Abstract
Syzygium aromaticum, commonly called clove, is a culinary spice with medical uses. Clove is utilized in cosmetics, medicine, gastronomy, and agriculture due to its abundance of bioactive components such as gallic acid, flavonoids, eugenol acetate, and eugenol. Clove essential oil has been revealed to have antibacterial, antinociceptive, antibacterial activities, antifungal, and anticancerous qualities. Anti-inflammatory chemicals, including eugenol and flavonoids, are found in clove that help decrease inflammation and alleviate pain. The anti-inflammatory and analgesic qualities of clove oil have made it a popular natural cure for toothaches and gum discomfort. Due to its therapeutic potential, it has been used as a bioactive ingredient in coating fresh fruits and vegetables. This review article outlines the potential food processing applications of clove essential oil. The chemical structures of components, bioactive properties, and medicinal potential of clove essential oil, including phytochemical importance in food, have also been thoroughly addressed.
Keywords: Clove essential oil, Antioxidant properties, Therapeutic uses, nanoemulsion, Shelf life
1. Introduction
Syzygium aromaticum, sometimes known as clove, is a Myrtaceae family plant. Clove is economically farmed in Sri Lanka, India, Madagascar, southern China, and Indonesia. Clove Essential Oil (CEO) is a common seasoning for pastries, condiments, and sauces. It's also used in medicine, particularly in the preparation of gums and teeth. In coastal places, clove trees are regularly planted up to 200 m above sea level. Flower buds, the tree's commercialized part, start to grow four years after it is planted. Flower buds are harvested all during the maturation stage, prior to blossoming. According to Filho et al., 2013 the collection could be carried out chemically or manually using a natural phytohormone that causes early maturation by producing ethylene in the vegetal tissue [1,2]. The biological qualities of clove bud oil include being antifungal, insecticidal, antibacterial, and antioxidant, and it has long been used in food as a flavoring and antimicrobial component. CEO has a large amount of eugenol, which has antimicrobial characteristics. According to Chaieb et al. (2007), the primary ingredients of essential oil of clove flower buds are phenylpropanoids such as cinnamaldehyde, eugenol, carvacrol, and thymol [3]. The main bioactive component of clove, hydroxy eugenol, has concentrations that range from 9381.70 to 14650.0 mg per 100 g of oil. Clove is high in phenolic compounds such as hydroxycinnamic acids, hydroxyphenyl propens flavonoids, and hydroxybenzoic acids (Cortés-Rojas et al., 2014). CEO consists mostly of phenylpropanoids, like eugenol and derivatives of eugenol, with negligible levels of the chemical components - humulene and - caryophyllene (El et al., 2015). Clove essential oil is beneficial in the pharmaceutical, active packaging, cosmetics, biomedical, culinary, and sanitary industries because of its physiological properties, which include antiseptic, antibacterial, antioxidant, anticarcinogenic, pesticide, & analgesic action. CEO is frequently utilized in food as a spice, preservative, and natural colorant [4].
The possibility of encasing CEOs in films, microcapsules (MCs) and nanocapsules (NCs), or microparticles and nanoparticles (MPs and NPs), nanocomposite materials has been raised. These methods improve CEO's stability in aqueous media, which increases their bioavailability, lessens their negative effects, provides a controlled release for the encapsulated material, protects them from the environment, or masks their pungent odour. Because of their corresponding increases in reactivity and larger surface-to-volume ratios, nanocarriers and microcarriers with customized features are particularly appealing (Pandey et al., 2022). These systems frequently depend on polymers, lipids, or a mixture of two other substances. Furthermore, when it comes to some factors, microcarriers and nanocarriers differ from one another with their entry into cells, potential tissue reactions, behavior after application, and capacity to cross particular biological barriers, which, depending on the application, will affect the decision to select one over the other. Encapsulation has been used to increase the value addition of the CEO by achieving controlled release, suggesting new applications, and increasing its physical-chemical stability and shelf life. Numerous encapsulation methods have been developed and documented in the literature that use a variety of carriers, often with unexpected results. The end results could be micelles, Emulsions, complexes, liposomes, or capsules. Most studies have concentrated on enhancing its bioavailability and stability and maintaining its valuable biological characteristics during storage and processing. In contrast, some have highlighted its effectiveness against certain bacterial strains. This review article outlines the various applications of clove essential oil. The molecular structures, bioactive properties, medicinal potential, and phytochemical value of clove essential oil were all investigated. A brief description of nanoemulsion, which has the same protective properties as essential oils for fruits and vegetables, is also provided in this article.
2. Chemical composition
Numerous lifestyle-related risk factors have long been treated and prevented with several medicinal plants that have phytogenic components. Traditional medicine has recommended extracts from numerous plant parts to treat inflammatory diseases, cardiovascular diseases, pneumonia, diarrhea, skin conditions, cancer, hyperlipidemia, liver problems, and arthritis [5]. These plants are believed to be effective due to the numerous bioactive substances they contain. The most common plant-based functional ingredients having actual health benefits and a variety of uses in the creation of nutraceutical and functional foods are phenols, tocopherols, sterols, flavonoids, and organic acids. This criticism focuses on eugenol's medicinal potential to treat frequently persistent disorders [6]. The composition and properties of clove essential oil are presented in Table 1.
Table 1.
Composition and properties of clove essential oil.
| Compound | Amount (%) | Chemical Structure | Properties | Uses & Application | References |
|---|---|---|---|---|---|
| Eugenol | 74.28 | 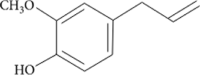 |
Phenylpropanoid compound volatile in nature low water solubility |
Antimicrobial Insecticidal Anti-inflammatory Wound healing Antioxidant Anticancerous against breast, prostate, colon, gastric, and skin cancer |
[43] |
| β caryophyllene | 24.8 | 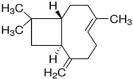 |
Sesquiterpene Found in clove, hemp, black pepper, guava leaves Insoluble in water but soluble in ethanol |
Anti-carcinogenic Anti-inflammatory Anxiolytic Antioxidant Anaesthetics effects Act as a Chemosensitizer |
El saber et al., 2020 |
| α humelene | 3.1 | 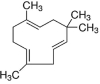 |
Found in Senecio brasiliensis S. aromaticum L., Humulus lupulus L Sesquiterpene |
Anti-tumour activity against in colon, prostate, lung, and breast cancer Antiproliferative activity Anticancerous bioactivities |
[44] |
| Eugenol acetate | 2.7 | 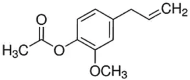 |
Phenylpropanoid Second in abundance in clove after eugenol Crystalline solid Yellowish fluid with fruity tint |
Antimicrobial Anti-bacterial Use in perfumes because of volatile nature Anti-inflammatory |
Hu et al., 2022 |
| α Copaene | 0.17 | 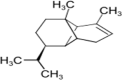 |
Sesquiterpene hydrocarbon Colourless, clear and viscous in nature |
Antimicrobial Antiproliferative Anti-genotoxic Anti-oxidant Cytotoxic activity |
[45] |
| Chavicol | 0.08 | 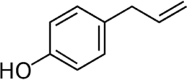 |
Natural phenylpropene Organic compound Colourless liquid Found together with terpenes |
Use in perfumes Act as analgesic Anti-inflammatory Anti-toxic |
Kose et al., 2021 |
| Methyl salicylate | 0.20 | 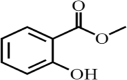 |
Organic ester Colourless, viscous liquid having fruity tint |
Flavoring agent Metabolite Antibacterial Ant irritant Antiproliferative |
Alfkri et al., 2020 |
| 4-Allylanisole | 0.13 | 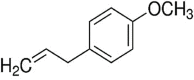 |
Phenylpropene Natural organic compound Colourless liquid |
Use in essential oil Perfume making Medicinal benefits Anticarcinogenic Antigenotoxic |
Wei et al., 2016 |
| Benzyl acetate | 0.07 | 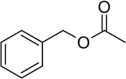 |
Organic ester Possess sweet and pleasant aroma |
Application in personal hygiene and health care products Industrial use in extraction Anti-irritant Antimicrobial |
[46] |
2.1. Eugenol
Several different plant sources for eugenol, a phenolic compound, including cinnamon extract, nutmeg oil, clove oil, and other plants. It has good health benefits, making it a useful natural component. The first-time eugenol was isolated from clove leaves and buds or Eugenia caryophyllata [7]. The pharmacological properties of eugenol have been claimed to include antioxidant capacity, antibacterial, neuroprotective ability, hypolipidemic efficiency anti-inflammatory action, anti-carcinogenic effects, and anti-diabetic effectiveness. The GRAS (generally recognized as safe) has designated eugenol and the World Health Organization (WHO) has determined that it is not mutagenic [8]. Numerous plants contain eugenol, including tulsi leaves, pepper, ginger, oregano, cinnamon bark and leaves, clove buds, and thyme. Additionally, a number of other aromatic plants, such as basil, mace, nutmeg, marjoram, and bay, should have a large eugenol content. Eugenol is primarily found in aerial plant parts like flowers, leaves, and bark because these sections also contain many essential oils. Additionally, there is a substantial amount of eugenol in tulsi leaves, often between 41 and 72 %. However, the amount of eugenol in numerous plant sections fluctuates according to the season. According to studies, fall harvests of eugenol produce the highest yields when compared to summer types [9].
2.2. Eugenyl acetate
A eugenol derivative known as eugenyl acetate has antibacterial, anticancer, antimutagenic, antioxidant, and antivirulence properties. It inhibited Rhizoctonia solani, Harpophora oryzae, and Fusarium moniliforme with 94.5, 92.1, and 100 %, respectively, at 200 g/mL. Eugenyl acetate, a capable antioxidant, demonstrated a 90.31 % DPPH free radical scavenging rate at a concentration of 35 gm/mL as well as 89.31 % No scavenging for free radicals at a concentration of 60 g/mL (Cavar et al., 2021). It also decreased the capacity to build biofilms and showed potential antifungal activity against Candida species. According to Gioffre et al. (2020), human scabies mites are highly poisonous to the substance. At 0.3 g/mL, eugenol acetate proved to be 100% harmful to Artemia salina. Eugenyl acetate low fatal quantities, is also hazardous to other creatures, like insect larvae that spread disease. The larvicide potential of eugenyl acetate against Aedes aegypti was demonstrated by its LC50 value of 0.01 mg/ml. The octopinergic system's interference is the primary cause of larvicidal activity. Its demand has grown in the cosmetic and food industries in light of its anticancer, antioxidant, antibacterial, and larvicidal characteristics (Golmakani et al., 2017).
