Abstract
Objective:
This study assessed diet diversity and consumption of ultra-processed foods and explored its impact on macronutrient intake and risk of micronutrient inadequacy.
Design:
Cross-sectional, non-probability snowball sampling.
Setting:
Nutrient intake was assessed using 24-h dietary recall method and diet diversity through FAO-diet diversity score (DDS). Mann–Whitney U test was used to assess differences in risk of inadequacy across gender. Spearman’s rank correlation assessed associations between energy contributed by ultra-processed food and risk of nutrient inadequacy.
Participants:
A total of 589 adults (20–40 years) belonging to upper-middle and high-income groups.
Results:
The average individual DDS was 4·4 ± 0·6. Most of the participants (>80 %) had intakes less than national recommendations of pulses/eggs/flesh foods, milk/milk products, fruits, vegetables and nuts. Ultra-processed foods contributed to 17 % of total energy intake, 12 % of protein, 17 % of carbohydrate, 29 % of added sugar, 20 % of total fat and 33 % of Na intake. The average risk of nutrient inadequacies for Zn (98 % v. 75 %), folate (67 % v. 22 %) and niacin (83 % v. 44 %) was higher among males than females (P < 0·001). The average risk of nutrient inadequacies for Fe (58 % v. 7 %), vitamin B6 (95 % v. 90 %) and vitamin A (68 % v. 44 %) was higher among females than males (P < 0·001). There was a positive correlation between energy contributed by ultra-processed food and risk of niacin (ρ = 0·136, P = 0·001) and folate (ρ = 0·089, P = 0·049) inadequacy.
Conclusion:
Reformulating ultra-processed food to reduce fat, sugar and salt and increase micronutrients and behaviour change communication strategies that promote dietary diversity will improve micronutrient adequacy and diet quality.
Keywords: Diet quality, Adequacy, Micronutrient, Risk assessment
In India, the prevalence of obesity and diet-related non-communicable diseases such as diabetes and hypertension has risen in the past 5 years(1). Overconsumption of energy-dense and nutrient-poor diets is one of the major factors that increase the risk of development of such diseases(2). Many adults prefer ultra-processed foods that are energy dense, high in fat, sugar and salt and low in nutrient content(3).
The NOVA food classification system groups foods into unprocessed or minimally processed foods (such as fresh fruits and vegetables, grains, milk, eggs), processed foods that are made by adding processed culinary ingredients like salt, oil, butter or sugar to foods (such as canned vegetables and legumes, freshly made unpackaged breads and cheeses) and ultra-processed foods that are made by industrial processes using preservatives, additives, synthetic flavour enhancers in order to enhance the taste, texture, shelf life and convenience (such as ready to consume packaged products like carbonated soft drinks, snacks, chocolate, confectionary, ice cream, breads, spreads, biscuits, cakes, breakfast cereals, fruit drinks, pre-prepared ready to heat foods, instant soups, noodles and desserts)(4). An increased consumption of ultra-processed foods can cause many health challenges such as abdominal obesity, dyslipidaemias, high blood pressure and hyperglycaemia to the population if diets are not modified(5). A study conducted in India showed that the consumption of ultra-processed food has reached all socio-economic segments of the society(6). The main drivers towards the consumption of packaged ultra-processed food among adults are busy schedule, lack of time to prepare cooked meals, irregular work hours and lack of willpower to resist tasty food and adopt healthy eating practices(7).
India is in a state of ‘triple burden of malnutrition’ with rise in diet-related non-communicable diseases and micronutrient deficiencies along with undernutrition(8). Studies have shown that micronutrient deficiencies arise due to inadequate intake of foods and nutrients in the diet. The universally standardised FAO-diet diversity score (FAO-DDS) is a tool often used to assess diet quality using categories of food groups(9). In India, a majority of the population have dietary intake of vegetables (83 %) and fruits (71 %) lower than the MyPlate Indian Council of Medical Research – National Institute of Nutrition (ICMR-NIN) recommendations(10). A study showed that adults in India had greater than 80 % risk of deficiencies of Ca, vitamin A and folate in the diet(11). The national survey also showed that the average nutrient intake of protein, Ca, Fe, thiamine, riboflavin, niacin and vitamin A was below the recommendations among urban adult population(12). Assessing the present dietary situation is a step towards addressing nutrient deficiencies to improve the public health(13). This study aims to assess the contribution of ultra-processed foods to macronutrient intake and the risk of micronutrient inadequacy in the diet.
