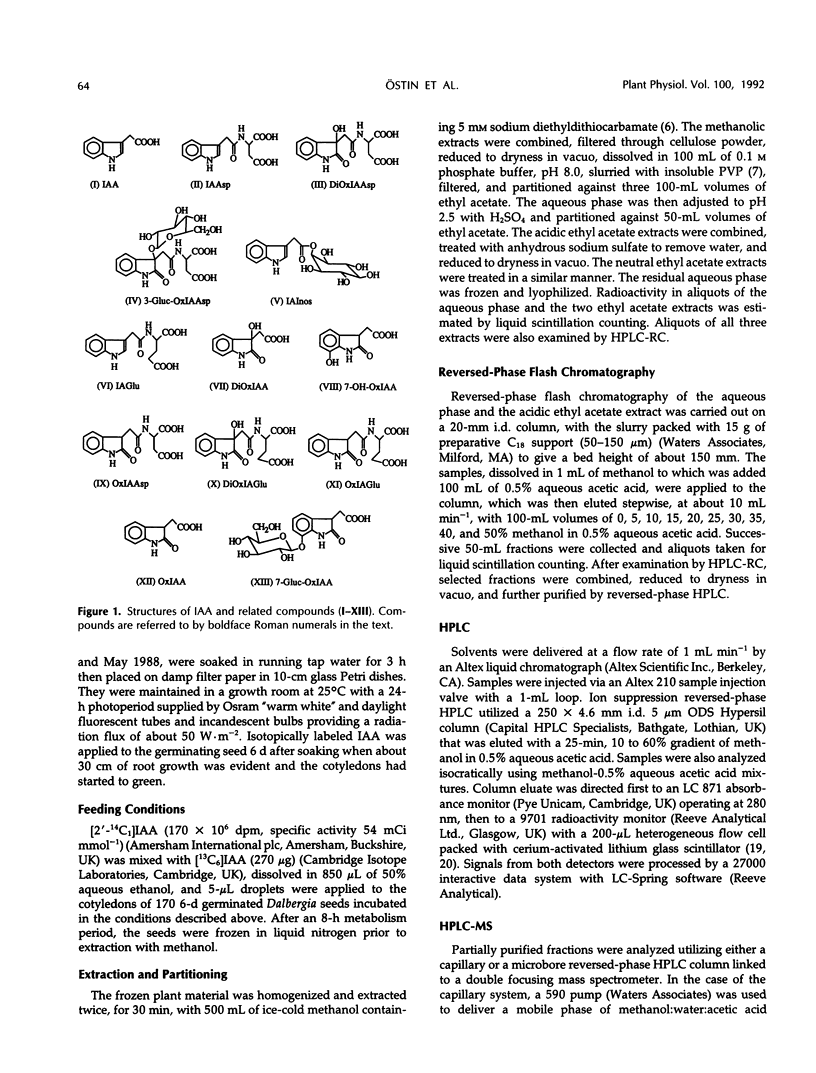
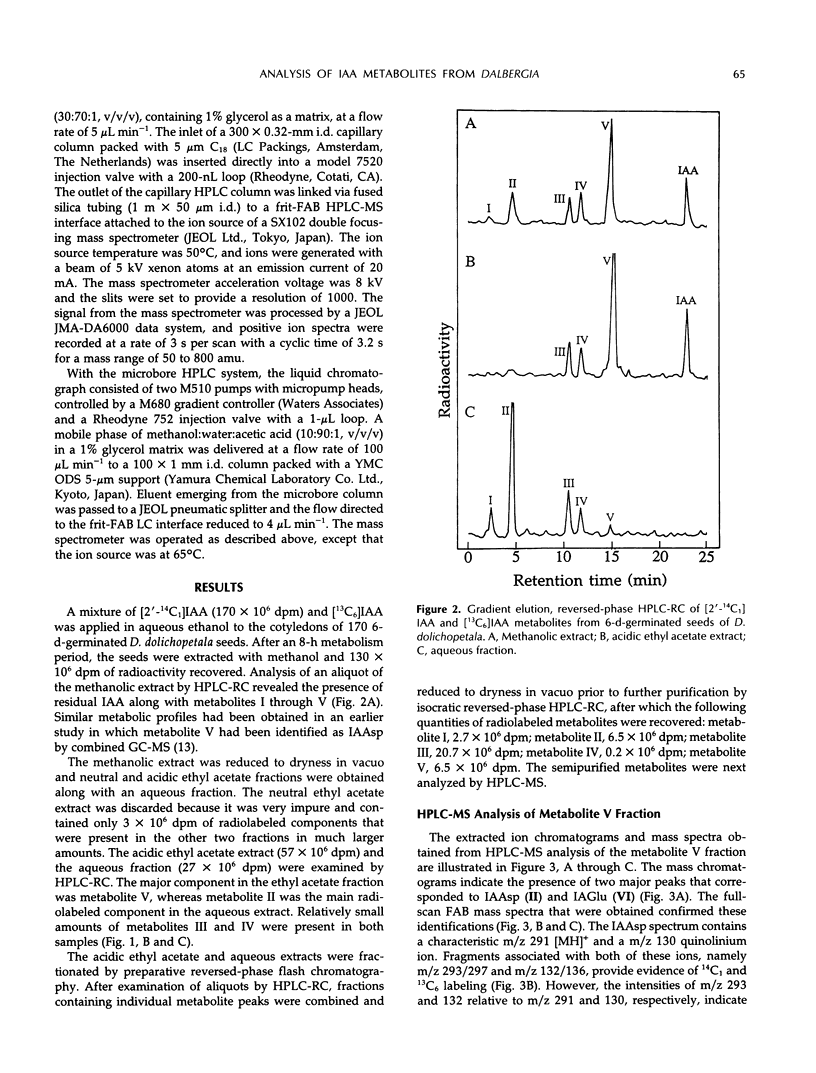
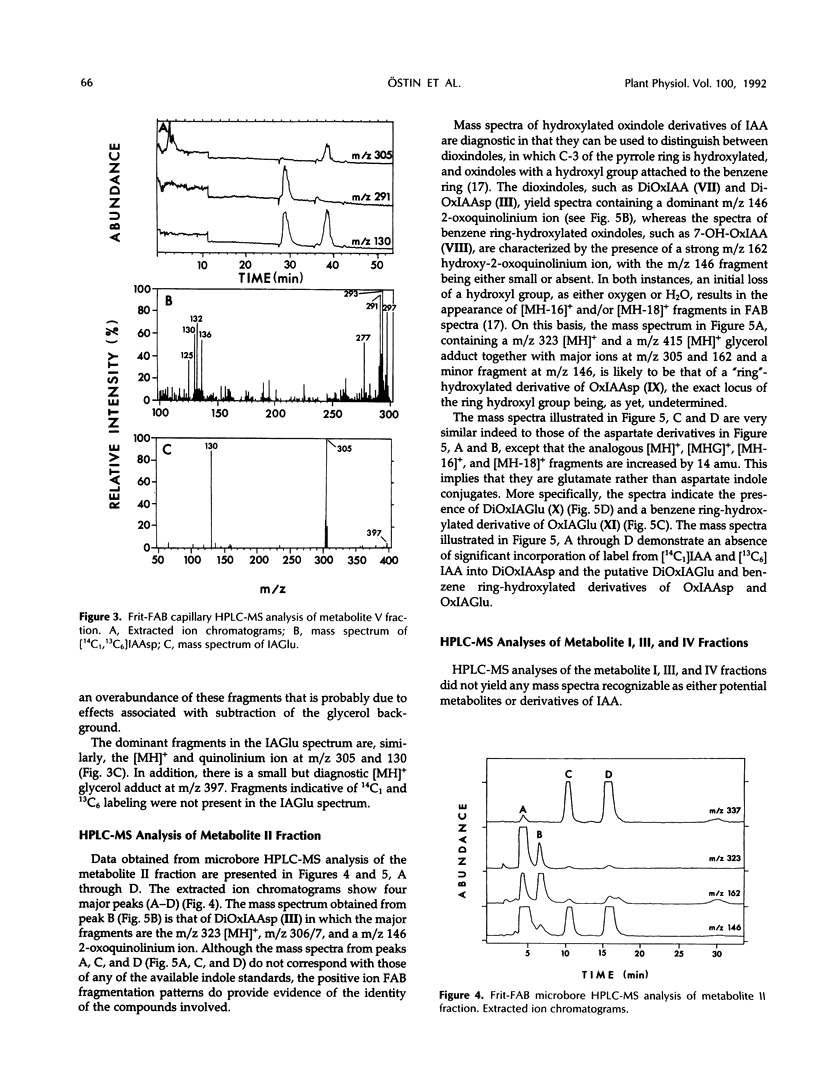
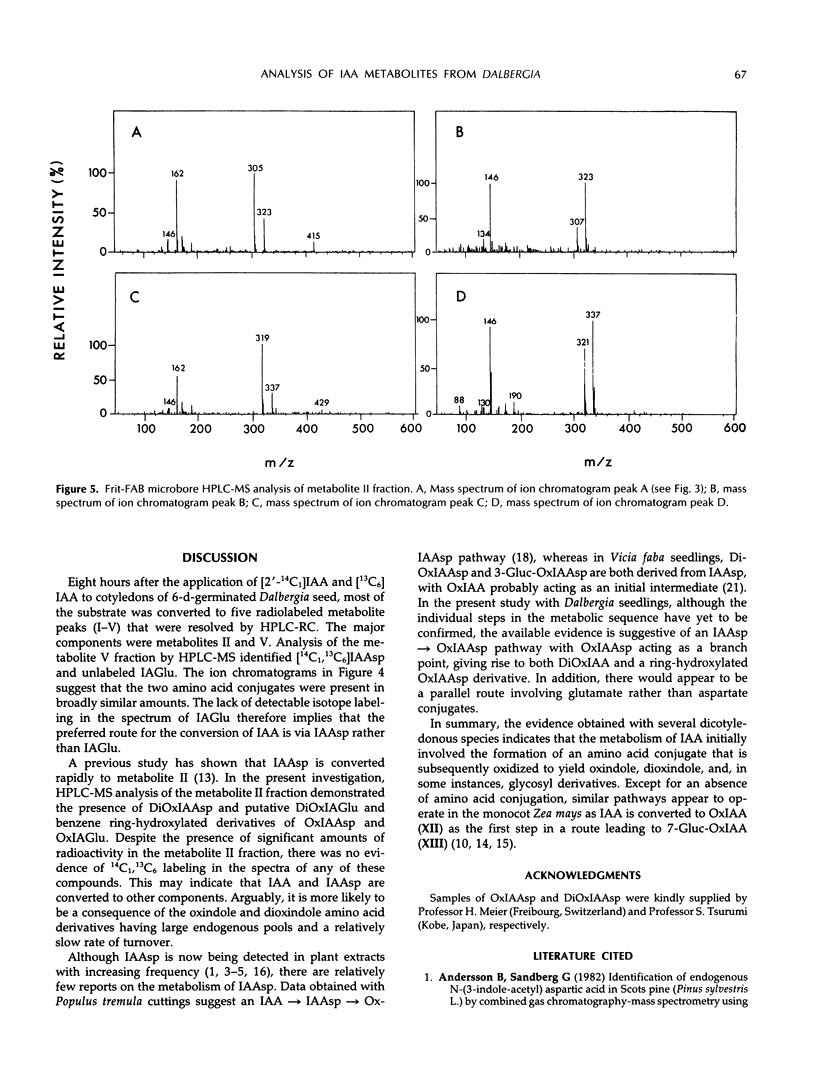
A mixture of [2-14C1] and [13C6]indole-3-acetic acid was applied to the cotyledons of 6-day-germinated seeds of “jacarandá do cerrado” (Dalbergia dolichopetala) and after 8 hours the seeds were extracted. Analysis of the fractionated extract by reversed-phase high performance liquid chromatography-radiocounting revealed the presence of five radiolabeled metabolite peaks (I-V). After further purification, the individual peaks of radioactivity were analyzed by combined high performance liquid chromatography-steel filter-fast atom bombardment-mass spectrometry. The metabolite fraction V was found to contain [14C1, 13C6]indole-3-acetylas-partic acid and unlabeled indole-3-acetylglutamic acid. Analysis of the metabolite fraction II revealed the presence of dioxindole-3-acetylaspartic acid and putative dioxindole-3-acetylglutamic acid as well as