2.3. β-Caryophyllene
Eugenia cuspidifolia, Eugenia tapacumensis, hemp (Cannabis sativa L.), Clove (Syzygium aromaticum), black pepper (Piper nigrum L.), and guava leaves all contain the sesquiterpene caryophyllene (Psidium cattleianum Sabine). Caryophyllene is ethanol soluble but not water soluble. It has proven to have local anesthetic effects, antibacterial, anxiolytic-like, antioxidant, anticarcinogenic, anti-inflammatory effects, and anticancer activities, including those against breast, cervical, prostate, and pancreatic cancer [10]. These studies show that caryophyllene suppresses colon cancer cell growth and proliferation, interferes with the stages of tumour formation, and decreases extracellular matrix metalloproteinase activity. Caryophyllene has the ability to chemosensitize, enhancing the effectiveness of drugs against cancerous cells. Additionally, it works well against Anopheles subpictus, Culex tritaeniorhynchus, Aedes albopictus, and (LC50 values of 41.66 g/mL, 44.77 g/mL, and 48.17 g/mL, respectively). According to Ref. [11]; the radical scavenging capacities of -caryophyllene using the FRAP and DPPH scavenging methods, respectively, were around 1.25 and 3.23 M. These findings suggest that -caryophyllene has a strong antioxidant capacity.
2.4. α-Humulene
Senecio brasiliensis, Salvia officinalis L., Humulus lupulus L., and S. aromaticum L. all contain the sesquiterpene humulene. The anticancerous and anti-inflammatory properties of the substance have been demonstrated in prostate, lung, colon, and breast cancer. Several investigations showed colon cancer cells showed altered mitochondrial cell membranes and antiproliferative activity [12]. Additionally, it can enhance the additional anticancer bioactivities and antiproliferative effects of cytostatic medications. According to Ref. [13] a drug-metabolizing enzyme, the CYP3A enzyme is inhibited by -humulene in the liver microsomes of rats and people [14]. In model rats and mice, oral therapy with humulene and caryophyllene (50 mg/kg) displayed equivalent anti-inflammatory benefits to therapy with dexamethasone. Humulene prevents TNF synthesis, but -Caryophyllene only slows its release. Cyclooxygenase, nitric oxide synthase, and Prostaglandin E2 production are also decreased, as is their inducible expression. - Humulene was safe for Gambusia affinis as demonstrated (LC50 = 1024.95 g/mL). However, it shows a larvicidal effect against the three vector mosquitoes, Culex tritaeniorhynchus, Aedes albopictus and Anopheles subpictus (LC50 = 10.26 g/mL, 11.15 g/mL, and 12.05 g/mL, respectively). It displayed larvicidal EC50 of 77.10 gm/mL and LC50 of 20.86 gm/mL against eggs of Helicoverpa armiger. Additionally, the usefulness of humulene towards insect species that feed on products in storage has been examined. With an LC50 value of 4.61 L/mL and a reduction in respiration rate at 1–3 h once exposed, Sitophilus granarius was toxic to humulene [15].
3. Extraction of CEO
3.1. Solvent extraction
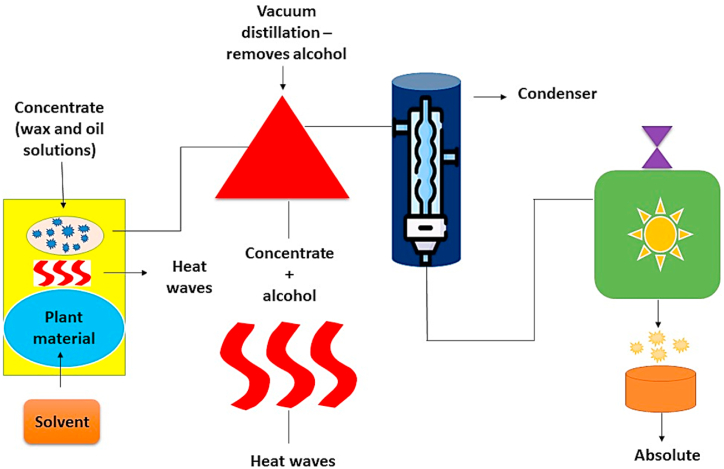
Solvent extraction is one of the most well-known and widely utilized processes to extract essential oils from plants. In order to extract eugenol, various solvents such as ether, n-hexane, ethanol, petroleum, and methanol are used. The main negatives of solvent extraction are that it alters the flavor of the food and leaves behind additional soluble residues [16]. However, employing this technique, other essential oils and eugenol can be obtained from a number of volatile herbs. The clove buds are smashed and covered in filter paper for the classic solvent extraction of eugenol using clove buds. The extraction process is then applied to the filter paper and placed into a 500 mL capacity reflux flask. In a Soxhlet apparatus, extraction is performed with a suitable organic solvent. The process is completed by utilizing a rotary vacuum evaporator to concentrate the extracted extracts at 50 °C. The standard solvent extraction approach has undergone a number of advancements in comparison to the original method, increasing efficiency. Soxhlet extraction is less desirable than batch extraction, for instance. This technique makes use of a reactor with a four-bladed agitator and a motor operating at 1200 rpm. In a recent study [17], used methanol as a solvent to extract eugenol from tulsi plant leaves and reported excellent extraction efficiency. Additionally, they asserted that the rate of agitation had no bearing on the effectiveness of eugenol extraction [18]. The diagrammatic representation of solvent extraction method is presented in Fig. 1.
Fig. 1.
Diagrammatic representation of Solvent extraction.
3.2. Hydro distillation
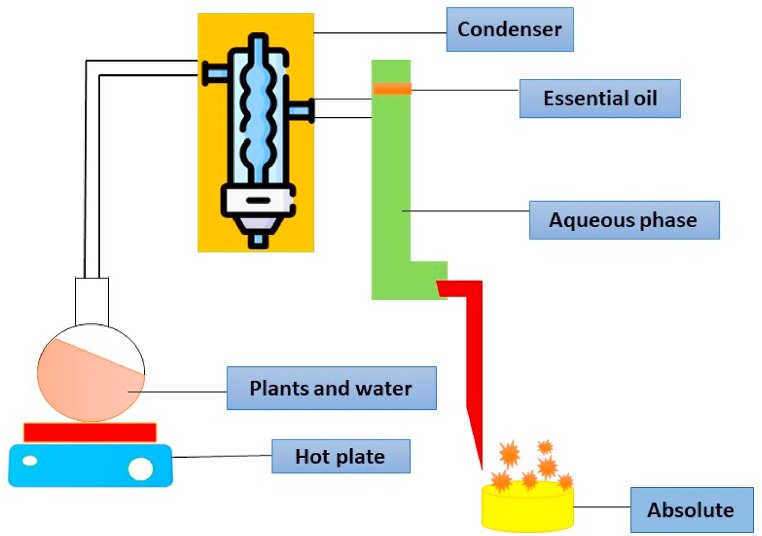
Hydro-distillation is among the most practical methods to get essential oils (Li et al., 2020). During the hydro distillation procedure, the dried powder (100 g of dried and crushed clove buds) is steeped in water. A dried clove sample is placed in a 500 mL volumetric flask and allowed to sit for four to 6 h to perform hydrodistillation. The addition of petroleum ether or other consisting of organic solvent before collecting and saturating the volatile distillate with sodium chloride. Using anhydrous sodium sulphate, Dehydrating and separating the hydro and ether compounds. The sample is then heated to 60 °C in the water bath to recover the ether and concentrate the extract. With eugenol values ranging from 50.5 to 53.5%, the average hydrodistillation yield is about 11.5% [19]. Reducing the clove bud powder's particle size can increase the extraction yield [20]. The pictorial presentation of hydro distillation extraction process is shown in Fig. 2.
Fig. 2.
Method of Hydro distillation extraction.
3.3. Microwave-assisted extraction of eugenol
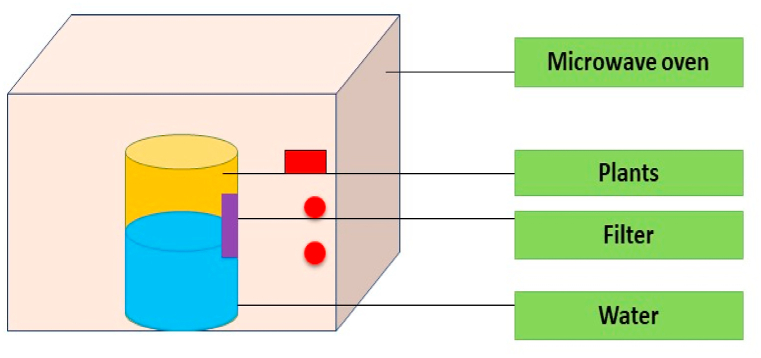
Hydrolysis, leaching, and the effects of heat on various scent ingredients are all issues with traditional methods for extracting eugenol from several sources of plants (Saricaiglu et al., 2019). To address these concerns, Modern extraction techniques have come up with a number of ways to achieve high extraction yields with less processing time and energy. Microwave-assisted extraction is one of these technologies (MWAE). It is acknowledged as a natural extraction technique capable of creating eugenol and other volatile oils with comparable sensory characteristics and quality to those obtained using conventional techniques [21]. The process of microwave assisted extraction is presented in Fig. 3.
Fig. 3.
Microwave assisted extraction.
3.4. Supercritical carbon dioxide extraction
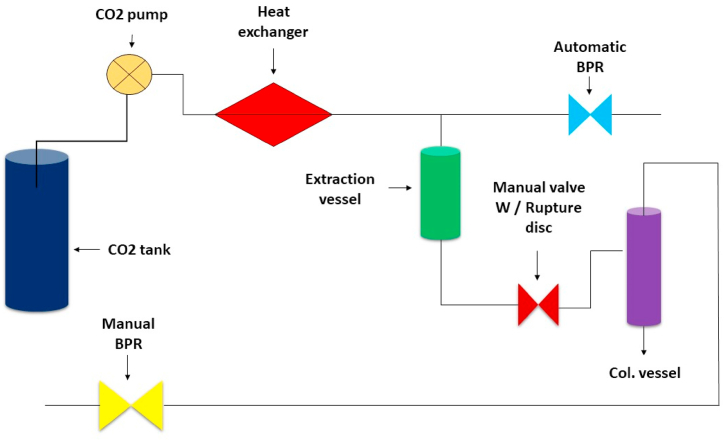
Supercritical CO2 is another efficient method for extracting eugenol from clove buds (Jonusaite et al., 2021). The effectiveness of SC-CO2 extraction has been investigated in a number of investigations. Han et al., 2017 compared the efficacy of SC-CO2 extraction and liquid and discovered that SC-CO2 extraction generated a high clove oil extraction rate. Similarly [22], found that increasing eugenol synthesis during supercritical extraction can increase the CO2 flow rate. In addition [23], compared the characteristics of the SC-CO2 extracted clove bud oil to those extracted by other standard procedures and found that the two methods gave remarkably similar results. Although various studies have demonstrated the effectiveness of SC-CO2 extraction for the extraction of CEO, few studies have focused on improving the yield of SC-CO2 extracted eugenol. As a result, more research is necessary to show that the SC-CO2 extraction method can successfully extract eugenol from clove oil. With the use of ultrasonography, eugenol may be extracted. Even though many non-traditional approaches successfully alleviate the issues associated with traditional methods, their extraction performance varies. Another environmentally friendly extraction method, ultrasound (US) extraction, was recently introduced, significantly accelerating the extraction process while consuming less energy [24]. compared the efficiency of traditional maceration to ultrasound-assisted extraction for clove oil extraction. They employed three various flow modes for US extraction (450, 900, and 1350 mL min−1), with the 1350 mL min−1 flow mode yielding the highest extraction rate. [25]; for example, used a central composite design to extract clove essential oils using a US extraction method (CCD). The study's independent variables were temperature (32–52 °C), plant concentration (3–7%), and extraction time (30–60 min). The dependent variable was clove extract. The frequency of the ultrasonic bath was set to 53 kHz. The temperature had a substantial impact on extract production, according to the findings of the study. The extract contained eugenol, acaryophyllene, and 2-methoxy-4-(2-propenyl) phenol acetate. This research discovered that ultrasound extraction is a beneficial technology that could lead to commercial applications. The flow diagram of supercritical carbon dioxide extraction is shown in Fig. 4.