Methods
Study design
The study had a cross-sectional survey design with non-probability purposive sampling. A multistage non-probability sampling was used, whereby residential areas were selected from each of the four geographical zones of the city by purposive sampling and participants in each selected residential area were selected by snowball sampling. The city of Delhi was geographically divided into four zones: north, south, east and west. A total of twenty-three residential areas from four geographical zones of the city, north (6), south (6), east (5) and west (6), were purposively selected depending on the ease of accessibility and permission from resident welfare associations. Residential areas representing the upper-middle and high-income groups were included based on the categorisation given by the city’s municipal corporation(14). A total of 589 adults in the age group 20–40 years belonging to upper-middle-income group and high-income group were selected, who engaged in the purchase of packaged and ultra-processed food items. We purposively looked at those in the higher income groups because we wanted to explore dietary consumption when there are no financial constraints. Resident welfare associations of residential areas were contacted, and key informants were identified, who helped to identify households where further sample size of the study could be completed. One participant was selected from the identified household and thereafter snowball sampling was applied. Selection of participants depended on the family income also and not solely on the location of the house. The Modified Kuppuswamy’s Socio-economic Scale(15) was applied to categorise participants into, that is, upper-middle-income and high-income groups.
This study was part of a wider research work where the sample size was calculated on the basis of proportion of adults with a low fruit and vegetable consumption, because it is one of the main indicators of diet quality. According to the WHO guidelines, a low fruit and vegetable consumption is defined as consuming less than five servings of fruits and/or vegetables per day(16). A study reported the proportion of adults with low fruits and vegetable consumption to be 74·4 % among males and 74 % among females(17) in India. The sample size for males and females was calculated separately with a CI of 95 % and confidence limits set at 5 %. Assuming a dropout rate of around 10 %, around 684 participants were enrolled in the study; after an attrition of about 14 %, data collection was stopped when 589 participants were enrolled from the four geographical zones of the city based on the inclusion criteria. The sample size estimation is provided in supplemental file (Table 1).
Table 1.
Proportion of individuals consuming different food groups (n 589)
| Food groups | Total | Male | Female | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Starchy staples | 589 | 100 | 293 | 100 | 296 | 100 |
| Dark green leafy vegetables | 69 | 12 | 42 | 14 | 27 | 9 |
| Other vitamin A-rich fruits and vegetables | 15 | 2·5 | 10 | 4 | 5 | 2 |
| Other fruits and vegetables | 589 | 100 | 293 | 100 | 296 | 100 |
| Organ meat | 5 | 1 | 2 | 1 | 3 | 1 |
| Meat and fish | 131 | 22 | 69 | 24 | 62 | 21 |
| Eggs | 91 | 15 | 46 | 16 | 45 | 15 |
| Legumes, nuts and seeds | 589 | 100 | 293 | 100 | 296 | 100 |
| Milk and milk products | 564 | 95 | 287 | 98 | 277 | 94 |
Note: All responses are presented as number (percentage) of participants.
Data collection
The dietary and nutrient intakes were assessed using the 24-h dietary recall method repeated on two non-consecutive days (one working and one non-working). Participants were asked to recall all the food items consumed starting from morning till night over the past 24 h. They were probed on the method of food preparation such as consistency like thin/thick, greasiness of food item and ingredients used in preparation. The amount of food items consumed was recorded by using two- and three-dimensional food models of standardised spoons, ladles, bowls, plates and glass. A calibrated electronic digital weighing scale was used for standardising the amounts of food stuffs consumed by respondents as reported in the 24-h recalls. In the case of ultra-processed packaged food products, nutrient content was taken from the respective food labels and, in the case of composite food dishes, standardised recipes were used for recording ingredients to capture the nutrient contents of dishes(18,19).