putative benzene ring-hydroxylated derivatives of oxindole-3-acetylaspartic acid and oxindole-3-acetylglutamic acid. There was no evidence of significant incorporation of label from [2′-14C1] or [13C6]indole-3-acetic acid into any of these conjugated indoles.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bialek K., Meudt W. J., Cohen J. D. Indole-3-acetic Acid (IAA) and IAA Conjugates Applied to Bean Stem Sections: IAA Content and the Growth Response. Plant Physiol. 1983 Sep;73(1):130–134. doi: 10.1104/pp.73.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D. Identification and Quantitative Analysis of Indole-3-Acetyl-l-Aspartate from Seeds of Glycine max L. Plant Physiol. 1982 Sep;70(3):749–753. doi: 10.1104/pp.70.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. J., Bandurski R. S. [3H]Indole-3-acetyl-myo-inositol hydrolysis by extracts of Zea mays L. vegetative tissue. Plant Physiol. 1986;80:374–377. doi: 10.1104/pp.80.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoszynski M., Bandurski R. S. Transport and metabolism of indole-3-acetyl-myo-inositol-galactoside in seedlings of Zea mays. Plant Physiol. 1986;80:961–964. doi: 10.1104/pp.80.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewer P., Bandurski R. S. Occurrence and metabolism of 7-hydroxy-2-indolinone-3-acetic acid in Zea mays. Phytochemistry. 1987;26(5):1247–1250. doi: 10.1016/s0031-9422(00)81790-2. [DOI] [PubMed] [Google Scholar]
- Nonhebel H. M., Bandurski R. S. Oxidation of indole-3-acetic acid and oxindole-3-acetic acid to 2,3-dihydro-7-hydroxy-2-oxo-1H indole-3-acetic acid-7'-O-beta-D-glucopyranoside in Zea mays seedlings. Plant Physiol. 1984;76:979–983. doi: 10.1104/pp.76.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonhebel H. M., Kruse L. I., Bandurski R. S. Indole-3-acetic acid catabolism in Zea mays seedlings. Metabolic conversion of oxindole-3-acetic acid to 7-hydroxy-2-oxindole-3-acetic acid 7'-O-beta-D-glucopyranoside. J Biol Chem. 1985 Oct 15;260(23):12685–12689. [PubMed] [Google Scholar]