Fig. 4.
Flow diagram of Supercritical Carbon Dioxide Extraction.
4. Bioactive characteristics of Clove essential oil
4.1. Anticancer activity
In cancer cell lines, clove essential oil has been demonstrated to exhibit cytotoxic or antimutagenic effects. On colon cancer, the HCT 116 cells (IC50: 31 g/mL), breast adenocarcinoma MCF-7 cells (IC50: 29.7 g/mL), and hepatocarcinoma HepG2 cells (IC50: 18.70 gm/mL) clove flower buds had powerful cytotoxic effects [26]. It has been studied that ethanol, water, and oil-based clove extracts have cytotoxic effects on cervical cancer. Oesophageal cancer TE-13 cells, prostate cancer DU-145 cells, HeLa cells, and breast adenocarcinoma MDA-MB-231 and MCF-7 cells were all negatively impacted, with normal human peripheral blood lymphocytes seeing just minimal impacts (Dwivedi et al., 2011). As a result, clove extract could be used as a cancer treatment herb, with 14 seeming one among the bioactive constituents [27]. The SOS response is a global response to DNA damage that stops the cell cycle and activates DNA repair. Clove methanol extract suppresses the SOS-inducing action of the mutagen furylfuramide in Salmonella typhimurium TA1535/pSK1002 [28]. Clove seed extracts were also found to have antimutagenic pr Cloves were found to inhibit the carcinogenesis process in a mouse model of skin cancer brought on by 9,10-dimethylbenz(a) anthracene [29]. The properties with antimutagenic activity vary from 34.11 to 79.74 % towards two mutant bacterial strains, S. typhimurium TA100 and S. typhimurium TA98 [30].
4.2. Antidiabetic activity
Numerous studies examined clove's potential as an antidiabetic medication. It has been found that clove and insulin work together in the same way to control the expression of genes linked to diabetes, such as glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK) [31]. Alpha-glucosidase and alpha-amylase are carbohydrate hydrolyzing enzymes that are present in the pancreas of rats. The dose-dependent inhibition of glucosidase highlights their medicinal potential for type 2 diabetes [32]. also demonstrated the impact of clove bud powder on metabolic indicators in a type 2 diabetes rat model, demonstrating its ability to lower hyperglycemia in type 2 diabetes settings [32]. S. aromaticum extracts have also been shown in animal models to increase blood sugar levels via muscle glycolysis and mitochondrial activity by regulating the AMP-activated protein kinase and sirtuin-1 pathways [33]. An ethanol extract found in the flower buds of this plant can significantly suppress glucose spikes in type 2 diabetic KK-A (y) mice, suggesting that it may be employed as a functional food component to treat type 2 diabetes. Dehydrodieugenol and dehydrodieugenol B, which have strong ligand-binding properties for the human PPAR-, and compound 14 to a lesser extent, are among the known bioactive components of the extract [34].
4.3. Antinociceptive and anti-inflammatory activities
In non-cytotoxic quantities, clove has anti-inflammatory and immunomodulatory effects on mouse macrophage cytokine production [35]. Interleukin (IL)-1, IL-6, and IL-10 production was effectively reduced in one investigation at a concentration of 100 g per well, All cytokines were produced either before or after the lipopolysaccharide challenge; compound 1 decreased the production of IL-10 and IL-6 but had no effect on the production of IL-1 [35]. One of the key components of clove oil, -caryophyllene (3), has also been shown to have local anesthetic properties. The molecule can dramatically diminish electrically Generated dose-dependent convulsions of the rat's phrenic hemidiaphragm. With eugenol being the main component responsible for the antinociceptive properties, clove has been used as an analgesic for toothaches, joint discomfort, and antispasmodic since the 13th century. It is believed that the process by which ganglionar cells activate calcium and chloride channels evolved [2]. The voltage-dependent activities of eugenol influenced clove's analgesic efficacy on sodium and calcium channels and receptors expressed in the trigeminal ganglio [36]. Other research suggests that clove's ability to operate as a capsaicin agonist accounts for its analgesic effects [37] (see Table 1).
4.4. Antimicrobial activity
A number of different bacteria and fungus species have been demonstrated to be resistant to clove's antibacterial effects. Researchers investigated the antibacterial properties of cinnamon, mustard, mint, ginger, clove, and garlic, among other Indian spices [38]. Only sample that completely eliminated all foodborne pathogens tested Bacillus cereus, Staphylococcus aureus, and E. coli, was the clove aqueous extracted at a concentration level of 3%. A 1 % concentration of clove extract exhibited a significant inhibitory impact. At 1 % concentration, clove extract showed a potent inhibitory effect. Thyme, oregano, and clove have the broadest ranges of action. Clove, oregano (Origanum vulgare), pimenta racemosa, and thyme (Thymus vulgaris) essential oils' antibacterial effects against E. coli are inhibited to varying degrees O157:H7 [39]. Depending mostly on spore and micelle lysis, chromatographic tests showed that eugenol was the main ingredient relevant to the antimicrobial impact. A similar mode of action for eugenol-induced membrane rupture and macromolecule deformation was reported by Ref. [40]. (Fu et al., 2007). Eugenol and carvacrol were examined for their anticandidal action in microbiological and histological methods and the vaginal candidiasis model was utilized to compare the samples to the controls. According to the research, carvacrol, and eugenol may be effective antifungal medications for managing and preventing vaginal candidiasis [41]. In addition to eugenol's extensive range of antibacterial effects, a study discovered that at a concentration of 2 g/mL, both eugenol or cinnamaldehyde retarded the growth of 31 strains of Helicobacter pylori after 9–12 h of incubation, respectively, and were more effective than amoxicillin without leading to resistance. Helicobacter pylori lives in the stomach, so its action and stability were studied at low pH levels [42]. The antimicrobial nanoemulsions advantages in food products are presented in Table 2.
Table 2.
Antimicrobial nanoemulsions advantages in food products.
| Food products | Contaminating Microorganisms | Antimicrobial Nanoemulsions | Advantages | References |
|---|---|---|---|---|
| Meat products |
C. jejuni E. coli Salmonella S. aureus |
Oregano oil Geraniol, linalool Chitosan/Cinnamon oil Bunium persicum Boiss (BEO) and Zataria Boiss (ZEO) |
Ensure that these products' microbiological development is under control These nanoemulsions can be used as coatings, dips, sprays, coatings, or films on meat and fish items |
[47] |
| Milk and Milk product | Fennel (Foeniculum vulgare) oils Sea buckthorn (Hippophae rhamnoides) Basil (O. basilicum) Mint (M. piperita) |
Low-fat cheese's shelf life can be increased by using oregano oil-based nanoemulsion antimicrobial edible coatings, which stop the growth of yeasts, moulds, and bacteria Because of the antibacterial and antioxidant properties of the sea buckthorn, fennel, mint, basil, and m. piperita oils that were encapsulated in sodium alginate hydrogels, yogurt's quality features and shelf life were improved |
Take advantage of using antimicrobial nanoemulsions Casein-stabilized thymol oil nanoemulsions are efficient antimicrobial agents in dairy products After 48 h of storage, reduced fat milk showed a greater suppression of L. monocytogenes (5 log CFU/ml) than the full fat milk (3 log CFU/ml). |
[48] |
| Fish and Fish products | S. Paratyphi A Photobacterium damselae S. aureus Pseudomonas luteola Vibrio vulnificus K. pneumoniae Proteus mirabilis Serratia liquefaciens E. faecalis |
The antibacterial activity of thyme oil nanoemulsions against different food-borne diseases has been demonstrated. Strong antibacterial action against foodborne spoilage organisms or pathogens like S. paratyphi A, E. faecalis and S. aureus was demonstrated with sage oil nanoemulsions. |
Extending shelf life and decreased populations of lactic acid and heterotrophic bacteria in fish steaks formulated with sunflower oil nanoemulsion The shelf life and microbiological load of sea bream as well as sea bass fillets treated with sunflower oil nanoemulsions were both increased. Utilizing antimicrobial nanoemulsions which are plant-based as preservatives will increase the fish products' safety, shelf life, and quality. |
[49] |
| Cereals based products | Microorganisms prone to deterioration, such as moulds, yeast, and bacteria | Methylcellulose films infused with nanoemulsions of oregano oil (Origanum vulgare) or (S. aromaticum) clove bud oil Eugenol Origanum majorana L. oils α-terpineol-loaded chitosan nanoemulsion |
preventing the development of yeast and mould on sliced bread Strong antibacterial action against A. flavus was demonstrated by Myristica fragrans oil encapsulated within chitosan nanoparticles in preserved rice. Antifungal activity Anti-aflatoxigenic activity |
[50] |
| Fruits and Vegetables |
L. innocua B. cinerea S. Typhimurium E. coli |
Thymol oil Oregano oil Mint (M. piperita) Basil (O. basilicum) Sea buckthorn |
Chitosan-based edible coatings Mandarin oil Thymol nanoemulsions prevented the development of mould on chilled tomatoes. Freshly cut Fuji apples with better safety and quality thanks to lemon oil nanoemulsions. (B. cereus) a Gram-positive bacteria and Gram-negative bacteria were significantly reduced in their development by geraniol and carvacrol-loaded nanoemulsions (E. coli) |
[51] |
4.5. Antiviral activity
In general, essential oil constituents are toxic to viruses; however, phenylpropanoids, monoterpenals, and monoterpenols, have demonstrated antiviral efficacy in vitro. Syzygium aromaticum extract was reported to be particularly effective at inhibiting hepatitis C virus replication (90 % inhibition at 100 g/mL) by Ref. [52] (Hussein et al., Hussein). Kurokawa et al. (1998) isolated and identified an anti-HSV chemical, eugeniin, from Syzygium aromaticum extracts, which inhibited HSV-1 DNA polymerase activity with specificity [53].