Assessing dietary diversity and food group adequacy
The FAO-individual dietary diversity score (DDS), which has a standardised questionnaire for universal application, was used to assess dietary diversity in the diets of participants. For the purpose of scoring, the foods are classified into nine food groups, such as starchy staples, dark green leafy vegetables, other vitamin A-rich fruits and vegetables, other fruits and vegetables, organ meat, meat and fish, eggs, legumes nuts and seeds, milk and milk products. A score of 1 is given for each food group consumed over the past 24 h. The scores were then added to arrive at a final score. There are no established cut-off points, in terms of number of food groups to indicate adequate dietary diversity in the DDS. However, it is recommended to use the mean scores or distribution of scores. Hence, the scores were divided based on tertiles as less than or equal to 4, 5–6 and above 6(9). Food group intake adequacy was assessed on the basis of the ICMR-NIN MyPlate recommendations(10). ‘There were no significant differences in the DDS and adequacy of food groups based on income group of the participants’.
Measuring risk of nutrient inadequacy
First, the individual risk of inadequate intake was estimated based on the probability that the requirement is greater than the intake. The population distribution of the requirement of each nutrient was derived from reported estimated average requirement (EAR), RDA and type of distribution by ICMR. In case of symmetric requirement distribution, normal probability distribution with mean at µ = EAR and sd at σ = (RDA-EAR)/1·96 was assumed. For a positively skewed distribution, lognormal probability distribution with scale and shape parameters as µ = log (EAR) and σ = {log (RDA)-log (EAR)}/1·96 was assumed, respectively. Therefore, the individual risk of inadequate intake of a given intake x was derived as in the case of normal distribution as:
where
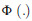 is the cumulative distribution function of standard normal distribution.
is the cumulative distribution function of standard normal distribution.
Statistical analysis
The period of data collection was from March 2019 to February 2020. The 24-h dietary intakes were entered in ‘DietCal’ software version 9.0 (Profound Tech Solutions, 2014), and the nutrient intakes were analysed. The software utilises the nutritive values of food items from the Indian Food Composition Tables(20). The contribution of ultra-processed foods to the daily macronutrient intakes was expressed as percentage. Statistical software R version 4.2.1 (R core Team, 2022) was used to analyse the risk of nutrient inadequacies in the diet (see online Supplemental file, Table 2). The average risk of inadequate intakes with 95 % confidence intervals was reported for each nutrient. Mann–Whitney U test was used to assess the difference in probability of nutrient inadequacy (average risk) across gender. The association between energy contributed by ultra-processed food and risk of micronutrient inadequacy was calculated by Spearman’s rank correlation. Statistical significance was assumed at 5 % level (P < 0·05).
Results
The sample (n 589) comprised of equal proportion of males (50 %) and females (50 %). Participants were divided into upper-middle-income group (n 118) and high-income group (n 471).
Diet diversity scores
Table 1 shows that all participants consumed starchy staples, other fruits and vegetables and legumes, nuts and seeds. Milk and milk products were consumed by most (95 %) of the participants. Few participants consumed meat and fish (22 %), eggs (15 %) and dark green leafy vegetables (12 %). Very few of the participants consumed foods from the other vitamin A-rich fruits and vegetables (2·5 %) and organ meats (1 %). A higher percentage of male participants consumed dark green leafy vegetables (14 % v. 9 %), meat and fish (24 % v. 21 %) and milk and milk products (98 % v. 94 %) compared with females. The average individual DDS among participants was 4·4 ± 0·6, and the range was (3–6) out of a total possible score of 9. Most of the (58 %) participants had DDS in the first tertile consuming only four food groups, 33 % of the participants had scores in the second tertile consuming five food groups and only 9 % had scores in the highest tertile consuming more than six food groups out of the nine food groups. Table 2 shows that the differences in the individual DDS across socio-demographic variables were significant for gender (P = 0·008) and eating habits (P < 0·001). Males and non-vegetarians had a higher dietary diversity score compared with females and vegetarians, respectively.