4.6. Antioxidant capacity
Plant phenolics, which are found in all parts of the plant, including the seeds, fruit, and leaves, are a rich source of natural antioxidants. Eugenol, the primary component of clove oil, is responsible for many of the antioxidant advantages [54]. Scavenging free radicals and chelating metal ions are two methods for producing antioxidant activity. Eugenol has high antioxidant and photocytotoxic capabilities in addition to being suspected to be engaged in photochemical reactions [54,55]1) [56]. discovered that 0.005% clove oil has the same antioxidant activity as 0.01% butylated hydroxytoluene. Due to its strong chelating capacity for Fe+3, it can stop the production of hydroxyl radicals [56]. Clove oil has potent antioxidant capabilities and can be used in both pharmacological applications as well as a handy source of natural antioxidants [57].
4.7. Insecticidal activity
Medicines used to control parasitic arthropods face a number of obstacles, including drug resistance and environmental pollution. Eugenia caryophyllata oil has been studied for biological activity against a wide variety of parasites. It has been demonstrated to have an insecticidal effect against Anopheles dirus mosquitoes, Pediculus capitis, Culex pipiens larvae, Sitophilus zeamais and Tribolium castaneum, as well as to decrease the offspring growth of Sitophilus zeamais when combined with isoeugenol [58]. Eugenol, an acaricidal substance found in clove essential oil, has been reported to have acaricidal effects on Dermatophagoides pteronyssinus and Dermatophagoides farinae. Additionally, eugenol congeners were discovered to be effective acaricides against the mite species [59]. According to one study, clove oil is a new fumigant that could be used to combat Japanese termites [60].
5. Applications of Clove essential oil
5.1. Food Sector
Compared to synthetic antioxidants, it is used as a protective antioxidant in baked goods, slows down oxidation, and reduces the formation of oxidation products [61]. In comparison to ginger and garlic, clove powder was integrated at 0.2%. Until the conclusion of storage, raw chicken meat emulsion was demonstrated to have the lowest TBA value [62]. At 7–9 °C, The shelf life of paneer packaged in LDPE was five days, while samples treated with cloves had a shelf life of ten days [63]. It is strongly advised to utilize paneer because it is unstable and has a limited lifespan.
5.1.1. Baked foods
The baked food industry places a high priority on upholding nutrition and safety while also limiting the spread of mould. Some methods for preserving baked products include irradiation, altered storage conditions, preservative acids, and aseptic packing. However, due to their detrimental effects on human health, the use of organic acids (such as benzoic, propionic, and sorbic acids) has been prohibited in many nations [64]. In contrast to harmful bacteria such Aspergillus species, Escherichia coli, Penicillium species, and Staphylococcus aureus, eugenol gives CEO broad-spectrum activity. Its addition can lengthen the time baked goods can be stored without losing their natural flavour, texture, taste, appearance, or sensory acceptance [65].
5.1.2. Milk products
Consumption of dairy products like cheese has been connected to outbreaks of foodborne illness. Ahmed et al. utilized about 1 kg of CEO and 200 L of raw milk as an antibacterial agent for cheese production. The CEO demonstrated high antibacterial activity for a month at 4 °C without changing organoleptic properties, indicating a potential cost-effective application [66].
5.1.3. Processed foods
The industry for ready-to-cook food products (pre-cooked dishes) has recently grown as a result of changes in lifestyle and the expansion of chilled delivery networks. Due to foul odours, colour changes, stickiness, sedimentation, emissions, and pH changes, The health of consumers is at stake since the grade of processed food items has declined. Its use has focused on employing it as a flavoring factor with antibacterial and antioxidant properties, as it has been shown that adding 5 % (w/w) CEO to processed foods has a negative impact on their organoleptic quality [67].
5.1.4. Meat, poultry, and seafood
Applying CEO to meat products reduces the negative reactions that lead to the loss of sensory attributes of flavour, fragrance, colour, and texture. Because of its antioxidant properties, it has an antibacterial effect that reduces the quantity of bacteria, the production of hydroperoxide, and the ability of non-protein nitrogenous compounds to breakdown. Hydroperoxide dissolution, free radical interaction, Chain reaction inhibition, and Transition metal binding are the mechanisms by which CEO's antioxidant effects operate [68]. The CEO label has been applied on fish fillets, ground beef, white shrimp, salmon burgers, chicken breast, and chicken patties meat for storage in the refrigerator or freezer. For up to 45 days, CEO-fortified films can prevent microbial development in foods of animal origin, water activity, color change, lipid oxidation, and weight loss. Twelve days in the refrigerator and up to 45 days when heated, CEO fortified films can delay, microbial development in foods of animal origin, weight loss, colour change, water activity, and lipid oxidation [69].
5.1.5. Vegetables-
Significant financial losses are caused in the supply chain by the deterioration of post-harvest vegetables during transportation and storage. Due to CEO's antibacterial properties, it can serve as a good substitute for synthetic fungicides by minimizing negative health effects and vegetable fungal rot. Its antibacterial effectiveness is increased by combining it with different packaging or UV-C light treatment [70]. These procedures extend the useable life of vegetables without compromising their organoleptic qualities because they effectively regulate and preserve their physicochemical quality and post-harvest breakdown. CEO is used to wash newly cut veggies instead of chlorine-based disinfectants, sodium bicarbonate, and acetic acid to reduce microbial risks and increase shelf life. Additionally, CEO wash did not impact the composition, bioactive substances, or color properties [71].
Additionally, CEO wash does not affect the compositional, sensory, bioactive, or color properties. In order to bring veggies to the marketplace with enhanced and longer-lasting post-harvest quality and enhanced customer acceptance, CEO preparation in conjunction with cold storage is a successful ecological replacement that could be further developed for commercial uses.
5.1.6. Packaging materials
Recently, unique natural polymer-based Materials for biodegradable packaging have been created (lipids, proteins, polysaccharides). Essential oils can be used to enhance foods to increase their antioxidant and antibacterial properties, reducing or suppressing the growth of microorganisms and increasing shelf life that causes foodborne illness. By adding EO to coated films, functional characteristics such as water vapour permeability and antibacterial and antioxidant capabilities can be changed. CEO-fortified films displayed growth inactivation and antibacterial properties for up to 21 days due to CEO chemicals' penetration and cell structural disintegration. By making thicker films and adding more space inside the film matrix, CEO can change the moisture content of packaging materials [72].
When CEO is added to a film network, weaker connections (polymer-oil) partially replace stronger polymer-polymer interactions. This leads to a more centralized environment and irregular microstructure by rearranging the polymers. Additionally, the addition of CEO has a plasticizing impact that lowers the film's elastic modulus and glass transition temperature. When CEO was added to mechanically deboned chicken protein films, Sarcaoglu and Turhan saw a decrease in tensile strength and elastic modulus. Despite this, the tensile strength was kept above 3.5 MPa, which is the desired value for edible food coating film. These structural changes caused by the introduction of CEO resulted in rougher and more porous films [73].
5.2. Agricultural and larvicidal uses
Additionally, clove essential oil can be utilized as a pesticide. Clove essential oil is used to control the Reticulitermes speratus Kolbe, a Japanese termite, according to Park & Shin (2005) [60]. Similar to this, Eamsobhana et al. (2009) found that clove essential oil at 5.1 % had 100.2 % repelling effect against Leptotrombidium imphalu, a chigger, indicating that it might be a more affordable and secure substitute for synthetic repellents that have been associated with negative side effects [74]. Anopheles dirus Peyton and Harrion and Aedes aegypti (L.) bites were both effectively treated by a formulation containing 10 % clove essential oil, with protection periods of 80.3 and 56 and 60 and 10 h, respectively. As a guide, soybean oil was employed [75]. The effectiveness of synthetic eugenol and the principal derivatives of clove oil against Aedes aegypti (Diptera: Culicidae) larvae was examined in a recent study. Given that there is no vaccine or treatment for dengue, larvicidal methods are among the most effective ways to fight the disease. Eugenol demonstrated a positive impact and may provide an effective substitute for commonly used insecticides [76]. Eugenol, beta-caryophyllene, and eugenol acetate were found to be eugenol act the most quickly and are effective at driving away Solenopsis invicta (Hymenoptera: Formicidae) a red imported fire ant [77]. Paper wasps like Polistes dominulus and Pestiferous social wasps like Vespula pensylvanica (Saussure) were spatially repelled by clove oil (Christ) [78]. A variety of fish can be put to sleep with clove oil. On the other hand, longer exposures may cause subacute morbidity and mortality [79]. The optimal dose to put the angelfish to sleep was found by Ergun and Hekimoglu in 2012 [80]. One of the most challenging fish to handle in an aquarium, this research will help with shipping and handling. The enzyme activity of superoxide dismutase, polyphenol oxidase, catalase, peroxidase, and glutathione-S-transferase are all inhibited by clove oil, which could be utilized to restrict potato tuber germination [81].
5.3. Medicinal uses
Clove has the potential to alleviate toothache. A whole clove can be crushed and placed on the gum where the toothache is, or a tiny amount of ground cloves can be inserted in a piece of coffee filter, wrapped, soaked, and placed between the gum and the lip. Any of these remedies will help to relieve pain; the stronger the potency, the faster the alleviation. Clove oil can not only relieve pain, but it will also help to clear any infection from an abscess. If you have an earache, dilute clove oil with carrier oil (never water), place it on a cotton ball, and insert it just within the ear canal. The discomfort will fade rapidly, and it will aid in the reduction of infection if any is present. Furthermore, clove tea has a distinct flavor and aroma. The best aspect about drinking clove tea is how quickly it relieves nausea and leaves you with fresh breath. In fact, chewing on a clove from time to time is the finest breath mint ever, as well as a natural remedy for nausea and heartburn. Heartburn can be caused by drinking too much tea or chewing on cloves, especially if done on an empty stomach. Chewing on cloves for at least six weeks or longer can also assist in lowering blood pressure. Clove oil can be applied to burns, small open wounds, and cuts to relieve pain, prevent infection, and speed up healing. Simply sprinkling ground cloves on minor open wounds and cuts will help stop bleeding and relieve pain right away [82].
6. Clove essential oil nanoemulsion
The possibility of natural food use of essential oils preservation agents has been investigated. However, its limited water solubility, high volatility, and low long-term stability are limiting considerations for its usage as a natural preservative to replace conventional preservatives. The production of essential oil nanoemulsions has become a promising alternative due to nanotechnology advances. Because of their nanometric dimension, nanoemulsions offer unique properties such as physicochemical stability and increased contact surface area. Improved packaging materials with better mechanical strengths, antimicrobial film to nano sensing for detecting infections, barrier qualities, and educating consumers about the state of food safety are all advantages of nano-based "active" and "smart" food packaging [83]. The nanoencapsulation technique, in particular, encapsulates molecules with a variety of coating materials at the atomic, molecular, and supramolecular scales, typically 1–100 nm. However, some nanomaterials can be as small as 600 nm [84]. Food components are nano-encapsulated using various nanotechnologies to allow for the delivery of nutrients, bioactive compounds, and other nutraceuticals into body cells while remaining safe from the rigors of food preparation and harmful environmental variables. Cloves (Syringa oblata) are high in essential oils, which stop the expansion and proliferation of a variety of bacteria [85]. Utilizing clove essential oils as a natural preservative to lengthen the shelf life of goods in food and other industries. The mixed clove essential oil may provide enhanced antibacterial advantages due to the potential synergistic effects of the essential oils from various sources [86].
Nanoemulsions are a type of emulsion with droplet diameters between 1 and 100 nm (Hoar & Schulman, 1943). Because of their new physicochemical features and improved bioavailability of the encapsulated active components, nanoemulsions are gaining popularity in terms of formulation, production, and application (Sznitowska, Zurowska-Pryczkowska, Dabrowska, & Janicki, 2000; Mason, Wilking, Meleson, Chang, & Graves, 2006; McClements, 2011). Due to their distinct subcellular size, nanoemulsions can improve the dispersion of antimicrobial drugs in food matrices where bacteria prefer to reside, resulting in increased antimicrobial activity. Additionally, the physical stability of the encapsulating active chemicals is significantly increased in the nanoscale form (Weiss, Gaysinsky, Davidson, & McClements, 2009). Essential oil-based nanoemulsions are shown to have good antibacterial effects against a variety of microbes (Donsì, Annunziata, Sessa, & Ferrari, 2011). As a result, the nanoemulsion of clove essential oils is projected to have more stability and antibacterial action than non-nano state equivalents. Perishable commodities like Fruits and Vegetables require advanced technologies for their extended post-harvest shelf life. In addition to refrigerated environments and/or cold storage, fresh fruits, and vegetables have been preserved using edible coverings. In recent years, nanotechnology has developed as a novel technique for increasing coating qualities. In comparison to coatings based on traditional emulsions, The water barrier of coatings based on plant-sourced nanoemulsions is higher, as well as improved mechanical, optical, and microstructural features. Nanocoatings, as opposed to conventional emulsions, enable a more progressive and controlled release of antibacterial and antioxidant substances during food storage, resulting in higher bioactivity, a longer shelf life, and better nutritional produce quality.
6.1. Nanoemulsion formulation
In general, an emulsion or a colloidal dispersion composed of two immiscible liquids is called a nanoemulsion. In food applications, these liquids are commonly oil and water, and tiny droplets of one of the substances are scattered throughout the other (McClements, 2011). The fundamental factor separating emulsions from nanoemulsions is the size of the droplets. As previously indicated, conventional emulsions often have mean droplet diameters (d) larger than nanoemulsions (d). Even if the free energy of the separated water and oil phases is comparably smaller than that of the emulsion or nanoemulsion, neither system is thermodynamically stable. Therefore, a thermodynamic force still favors the slow disintegration of a nanoemulsion. It is possible to create nanoemulsions with a comparatively long shelf life by making sure that the kinetic energy barrier between separate and emulsified systems is sufficiently high. The system can be stabilized by adding emulsifiers or other stabilizers to accomplish this. Methods of formulations are depicted below.
6.1.1. Low energy emulsification method
Phase inversion temperature (PIT), emulsion inversion point (EIP), Spontaneous emulsification (SE), and Phase inversion composition (PIC) are all low-energy approaches for preparing nanoemulsions (Salvia-Trujillo et al., 2016). These techniques all rely on the sporadic creation of tiny oil droplets when specific system components or environmental circumstances change (Anton et al., 2008). The kind and concentration of oils and surfactants employed determine the stability and size of the oil droplets generated; hence these factors should be carefully optimized (Bouchemal et al., 2004). For instance, using Tween 80 as a non-ionic surfactant, PIT method antibacterial black pepper oils nanoemulsions were made (Vinh et al., 2020). For instance, a mixture of oil (4.3 %), surfactant (9.7 %), water (86 %), and which is typically used to make emulsions, was heated to 75 °C for 30 min. Then the mixture was quickly cooled to 5 °C for 15 min. The PIT approach was used by Moraes Lovison et al. (2017) to create oregano nanoemulsions, which demonstrated strong antibacterial efficiency against E. coli and Staphylococcus. The addition of these nanoemulsions did not alter the desired physicochemical properties of the chicken pate.
6.1.2. High energy emulsification
In the industrial setting, high-energy techniques, including microfluidization, high-pressure homogenization, and sonication, are frequently used to create nanoemulsions (Salvia-Trujillo et al., 2016). Sonication is used in an ultrasonic probe to create powerful disruptive forces in a solution of water, oil, and an emulsifier (Jafari et al., 2008). Particularly, the cavitation effects brought on by the frequent rise of pressure changes brought by intense ultrasonic waves, the mixture of the oil and aqueous phases together and break up tiny drops from larger ones, cause microbubbles to form inside the mixture (Modarres-Gheisari et al., 2019). Different forms of antibacterial nanoemulsions have been made using high-intensity techniques. Antimicrobial nanoemulsions made from Tween 80 and Cleome viscosa essential oil have been created via sonication and are proven to be effective against P. aeruginosa, E. coli, S. aureus, K. pneumoniae, and Streptococcus pyogenes (Krishnamoorthy et al., 2018). Additionally, chitosan/eugenol oil nanoemulsions with potent antibacterial properties have been created by sonication (Shao et al., 2018).
6.1.3. Microencapsulation
A nanoemulsion can be transformed into a very fine powder via spray-drying, which enhances its storage, handling, stability, and transport (Gharsallaoui et al., 2007). When oil is created in an aqueous condition, it is then sprayed into a hot chamber via a nozzle, which causes the water phase to evaporate rapidly. Nielsen et al. (2016) demonstrated that isoeugenol oil nanoemulsions could also be turned into powders by spray drying. These powders had high loading capacity for essential oils and showed antibacterial solid action against bacteria. The same authors demonstrated that these nanoemulsions might disperse pathogenic bacteria's biofilms (Nielsen et al., 2017). Eugenol nanoemulsions have also been transformed into powders via spray drying (Talón et al., 2019). When mixed with water, the resulting powders, against E. coli, displayed strong antibacterial activity and high encapsulation efficiencies (95–98 %).
6.1.4. Solvent evaporation method
The formulation of nanoemulsions frequently uses the solvent evaporation process. The oil phase and surfactants are dissolved using organic solvents, and the resulting solution is then dispersed into an aqueous phase. The organic solvent is subsequently evaporated, creating nanoemulsions with tiny droplet sizes. Simpleness, scalability, and the capacity to encapsulate lipophilic molecules are some benefits of the solvent evaporation approach. However, due to the possibility of some organic solvents being harmful or leaving residue in the finished product, caution must be taken when choosing them. To obtain the appropriate droplet sizes and long-term stability, it is crucial to optimize the formulation conditions, including the solvent evaporation rate, stirring speed, and surfactant concentration [87].
6.1.5. Coacervation phase separation method
The coacervation phase separation method is a method for creating nanoemulsions. It entails the division of a mixture of oil, water, and the proper surfactants into two different phases: an oil-rich coacervate phase, and a diluted watery phase. Nanoemulsions are created when the coacervate phase is subsequently dispersed into the continuous phase. The coacervation phase separation process has benefits like simplicity, the capacity to encapsulate hydrophobic substances, and potential control over droplet size distribution [88,89]. To accomplish the desired phase separation and stable nanoemulsion generation, parameters including temperature, pH, and surfactant concentration must be carefully optimized. Coacervate droplets can form when the surfactants in the system go through associative or segregative phase separation. The droplets of the coacervate phase, which are oil-rich, are then gently agitated or mixed with the continuous phase, which is usually the watery phase. A nanoemulsion is created at this step when the distributed coacervate droplets are enclosed within the continuous phase. For the purpose of stabilizing the resulting nanoemulsion, the surfactants in the coacervate droplets serve as emulsifiers. To decrease droplet size and improve the stability of the nanoemulsion, processes like high-pressure homogenization, sonication, or other physical techniques can be used [90]. These post-processing processes assist in obtaining the correct droplet size distribution and enhance the formulation's long-term stability. Droplet size, polydispersity index, zeta potential, and physical stability are just a few of the characteristics that are used to describe the final nanoemulsion. The quality and functionality of the nanoemulsion are revealed by these characterizations. To preserve its stability during storage and transit, the nanoemulsion is normally stored in the proper containers under regulated temperature and light conditions after being characterized. The coacervation phase separation technique provides flexibility in terms of encasing lipophilic substances and managing droplet size. Wherever stable and finely dispersed nanoemulsions are sought, it has found applications in a variety of industries, including food, cosmetics, and medicines [91,92].
6.2. Applications of CEO nanoemulsion in food industry
Consumer demand for safer and healthier food products has increased, prompting producers to rework their goods using more organic and natural ingredients. These substances must still possess the necessary functional qualities, though. Foods with more moisture content, pH values, or nutritional levels typically degrade quickly and cannot be kept for extended periods. Microbial contamination and development possibilities are frequently increased during post-harvest handling and processing procedures, which lowers food quality and shelf life [93]. Many foods' shelf lives can be extended, quality is maintained, and microbial growth is reportedly suppressed using antimicrobial nanoemulsions [94]. These systems are demonstrated to be efficient against both kinds of bacteria [95], attributed to their capacity to disrupt intercellular signaling or alter the composition and microbial cell wall's function (Eddin et al., 2019). They can also prevent the formation of biofilms by damaging microbial cell walls. Essential oils and various phytochemicals, including curcumin, cinnamaldehyde, eugenol, limonene, thymol, and carvacrol, is shown to have potent antibacterial function against a wide range of spoilage pathogens in a variety of food systems [96].