Table 2.
Diet diversity scores (DDS) among participants (n 589)
| Variables | DDS | Mann–Whitney U test | |
|---|---|---|---|
| Total (n 589) | 4 | 4,5 | |
| Median | IQR | P value | |
| Gender | |||
| Male (n 293) | 5·3 | 4·4–6·2 | 0·008 ** |
| Female (n 296) | 4·8 | 4·3–5·7 | |
| Age | |||
| 20–30 years (n 452) | 4·5 | 3·5–4·6 | 0·88 |
| 30–40 years (n 137) | 4·6 | 3·7–4·5 | |
| Marital status | |||
| Married (n 129) | 4·6 | 3·1–4·8 | 0·77 |
| Unmarried (n 460) | 4·4 | 3·5–4·6 | |
| Eating habit | |||
| Vegetarians (n 382) | 4·5 | 4·1–4·8 | <0·001 ** |
| Non-vegetarians (n 207) | 5·4 | 4·3–6·4 | |
Significant at P < 0·01.
Food group adequacy
Table 3 shows the intake of food groups by participants in comparison with the ICMR-NIN MyPlate recommendations. A majority of participants had dietary intake of cereals (71 %), total fat (83 %) and other foods such as chips, chocolate and packaged foods (86 %) more than the MyPlate recommendations. The intake of most of the participants was less than the MyPlate recommendations for food groups like pulses/eggs/flesh foods (98 %), milk and milk products (90 %), fruits (93 %), vegetables (80 %) and nuts (92 %). The intake of cereals (37 % v. 21 %, P < 0·001) and other foods (18 % v. 10 %, P = 0·005) was within the recommended intakes of a higher proportion of females. A higher percentage of males consumed pulses/eggs/flesh foods (P = 0·03) within the recommended range. A higher percentage of participants belonging to high-income group consumed milk and milk products, vegetables and fruits above the recommendations compared with those belonging to upper middle income. A higher percentage of participants belonging to upper-middle-income group consumed food from other groups within the recommended range compared with those belonging to high income.
Table 3.
Percentage adequacy of dietary intake among participants (n 589) based on ICMR-NIN MyPlate recommendations
| Food groups | Gender | Chi-square test | |||||
|---|---|---|---|---|---|---|---|
| Total (n 589) | Male (n 293) | Female (n 296) | |||||
| n consuming recommended intakes | % | n consuming recommended intakes | % | n | % | ||
| Cereals (≤40 % E) | 173 | 29 | 63 | 21 | 110 | 37 | χ2 = 17·408, P < 0·001 ** |
| Pulses/eggs/flesh foods (≥17 % E) | 11 | 2 | 45 | 15 | 39 | 13 | χ2 = 4·467, P = 0·03 * |
| Total fat (20–30 % E) | 98 | 17 | 57 | 20 | 41 | 14 | χ2 = 3·332, P = 0·06 |
| Milk and milk products (≥300 ml/g) | 61 | 10 | 26 | 9 | 35 | 12 | χ2 = 1·525, P = 0·21 |
| Vegetables (≥300 g) | 115 | 20 | 62 | 21 | 53 | 18 | χ2 = 0·993, P = 0·31 |
| Fruits (≥75 g) | 41 | 7 | 14 | 5 | 27 | 9 | χ2 = 3·065, P = 0·08 |
| Nuts (≥30 g) | 49 | 8 | 48 | 16 | 41 | 14 | χ2 = 0, P-value = 0·98 |
| Others (<10 % of E) | 80 | 14 | 28 | 10 | 52 | 18 | χ2 = 8·051, P = 0·005 * |
Significant at P < 0·05,
Significant at P < 0·01.
Other foods include chips, chocolate and packaged foods.
Eggs/Flesh foods are consumed as alternatives to pulses in Indian diets.