7. Conclusion
Based on the information gathered, it is possible to infer that the intriguing plant known as clove is a rich source of antioxidants and contains great potential as a food preservative. Its biological activity has been proven, showing the production of medications for both humans and animals and demonstrating the plant's long-term usage. Clove oils offer various agricultural, pharmaceutical, and food applications. They are also used as an antioxidant and antibacterial to help increase the shelf life of food and protect it from foodborne infections. It is crucial to conduct a study on CEO use in the food business, particularly how it can function as an antibacterial or antioxidant agent without affecting the flavor, color, or texture of food. There is not much research on CEO encapsulation's effects on the major biological and physicochemical traits. Research is still needed to determine how encapsulation techniques affect organoleptic qualities, shelf life, solubility, absorption, and bioavailability by inhibiting thermal and photo deterioration of oxidative energy. The use of nanotechnology to create edible coatings or films is an area of research that is becoming increasingly exciting. If active ingredients are included, this research could result in the creation of nanocapsules. More research is needed to fully understand the many drugs' modes of action of active ingredients with different coatings and edible films and how they affect the sensory and mechanical properties.
Data availability
No data was used for the research described in the article.
Funding
Project No. TKP2021-NKTA-32 has been implemented with support from the National Research, Development, and Innovation Fund of Hungary, financed under the TKP2021-NKTA funding scheme.
CRediT authorship contribution statement
Vinay Kumar Pandey: Writing - original draft, Visualization, Validation, Software, Resources, Methodology, Formal analysis, Data curation, Conceptualization. Shivangi Srivastava: Writing - original draft, Formal analysis, Data curation. Ashish: Writing - original draft, Formal analysis, Data curation. Kshirod Kumar Dash: Writing - review & editing, Writing - original draft, Visualization, Supervision, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Rahul Singh: Writing - review & editing, Supervision, Software, Resources, Project administration. Aamir Hussain Dar: Writing - review & editing, Project administration, Formal analysis. Tripti Singh: Software, Resources, Data curation. Alvina Farooqui: Validation, Software, Data curation. Ayaz Mukkaram Shaikh: Writing - review & editing, Funding acquisition. Bela Kovacs: Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All the authors are thankful to the Department of Bioengineering, Integral University, Lucknow, Uttar Pradesh, India for providing the manuscript number IU/R&D/2023-MCN0002157.
Contributor Information
Kshirod Kumar Dash, Email: kshirod@tezu.ernet.in.
Rahul Singh, Email: rahulsingh.jnu@gmail.com.
Bela Kovacs, Email: kovacsb@agr.unideb.hu.
References
- 1.Filho G.A., Cesar J.O., Ramos J.V. [Cravo from India] [online] 2013. http://www.ceplac.gov.br/radar.htm Portuguese. CEPLAC.
- 2.Cortés-Rojas D.F., de Souza C.R.F., Oliveira W.P. Clove (Syzygium aromaticum): a precious spice. Asian Pac. J. Trop. Biomed. 2014;4(2):90–96. doi: 10.1016/S2221-1691(14)60215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaieb K., Hajlaoui H., Zmantar T., Kahla‐Nakbi A.B., Rouabhia M., Mahdouani K., Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (syzigium aromaticum L. Myrtaceae): a short review. Phytotherapy research: an international journal devoted to pharmacological and toxicological evaluation of natural product derivatives. Phytother Res. 2007;21(6):501–506. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- 4.Hadidi M., Pouramin S., Adinepour F., Haghani S., Jafari S.M. Chitosan nanoparticles loaded with clove essential oil: characterization, antioxidant and antibacterial activities. Carbohydrate Polymers. 2020;236 doi: 10.1016/j.carbpol.2020.116075. [DOI] [PubMed] [Google Scholar]
- 5.Saricaoglu F.T., Turhan S. Performance of mechanically deboned chicken meat protein coatings containing thyme or clove essential oil for storage quality improvement of beef sucuks. Meat Sci. 2019;158 doi: 10.1016/j.meatsci.2019.107912. [DOI] [PubMed] [Google Scholar]
- 6.Hosseini M., Jamshidi A., Raeisi M., Azizzadeh M. Effect of sodium alginate coating containing clove (Syzygium aromaticum) and lemon verbena (Aloysia citriodora) essential oils and different packaging treatments on shelf life extension of refrigerated chicken breast. J. Food Process. Preserv. 2021;45(3) doi: 10.1111/jfpp.14946. [DOI] [Google Scholar]
- 7.Yang Y.C., Wei M.C., Hong S.J. Ultrasound-assisted extraction and quantitation of oils from Syzygium aromaticum flower bud (clove) with supercritical carbon dioxide. Journal of Chromatography. A. 2014;1323:18–27. doi: 10.1016/j.chroma.2013.10.098. [DOI] [PubMed] [Google Scholar]
- 8.Overly K.R. Microwave-assisted isolation of eugenol from cloves. J. Chem. Educ. 2019;96(11):2665–2667. doi: 10.1021/acs.jchemed.8b01022. [DOI] [Google Scholar]
- 9.Mohamed Y., Mohamed I., Elsadek M., Ali M., Ghatas Y. Improving growth, productivity, and chemical composition of Trachyspermum ammi L. by using organic and chemical fertilization in the presence of boron. Ind. Crop. Prod. 2021;169 doi: 10.1016/j.indcrop.2021.113637. [DOI] [Google Scholar]
- 10.Hatami T., Johner J.C.F., Zabot G.L., Meireles M.A.A. Supercritical fluid extraction assisted by cold pressing from clove buds: extraction performance, volatile oil composition, and economic evaluation. J. Supercrit. Fluids. 2019;144:39–47. doi: 10.1016/j.supflu.2018.10.003. [DOI] [Google Scholar]
- 11.Kennouche A., Benkaci-Ali F., Scholl G., Eppe G. Chemical composition and antimicrobial activity of the essential oil of Eugenia caryophyllata Cloves extracted by conventional and microwave techniques. Journal of Biologically Active Products from Nature. 2015;5:1–11. [Google Scholar]
- 12.Lu W., Cui R., Zhu B., Qin Y., Cheng G., Li L., Yuan M. Influence of clove essential oil immobilized in mesoporous silica nanoparticles on the functional properties of poly(lactic acid) biocomposite food packaging film. J. Mater. Res. Technol. 2021;11:1152–1161. doi: 10.1016/j.jmrt.2021.01.098. [DOI] [Google Scholar]
- 13.Banerjee K., Madhyastha H., Sandur R., N T M., N T., Thiagarajan P. Anti-inflammatory and wound healing potential of a clove oil emulsion. Colloids and Surfaces. B, Biointerfaces. 2020;193 doi: 10.1016/j.colsurfb.2020.111102. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed J., Mulla M., Jacob H., Luciano G., T.b B., Almusallam A. Polylactide/poly(ε-caprolactone)/zinc oxide/clove essential oil composite antimicrobial films for scrambled egg packaging. Food Packag. Shelf Life. 2019;21 doi: 10.1016/j.fpsl.2019.100355. [DOI] [Google Scholar]
- 15.Khalilzadeh E., Hazrati R., Saiah G.V. Effects of topical and systemic administration of Eugenia caryophyllata buds essential oil on corneal anesthesia and analgesia. Research in Pharmaceutical Sciences. 2016;11(4):293–302. doi: 10.4103/1735-5362.189297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaichi M., Mohammadi A., Badii F., Hashemi M. Triple synergistic essential oils prevent pathogenic and spoilage bacteria growth in the refrigerated chicken breast meat. Biocatal. Agric. Biotechnol. 2021;32 doi: 10.1016/j.bcab.2021.101926. [DOI] [Google Scholar]
- 17.Rajaei A., Hadian M., Mohsenifar A., Rahmani-Cherati T., Tabatabaei M. A coating based on clove essential oils encapsulated by chitosan-myristic acid nanogel efficiently enhanced the shelf-life of beef cutlets. Food Packag. Shelf Life. 2017;14:137–145. doi: 10.1016/j.fpsl.2017.10.005. [DOI] [Google Scholar]
- 18.Takahashi H., Nakamura A., Fujino N., Sawaguchi Y., Sato M., Kuda T., Kimura B. Evaluation of the antibacterial activity of allyl isothiocyanate, clove oil, eugenol and carvacrol against spoilage lactic acid bacteria. LWT. 2021;145 doi: 10.1016/j.lwt.2021.111263. [DOI] [Google Scholar]
- 19.Dehghani S., Hosseini S.V., Regenstein J.M. Edible films and coatings in seafood preservation: a review. Food Chem. 2018;240:505–513. doi: 10.1016/j.foodchem.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Ju J., Xie Y., Yu H., Guo Y., Cheng Y., Qian H., Yao W. A novel method to prolong bread shelf life: sachets containing essential oils components. LWT. 2020;131 doi: 10.1016/j.lwt.2020.109744. [DOI] [Google Scholar]
- 21.Liu X., Zhang C., Liu S., Gao J., Cui S.W., Xia W. Coating white shrimp (Litopenaeus vannamei) with edible fully deacetylated chitosan incorporated with clove essential oil and kojic acid improves preservation during cold storage. Int. J. Biol. Macromol. 2020;162:1276–1282. doi: 10.1016/j.ijbiomac.2020.06.248. [DOI] [PubMed] [Google Scholar]
- 22.Nilmini R.K., Kodituwakku T.D., Abeywickrama K., Kuruppu M. In vitro and in vivo application of eco-friendly treatments to control postharvest stem-end rot of naturally infected avocado (cv. Pollock) J. Agric. Sci.–Sri Lanka. 2021;16:283–299. [Google Scholar]
- 23.Park J.-B., Kang J.-H., Song K.B. Bin Clove bud essential oil emulsion containing Benzethonium chloride inactivates Salmonella Typhimurium and Listeria monocytogenes on fresh-cut pak choi during modified atmosphere storage. Food Control. 2019;100:17–23. doi: 10.1016/j.foodcont.2019.01.001. [DOI] [Google Scholar]
- 24.Vurmaz A.K., Gündüz G.T. Inhibition of mold growth on the surface of dried persimmons using combined treatments of UV-C light and clove oil. Innovative Food Sci. Emerging Technol. 2020;61 doi: 10.1016/j.ifset.2020.102336. [DOI] [Google Scholar]
- 25.Nisar T., Wang Z.C., Yang X., Tian Y., Iqbal M., Guo Y. Characterization of citrus pectin films integrated with clove bud essential oil: physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018;106:670–680. doi: 10.1016/j.ijbiomac.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 26.Abd El Azim M.H.M., El-Mesallamy A.M., El-Gerby M., Awad A. Anti-tumor, antioxidant and antimicrobial and the phenolic constituents of clove flower buds (Syzygium aromaticum) J. Microb. Biochem. Technol. 2014;10:s8. s007. [Google Scholar]
- 27.Liu H., Schmitz J.C., Wei J., Cao S., Beumer J.H., Strychor S., Cheng L., Liu M., Wang C., Wu N., Zhao X., Zhang Y., Liao J., Chu E., Lin X. Clove extract inhibits tumor growth and promotes cell cycle arrest and apoptosis. Oncology Research. 2014;21(5):247–259. doi: 10.3727/096504014X13946388748910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazawa M., Hisama M. Suppression of chemical mutagen-induced SOS response by alkylphenols from clove (Syzygium aromaticum) in the Salmonella typhimurium TA1535/pSK1002 umu test. J. Agric. Food Chem. 2001;49(8):4019–4025. doi: 10.1021/jf0103469. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee S., Das S. Anticarcinogenic effects of an aqueous infusion of cloves on skin carcinogenesis. Asian Pac. J. Cancer Prev. APJCP. 2005;6(3):304–308. [PubMed] [Google Scholar]
- 30.Sultana B., Anwar F., Mushtaq M., Aslam M., Ijaz S. In vitro antimutagenic, antioxidant activities and total phenolics of clove (Syzygium aromaticum L.) seed extracts. Pak. J. Pharm. Sci. 2014;27(4):893–899. [PubMed] [Google Scholar]
- 31.Prasad R.C., Herzog B., Boone B., Sims L., Waltner-Law M. An extract of Syzygium aromaticum represses genes encoding hepatic gluconeogenic enzymes. J. Ethnopharmacol. 2005;96(1–2):295–301. doi: 10.1016/j.jep.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Adefegha S.A., Oboh G., Adefegha O.M., Boligon A.A., Athayde M.L. Antihyperglycemic, hypolipidemic, hepatoprotective and antioxidative effects of dietary clove (Szyzgium aromaticum) bud powder in a high‐fat diet/streptozotocin‐induced diabetes rat model. J. Sci. Food Agric. 2014;94(13):2726–2737. doi: 10.1002/jsfa.6617. [DOI] [PubMed] [Google Scholar]
- 33.Tu Z., Moss-Pierce T., Ford P., Jiang T.A. Syzygium aromaticum L.(Clove) extract regulates energy metabolism in myocytes. J. Med. Food. 2014;17(9):1003–1010. doi: 10.1089/jmf.2013.0175. [DOI] [PubMed] [Google Scholar]
- 34.Kuroda M., Mimaki Y., Ohtomo T., Yamada J., Nishiyama T., Mae T., Kishida H., Kawada T. Hypoglycemic effects of clove (Syzygium aromaticum flower buds) on genetically diabetic KK-A y mice and identification of the active ingredients. J. Nat. Med. 2012;66(2):394–399. doi: 10.1007/s11418-011-0593-z. [DOI] [PubMed] [Google Scholar]
- 35.Bachiega T.F., de Sousa J.P.B., Bastos J.K., Sforcin J.M. Clove and eugenol in noncytotoxic concentrations exert immunomodulatory/anti-inflammatory action on cytokine production by murine macrophages. J. Pharm. Pharmacol. 2012;64(4):610–616. doi: 10.1111/j.2042-7158.2011.01440.x. [DOI] [PubMed] [Google Scholar]
- 36.Li H.Y., Lee B.K., Kim J.S., Jung S.J., Oh S.B. Eugenol inhibits ATP-induced P2X currents in trigeminal ganglion neurons. KOREAN J. PHYSIOL. PHARMACOL. 2008;12(6):315–321. doi: 10.4196/kjpp.2008.12.6.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohkubo T., Shibata M. The selective capsaicin antagonist capsazepine abolishes the antinociceptive action of eugenol and guaiacol. J. Dent. Res. 1997;76(4):848–851. doi: 10.1177/00220345970760040501. [DOI] [PubMed] [Google Scholar]
- 38.Sofia P.K., Prasad R., Vijay V.K., Srivastava A.K. Evaluation of antibacterial activity of Indian spices against common foodborne pathogens. Int. J. Food Sci. Technol. 2007;42(8):910–915. doi: 10.1111/j.1365-2621.2006.01308.x. [DOI] [Google Scholar]
- 39.Burt S.A., Reinders R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157: H7. Lett. Appl. Microbiol. 2003;36(3):162–167. doi: 10.1046/j.1472-765x.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- 40.Devi K.P., Nisha S.A., Sakthivel R., Pandian S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010;130(1):107–115. doi: 10.1016/j.jep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 41.Chami F., Chami N., Bennis S., Trouillas J., Remmal A. Evaluation of carvacrol and eugenol as prophylaxis and treatment of vaginal candidiasis in an immunosuppressed rat model. J. Antimicrob. Chemother. 2004;54(5):909–914. doi: 10.1093/jac/dkh436. [DOI] [PubMed] [Google Scholar]
- 42.Ali S.M., Khan A.A., Ahmed I., Musaddiq M., Ahmed K.S., Polasa H., Rao L.V., Habibullah C.M., Sechi L.A., Ahmed N. Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005;4(1):20. doi: 10.1186/1476-0711-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakry A.M., Abbas S., Ali B., Majeed H., Abouelwafa M.Y., Mousa A., Liang L. Microencapsulation of oils: a comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016;15(1):143–182. doi: 10.1111/1541-4337.12179. [DOI] [PubMed] [Google Scholar]
- 44.El Asbahani A., Miladi K., Badri W., Sala M., Aït Addi E.H.A., Casabianca H., El Mousadik A., Hartmann D., Jilale A., Renaud F.N.R., Elaissari A. Essential oils: from extraction to encapsulation. Int. J. Pharm. 2015;483(1–2):220–243. doi: 10.1016/j.ijpharm.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 45.Ameur E., Sarra M., Yosra D., Mariem K., Nabil A., Lynen F., Larbi K.M. Chemical composition of essential oils of eight Tunisian Eucalyptus species and their antibacterial activity against strains responsible for otitis. BMC Complementary Medicine and Therapies. 2021;21(1):209. doi: 10.1186/s12906-021-03379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frohlich P.C., Santos K.A., Palú F., Cardozo-Filho L., da Silva C., da Silva E.A. Evaluation of the effects of temperature and pressure on the extraction of eugenol from clove (Syzygium aromaticum L.) leaves using supercritical CO2. J. Supercrit. Fluids. 2019;143:313–320. doi: 10.1016/j.supflu.2018.09.009. [DOI] [Google Scholar]
- 47.Constantinescu A.M., Tiţa M.A., Georgescu C. Comparative analysis of yoghurts obtained with bioactive compounds. Series II – Forestry • Wood Industry • Agricultural Food Engineering. 2020;12(61):73–84. doi: 10.31926/but.fwiafe.2019.12.61.2.6. 2. [DOI] [Google Scholar]
- 48.Thakur R., Pristijono P., Scarlett C.J., Bowyer M., Singh S.P., Vuong Q.V. Starch-based films: major factors affecting their properties. Int. J. Biol. Macromol. 2019;132:1079–1089. doi: 10.1016/j.ijbiomac.2019.03.190. [DOI] [PubMed] [Google Scholar]
- 49.Chu Y., Gao C.C., Liu X., Zhang N., Xu T., Feng X., Yang Y., Shen X., Tang X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT. 2020;122 doi: 10.1016/j.lwt.2020.109054. [DOI] [Google Scholar]
- 50.Hasan S.M.K., Ferrentino G., Scampicchio M. Nanoemulsion as advanced edible coatings to preserve the quality of fresh-cut fruits and vegetables: a review. Int. J. Food Sci. Technol. 2020;55(1):1–10. doi: 10.1111/ijfs.14273. [DOI] [Google Scholar]
- 51.Miranda M., Sun X., Ference C., Plotto A., Bai J., Wood D., Assis O.B.G., Ferreira M.D., Baldwin E. Nano- and micro-carnauba wax emulsions versus shellac protective coatings on postharvest citrus quality. J. Am. Soc. Hortic. Sci. 2020;1:1–10. [Google Scholar]
- 52.Hussein G., Miyashiro H., Nakamura N., Hattori M., Kakiuchi N., Shimotohno K. Inhibitory effects of Sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytotherapy research: an international journal devoted to pharmacological and toxicological evaluation of natural product derivatives. Phytother Res. 2000;14(7):510–516. doi: 10.1002/1099-1573. (200011)14:7<510::aid-ptr646>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 53.Kurokawa M., Hozumi T., Basnet P., Nakano M., Kadota S., Namba T., Kawana T., Shiraki K. Purification and characterization of Eugeniin as an anti-herpesvirus compound from Geum japonicum and Syzygium aromaticum. J. Pharmacol. Exp. Therapeut. 1998;284(2):728–735. [PubMed] [Google Scholar]
- 54.Ogata M., Hoshi M., Urano S., Endo T. Antioxidant activity of eugenol and related monomeric and dimeric compounds. Chem. Pharm. Bull. 2000;48(10):1467–1469. doi: 10.1248/cpb.48.1467. [DOI] [PubMed] [Google Scholar]
- 55.Atsumi T., Iwakura I., Fujisawa S., Ueha T. Reactive oxygen species generation and photo-cytotoxicity of eugenol in solutions of various pH. Biomaterials. 2001;22(12):1459–1466. doi: 10.1016/s0142-9612(00)00267-2. [DOI] [PubMed] [Google Scholar]
- 56.Jirovetz L., Buchbauer G., Stoilova I., Stoyanova A., Krastanov A., Schmidt E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006;54(17):6303–6307. doi: 10.1021/jf060608c. [DOI] [PubMed] [Google Scholar]
- 57.Gülçin W., Şat İ.G., Beydemir Ş., Elmastaş M., Küfrevioǧlu Ö.İ. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.) Food Chem. 2004;87(3):393–400. doi: 10.1016/j.foodchem.2003.12.008. [DOI] [Google Scholar]
- 58.Ho S.H., Cheng L.P.L., Sim K.Y., Tan H.T.W. Potential of cloves (Syzygium aromaticum (L.) merr. And perry as a grain protectant against Tribolium castaneum (herbst) and Sitophilus zeamais motsch. Postharvest Biol. Technol. 1994;4(1–2):179–183. doi: 10.1016/0925-5214(94)90019-1. [DOI] [Google Scholar]
- 59.Kim E.H., Kim H.K., Ahn Y.J. Acaricidal activity of clove bud oil compounds against Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: pyroglyphidae) J. Agric. Food Chem. 2003;51(4):885–889. doi: 10.1021/jf0208278. [DOI] [PubMed] [Google Scholar]