Contribution of ultra-processed food to nutrient intake
According to the NOVA classification, the ultra-processed food items were categorised into the following categories that are packaged and mass produced, that is, beverages (carbonated, milk based, fruit based), savoury snacks (namkeen, chips), confectioneries, chocolate, ice cream, breads, biscuit/cookies, cake/pastries, breakfast cereals, noodles, pasta, processed meat products (ham, sausage, seekh), ready to eat dishes (Indian gravies and pulses), ready to cook (instant soup, frozen vegetarian snacks, frozen non-vegetarian snacks) and spreads/dressings.
Macronutrient contribution to daily energy intake by fat, protein and carbohydrate was 33 %, 9·5 % and 56 %, respectively. The amount of the ultra-processed foods consumed are presented in Table 4. Based on the consumption as recorded during the 24-h recall, 47 % of the participants consumed biscuits, 20 % namkeens (savoury snack), 19 % spreads and dressings, 19 % chips and 14 % breads. Rest of the foods were consumed by less than 10 % of the participants. Table 5 shows that ultra-processed foods contributed to 17 % of total energy intake, 12 % of protein, 17 % of carbohydrate, 29 % of added sugar, 20 % of total fat and 33 % of Na intake in the diet. Male participants had a significantly higher energy (P < 0·001), protein (P = 0·014), total fat (P < 0·001), carbohydrate (P < 0·001) and free sugar (P < 0·001) intake from ultra-processed foods in the diet compared with females.
Table 4.
Consumption of ultra-processed food items (g/ml) among participants (n 589)/d
| Food categories | n | % | Mean (g/ml) | SD | Range (min–max) | Median | IQR |
|---|---|---|---|---|---|---|---|
| Spreads and dressing | 111 | 19 | 11 | 5 | 5–20 | 10 | 10–15 |
| Breads | 85 | 14 | 61 | 15 | 30–90 | 60 | 60–70 |
| Namkeen (savoury snack) | 119 | 20 | 37 | 10 | 10–80 | 40 | 30–45 |
| Biscuits | 274 | 47 | 20 | 7 | 10–70 | 20 | 20–20 |
| Chips | 113 | 19 | 43 | 12 | 20–90 | 40 | 40–50 |
| Breakfast cereal | 23 | 4 | 47 | 12 | 20–80 | 40 | 40–50 |
| Ice creams | 56 | 10 | 49 | 14 | 10–90 | 50 | 40–60 |
| Chocolates | 15 | 3 | 17 | 5 | 10–20 | 20 | 10–20 |
| Cakes | 54 | 9 | 28 | 11 | 10–90 | 30 | 20–30 |
| Carbonated beverage | 52 | 9 | 280 | 93 | 100–500 | 300 | 200–350 |
| Milk-based beverage | 40 | 7 | 215 | 57 | 80–400 | 200 | 200–200 |
| Fruit juice | 22 | 4 | 232 | 41 | 160–360 | 220 | 220–240 |
| Noodles | 48 | 8 | 59 | 12 | 30–80 | 60 | 50–60 |
| Pasta | 35 | 6 | 70 | 14 | 50–90 | 60 | 60–90 |
| Processed meat products | 31 | 5 | 33 | 13 | 15–70 | 30 | 25–40 |
| Ready to eat | 25 | 4 | 90 | 41 | 40–160 | 80 | 48–120 |
| Instant soup | 26 | 4 | 7 | 5 | 2–23 | 5 | 3–10 |
| Frozen vegetarian snacks | 52 | 9 | 37 | 11 | 15–60 | 32 | 32–40 |
| Frozen non-vegetarian snacks | 58 | 10 | 49 | 11 | 5–20 | 45 | 45–60 |
Note: These foods were packaged, and the list of ingredients was checked to classify them as ultra-processed. All responses are presented as number (percentage) of participants.
Table 5.
Dietary intake and contribution of ultra-processed foods to the diet
| Macronutrient | Total (n 589) | Male (n 293) | Female (n 296) | Mann–Whitney U test P value | % nutrient contribution | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Median | IQR | Median | IQR | Median | IQR | Total (n 589) | Male (n 293) | Female (n 296) | ||
| Energy, kcal | 383 | 146 | 374 | 272–465 | 413 | 337–487 | 316 | 240–422 | <0·01 ** | 17 | 18 | 17 |
| Total fat, g | 16 | 7 | 15 | 10–23 | 18 | 12–23 | 12 | 10–20 | <0·01 ** | 20 | 22 | 20 |
| Carbohydrate, g | 52 | 23 | 50 | 36–65 | 52 | 40–67 | 45 | 30–60 | <0·01 ** | 17 | 17 | 17 |
| Protein, g | 6·4 | 3·3 | 6 | 4–8 | 6 | 4·5–7·9 | 5·4 | 3·1–8·3 | 0·014 * | 12 | 11 | 13 |
| Free sugar, g | 17 | 12 | 13 | 9–19 | 15 | 10–21 | 10 | 8–16 | <0·01 ** | 29 | 32 | 24 |
| Na, mg | 266 | 50 | 123 | 51–343 | 126 | 51–350 | 100 | 51–331 | 0·85 | 33 | 26 | 29 |
Significant at P < 0·05,
Significant at P < 0·01.
Note: Percent (%) contribution = nutrient intake from ultra-processed foods/total nutrient intake.
IQR: interquartile range, that is, P25–P75.
Risk of nutrient inadequacies
Nutrient adequacy was analysed by applying the EAR cut point method and by the probability approach (Table 6). Most of the participants had nutrient intakes of Ca (97 %), Zn (98 %), riboflavin (100 %), niacin (68 %), vitamin B6 (96 %), folate (43 %) and vitamin A (60 %) lower than the EAR. The nutrient requirements of Fe, folate and vitamin A are not normally distributed and log values were taken in these cases. The average risk of inadequate intake was considered as the estimate of probability of inadequate intake of the population(21). The nutrient intake was considered adequate if the probability of nutrient inadequacy was less than or equal to 50 %(22).
Table 6.
Average risk of nutrient inadequacy among adults (n 589)
| Nutrients | Intake | Requirements | Risk of inadequacy | P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n 589) | Male | Female | Male | Female | |||||||||||
| <EAR | Mean | sd | Mean | sd | EAR | RDA | EAR | RDA | Males (n 293) | Females (n 296) | |||||
| Mean | 95 % CI | Mean | 95 % CI | ||||||||||||
| Ca (mg) | 572 | 97 | 425 | 137 | 423 | 164 | 800 | 1000 | 800 | 1000 | 0·9575 | 0·94, 0·97 | 0·977 | 0·96, 0·98 | <0·001** |
| Mg (mg) | 11 | 2 | 463 | 68 | 411 | 64 | 320 | 385 | 270 | 325 | 0·029 | 0,1 | 0·021 | 0, 1 | <0·001 ** |
| Fe (mg) | 218 | 37 | 16 | 2 | 14 | 3 | 11 | 19 | 15 | 29 | 0·065 | 0, 0·28 | 0·5887 | 0·2, 0·9 | <0·001 ** |
| Zn (mg) | 581 | 98 | 10 | 1 | 9 | 1 | 14 | 17 | 11 | 13·2 | 0·980 | 0, 1 | 0·7543 | 0, 1 | <0·001 ** |
| Thiamine B1 mg) | 93 | 16 | 1·4 | 0·2 | 1·2 | 0·1 | 1·2 | 1·4 | 1·1 | 1·4 | 0·156 | 0, 0·6 | 0·2468 | 0, 0·9 | <0·001 ** |
| Riboflavin B2 (mg) | 589 | 100 | 0·5 | 0·1 | 0·5 | 0·1 | 1·6 | 2 | 1·6 | 1·9 | 0·999 | 0, 1 | 0·9999 | 0·9, 1 | 0·91 |
| Niacin B3 (mg) | 403 | 68 | 10 | 2 | 9 | 2 | 12 | 14 | 9 | 11 | 0·831 | 0, 1 | 0·4459 | 0, 1 | <0·001 ** |
| Vitamin B6 (mg) | 570 | 96 | 1·2 | 0·1 | 1·1 | 0·1 | 1·6 | 1·9 | 1·6 | 1·9 | 0·906 | 0·55, 1 | 0·9531 | 0·7, 1 | <0·001 ** |
| Folate B9 (µg) | 255 | 43 | 231 | 40 | 220 | 50 | 250 | 300 | 180 | 220 | 0·675 | 0,1 | 0·2203 | 0, 1 | <0·001 ** |
| Vitamin C (mg) | 27 | 5 | 92 | 23 | 90 | 25 | 65 | 80 | 55 | 65 | 0·117 | 0, 0·7 | 0·0522 | 0, 1 | <0·001 ** |
| Vitamin A (µg) | 353 | 60 | 503 | 110 | 330 | 191 | 460 | 1000 | 390 | 840 | 0·437 | 0·1, 0·77 | 0·680 | 0·1, 1 | <0·001 ** |
Significant at P < 0·01.