- 60.Park I.K., Shin S.C. Fumigant activity of plant essential oils and components from garlic (Allium sativum) and clove bud (Eugenia caryophyllata) oils against the Japanese termite (Reticulitermes speratus Kolbe) J. Agric. Food Chem. 2005;53(11):4388–4392. doi: 10.1021/jf050393r. [DOI] [PubMed] [Google Scholar]
- 61.Karunamay S., Badhe S.R., Shukla V., Singh N., Lali K., Patil S. Application of clove essential oil in food industry – a review. Journal of Food Research and Technology. 2019;7(4):23–25. [Google Scholar]
- 62.Singh S., Bond J., Singh A., Rustagi A. Evaluation of antibacterial properties of essential oils from clove and eucalyptus. Evaluation. 2014;7(5) [Google Scholar]
- 63.Khatkar A.B., Ray A., Kaur A. Effect of addition of clove essential oil on the storage stability of paneer. Journal of Pharmaceutical Innovation. 2017;6(9):39. A. [Google Scholar]
- 64.Shukla V., Mendiratta S.K., Zende R.J., Agrawal R.K., Kumar Jaiswal R. Effects of chitosan coating enriched with Syzygium aromaticum essential oil on quality and shelf-life of chicken patties. J. Food Process. Preserv. 2020;44(11) doi: 10.1111/jfpp.14870. [DOI] [Google Scholar]
- 65.Sung B., Prasad S., Yadav V.R., Aggarwal B.B. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr. Cancer. 2012;64(2):173–197. doi: 10.1080/01635581.2012.630551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fadilah F., Yanuar A., Arsianti A., Andrajati R. Phenylpropanoids, eugenol scaffold, and its derivatives as anticancer. Asian J. Pharmaceut. Clin. Res. 2017;10(3):41–46. doi: 10.22159/ajpcr.2017.v10i3.16071. [DOI] [Google Scholar]
- 67.Thapa D., Richardson A.J., Zweifel B., Wallace R.J., Gratz S.W. Genoprotective effects of essential oil compounds against oxidative and methylated DNA damage in human colon cancer cells. J. Food Sci. 2019;84(7):1979–1985. doi: 10.1111/1750-3841.14665. [DOI] [PubMed] [Google Scholar]
- 68.Perumal A.B., Nambiar R.B., Sellamuthu P.S., Emmanuel R.S. Use of modified atmosphere packaging combined with essential oils for prolonging postharvest shelf life of mango. LWT. 2021;148 doi: 10.1016/j.lwt.2021.111662. [DOI] [Google Scholar]
- 69.Cansian R.L., Vanin A.B., Orlando T., Piazza S.P., Puton B.M.S., Cardoso R.I., Gonçalves I.L., Honaiser T.C., Paroul N., Oliveira D. Toxicity of clove essential oil and its ester eugenyl acetate against Artemia salina. Braz. J. Biol. 2017;77(1):155–161. doi: 10.1590/1519-6984.12215. [DOI] [PubMed] [Google Scholar]
- 70.Phothisuwan S., Preechatiwong W., Matan N. Enhancement of antibacterial activity of essential oil vapor released from a paper egg tray in combination with UV-C radiation against pathogenic bacteria on chicken eggs. J. Food Process. Preserv. 2020;44(10) doi: 10.1111/jfpp.14794. [DOI] [Google Scholar]
- 71.Musthafa K.S., Hmoteh J., Thamjarungwong B., Voravuthikunchai S.P. Antifungal potential of eugenyl acetate against clinical isolates of Candida species. Microb. Pathog. 2016;99:19–29. doi: 10.1016/j.micpath.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 72.Kaur K., Kaushal S., Rani R. Chemical composition, antioxidant and antifungal potential of clove (Syzygium aromaticum) essential Oil, its major compound and its derivatives. Journal of Essential Oil Bearing Plants. 2019;22(5):1195–1217. doi: 10.1080/0972060X.2019.1688689. [DOI] [Google Scholar]
- 73.Barajas-Álvarez P., Castillo-Herrera G.A., Guatemala-Morales G.M., Corona-González R.I., Arriola-Guevara E., Espinosa-Andrews H. Supercritical CO2-ethanol extraction of oil from green coffee beans: optimization conditions and bioactive compound identification. Journal of Food Science and Technology. Arriola-Guevara. H. Supercritical. 2021;58(12):4514–4523. doi: 10.1007/s13197-020-04933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eamsobhana P., Yoolek A., Kongkaew W., Lerdthusnee K., Khlaimanee N., Parsartvit A., Malainual N., Yong H.S. Laboratory evaluation of aromatic essential oils from thirteen plant species as candidate repellents against Leptotrombidium chiggers (Acari: trombiculidae), the vector of scrub typhus. Exp. Appl. Acarol. 2009;47(3):257–262. doi: 10.1007/s10493-008-9214-2. [DOI] [PubMed] [Google Scholar]
- 75.Sritabutra D., Soonwera M., Waltanachanobon S., Poungjai S. Evaluation of herbal essential oil as repellents against Aedes aegypti (L.) and Anopheles dirus Peyton & Harrion. Asian Pac. J. Trop. Biomed. 2011;1(1):S124–S128. doi: 10.1016/S2221-1691(11)60138-X. [DOI] [Google Scholar]
- 76.Barbosa J.D., Silva V.B., Alves P.B., Gumina G., Santos R.L., Sousa D.P., Cavalcanti S.C. Structure–activity relationships of eugenol derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag. Sci. 2012;68(11):1478–1483. doi: 10.1002/ps.3331. [DOI] [PubMed] [Google Scholar]
- 77.Kafle L., Shih C.J. Toxicity and repellency of compounds from clove (Syzygium aromaticum) to red imported fire ants Solenopsis invicta (Hymenoptera: Formicidae) J. Econ. Entomol. 2013;106(1):131–135. doi: 10.1603/ec12230. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Q.H., Schneidmiller R.G., Hoover D.R. Essential oils and their compositions as spatial repellents for pestiferous social wasps. Pest Manag. Sci. 2013;69(4):542–552. doi: 10.1002/ps.3411. [DOI] [PubMed] [Google Scholar]
- 79.Javahery S., Nekoubin H., Moradlu A.H. Effect of anaesthesia with clove oil in fish. Fish Physiol. Biochem. 2012;38(6):1545–1552. doi: 10.1007/s10695-012-9682-5. [DOI] [PubMed] [Google Scholar]
- 80.Hekimoğlu M.A., Ergun M. Evaluation of clove oil as anaesthetic agent in fresh water angelfish, Pterophyllum scalare. Pakistan J. Zool. 2012;44(5):1297–1300. [Google Scholar]
- 81.Afify A.E.M.M., El-Beltagi H.S., Aly A.A., El-Ansary A.E. Antioxidant enzyme activities and lipid peroxidation as biomarker for potato tuber stored by two essential oils from caraway and clove and its main component carvone and eugenol. Asian Pac. J. Trop. Biomed. 2012;2(2):S772–S780. doi: 10.1016/S2221-1691(12)60312-8. [DOI] [Google Scholar]
- 82.Bhowmik D., Kumar K.S., Yadav A., Srivastava S., Paswan S., Dutta A.S. Recent trends in Indian traditional herbs Syzygium aromaticum and its health benefits. J. Pharmacogn. Phytochem. 2012;1(1):13–22. [Google Scholar]
- 83.Ribes S., Fuentes A., Barat J.M. Effect of oregano (Origanum vulgare L. ssp. hirtum) and clove (Eugenia spp.) nanoemulsions on Zygosaccharomyces bailii survival in salad dressings. Food Chem. 2019;295:630–636. doi: 10.1016/j.foodchem.2019.05.173. [DOI] [PubMed] [Google Scholar]
- 84.Ghule S.N., Desai M.A. Intensified extraction of valuable compounds from clove buds using ultrasound assisted hydrotropic extraction. Journal of Applied Research on Medicinal and Aromatic Plants. 2021;25 doi: 10.1016/j.jarmap.2021.100325. [DOI] [Google Scholar]
- 85.Ju J., Xu X., Xie Y., Guo Y., Cheng Y., Qian H., Yao W. Inhibitory effects of cinnamon and clove essential oils on mold growth on baked foods. Food Chem. 2018;240:850–855. doi: 10.1016/j.foodchem.2017.07.120. [DOI] [PubMed] [Google Scholar]
- 86.Ahmed L.I., Ibrahim N., Abdel-Salam A.B., Fahim K.M. Potential application of ginger, clove and thyme essential oils to improve soft cheese microbial safety and sensory characteristics. Food Biosci. 2021;42 doi: 10.1016/j.fbio.2021.101177. [DOI] [Google Scholar]
- 87.Sharma N., Madan P., Lin S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: a co-surfactant study. Asian J. Pharm. Sci. 2016;11(3):404–416. [Google Scholar]
- 88.Veis A. A review of the early development of the thermodynamics of the complex coacervation phase separation. Adv. Colloid Interface Sci. 2011;167(1–2):2–11. doi: 10.1016/j.cis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maurya A., Singh V.K., Das S., Prasad J., Kedia A., Upadhyay N.…Dwivedy A.K. Essential oil nanoemulsion as eco-friendly and safe preservative: bioefficacy against microbial food deterioration and toxin secretion, mode of action, and future opportunities. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.751062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hidajat M.J., Jo W., Kim H., Noh J. Effective droplet size reduction and excellent stability of limonene nanoemulsion formed by high-pressure homogenizer. Colloids and interfaces. 2020;4(1):5. [Google Scholar]
- 91.Sharma M., Mann B., Sharma R., Bajaj R., Athira S., Sarkar P., Pothuraju R. Sodium caseinate stabilized clove oil nanoemulsion: physicochemical properties. J. Food Eng. 2017;212:38–46. [Google Scholar]
- 92.Sharma M., Mann B., Pothuraju R., Sharma R., Kumar R. Physico-chemical characterization of ultrasound assisted clove oil-loaded nanoemulsion: as enhanced antimicrobial potential. Biotechnology Reports. 2022;34 doi: 10.1016/j.btre.2022.e00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brizzolara S., Manganaris G.A., Fotopoulos V., Watkins C.B., Tonutti P. Primary metabolism in fresh fruits during storage. Front. Plant Sci. 2020;11:80. doi: 10.3389/fpls.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mohamed S.A.A., El-Sakhawy M., El-Sakhawy M.A.-M. Polysaccharides, protein and lipid-based natural edible films in food packaging: a review. Carbohydrate Polymers. 2020;238 doi: 10.1016/j.carbpol.2020.116178. [DOI] [PubMed] [Google Scholar]
- 95.Dehghani P., Hosseini S.M.H., Golmakani M.-T., Majdinasab M., Esteghlal S. Shelf-life extension of refrigerated rainbow trout fillets using total Farsi gum-based coatings containing clove and thyme essential oils emulsions. Food Hydrocolloids. 2018;77:677–688. doi: 10.1016/j.foodhyd.2017.11.009. [DOI] [Google Scholar]
- 96.Arnon-Rips H., Poverenov E. Improving food products' quality and storability by using Layer by Layer edible coatings. Trends Food Sci. Technol. 2018;75:81–92. doi: 10.1016/j.tifs.2018.03.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.