The average risk of nutrient inadequacies for Zn, niacin and folate was higher among males than females (P < 0·001). The average intake of niacin was (10 ± 2) mg/d in males and (9 ± 2) mg/d in females, while the EAR is 12 mg/d for males and 9 mg/d for females and the average risk of niacin inadequacy among males was 83 % and 44 % among females, so the dietary niacin was inadequate for males and not for females (P < 0·001). The average intake of folate was (231 ± 40) µg/d in males and (220 ± 50) µg/d in females, while the EAR is 250 µg/d for males and 180 µg/d for females and the average risk of folate inadequacy among males was 67 % and 22 % among females so the dietary folate was inadequate for males and not for females (P < 0·001).
The average risk of nutrient inadequacies for Ca (97 % v. 95 %), Fe (58 % v. 7 %), vitamin B6 (95 % v. 90 %) and vitamin A (68 % v. 44 %) was higher among females than males (P < 0·001). The average intake of vitamin A was (503 ± 110) µg/d in males and (330 ± 191) µg/d in females, while the EAR is 460 µg/d for males and 390 µg/d for females and the average risk of vitamin A inadequacy among males was 44 % and 68 % among females so the dietary vitamin A was inadequate for females and not for males (P < 0·001).
The average risk of nutrient inadequacies for Mg, thiamine B1 and vitamin C was below 50 % for both genders, so the diets were more than adequate for these nutrients. The average risk of nutrient inadequacy for riboflavin among participants was 99 % and their diets were highly inadequate in these nutrients. There were significant associations (P < 0·05) between the risk of nutrient inadequacy and the percentage energy contributed by ultra-processed food (Table 7). There was weak negative correlation between the percentage energy contributed by ultra-processed food and the risk of Fe (ρ = −0·08, P < 0·05), vitamin A (ρ = −0·084, P = 0·041) and riboflavin (ρ = −0·152, P < 0·001) inadequacy. There was a positive correlation between energy contributed by ultra-processed food and the risk of niacin (ρ = 0·136, P = 0·001) and folate (ρ = 0·089, P = 0·049) inadequacy.
Table 7.
Association between percentage energy contributed by ultra-processed food and the individual risk of nutrient inadequacy
| Nutrients | Spearman’s rho (ρ) | P value |
|---|---|---|
| Ca (mg) | 0·044 | 0·29 |
| Mg (mg) | 0·019 | 0·64 |
| Fe (mg) | −0·088 | 0·03 * |
| Zn (mg) | 0·007 | 0·86 |
| Thiamine B1 mg) | −0·078 | 0·05 |
| Riboflavin B2 (mg) | −0·152 | <0·001 ** |
| Niacin B3 (mg) | 0·136 | <0·001 ** |
| Vitamin B6 (mg) | −0·062 | 0·13 |
| Folate B9 (µg) | 0·081 | 0·049 * |
| Vitamin C (mg) | −0·036 | 0·38 |
| Vitamin A (µg) | −0·084 | 0·04 * |
significant at P < 0·05,
significant at P < 0·01.
Discussion
In the present study, the mean dietary diversity score could only reach half of the total possible score. The major food groups missing from diets included meat and fish, eggs, vitamin A-rich fruits and vegetables and dark green leafy vegetables. However, it was heartening to see that all respondents were consuming legumes, nuts and seeds that are rich sources of protein, good fats, fibre and several vitamins and minerals. Legumes have been associated with several health benefits such as improved gut functioning, lowered risk of diabetes, heart diseases, hypertension and overweight(23). Compared with the ICMR-NIN MyPlate recommendations, the majority of the participants (>80 %) had dietary intake of pulses/eggs/flesh foods, milk and milk products, fruits, vegetables and nuts below the recommendations. These protective food groups are hence not being consumed in adequate quantities to derive their health benefits. Milk and milk products are the most bioavailable form of Ca, which is important for bone health. Fruits and vegetables are a store house of vitamins and minerals and also fibre, which protects against several diet-related non-communicable diseases. Similar to our results, a study conducted in Kolkata, India showed that less than half of the respondents consumed dark green leafy vegetables (45 %), vitamin A-rich fruits and vegetables (38 %), organ meats (35 %) and eggs (12 %) even in urban, high-income areas(24). In another study conducted in China, the mean DDS score was 5·2 ± 1·1, but the intake of vegetables, fruits and eggs was lower as per the recommendations(25). Most (82 %) of the participants had total fat intake more than 30 % of total energy per day and 18 % had total fat intake between 20 % and 30 % of total energy per day. None of the respondents had total fat intake less than 20 % of the total energy per day, while some (20 %) participants consumed more than 35 energy %. Most (65·5 %) of the participants consumed more than 10 % of total energy per day from added sugars.
The ‘MyPlate for the Day’ developed by the ICMR-NIN has been designed on the basis of RDA, which typically illustrates proportion of foods from different food groups to be sourced for a 2000 kcal Indian diet. In the present research, male participants and those who had non-vegetarian eating habit had a higher DDS. In comparison with the ICMR-NIN MyPlate recommendations, a higher percentage of males had dietary intake from pulses/eggs/flesh foods (P = 0·03) within the recommended intakes compared with females. A study conducted among adults showed that those with low intake of non-vegetarian foods have lower DDS with higher nutrient inadequacies(26). Generally, women opt for vegetarian foods due to the personal beliefs, consciousness towards body image and a feeling of aversion towards meat products(27). Also, a higher percentage of participants from the high-income group had food group adequacy from pulses/eggs/flesh foods, milk and milk products, vegetables and fruits compared with those belonging to upper-middle income. However, a higher percentage of participants from upper-middle-income group consumed food from others group within the recommended range compared with those belonging to high income. Participants from the income groups studied were not constrained by the price of food products. This shows that belonging to a higher socio-economic income group does not always ensure a healthy diet.
In a study conducted in Philippines, rise in socio-economic income led to increased intake of beneficial foods such as milk and chicken in the diet. However, it also resulted in an increased consumption of savoury snacks, ice creams, processed meat and sugar-sweetened beverages among adolescents(28). A study showed that an improved socio-economic condition could lead to increased access and affordability to food items, but choosing healthier food options requires knowledge and understanding of healthy diet(29). A study conducted using dataset from the Indian NFHS 4 survey showed that women in the highest income quintile (OR = 3·24, P < 0·001) and those who had high dietary diversity (OR = 1·21, P < 0·001) were more likely to be overweight and obese. Increased intakes of ultra-processed foods could be one of the potential causes as they tend to be high in fat, sugar and salt(30).
Results in this study showed that the majority of participants had the risk of micronutrient inadequacies in the diet. The risk of inadequate nutrient intake is defined as the area under the requirement distribution curve that is above the intake, where 50th percentile is the EAR and 97·5th percentile is the RDA(31). Micronutrient inadequacies lead to deficiencies, which cause impaired immune function and poor metabolic and cognitive functions in the human body(32). In a study conducted in India, the mean probability of adequacy for nutrients such as thiamine, riboflavin, niacin, Zn, Fe, vitamin A, vitamin C and Ca was only 37 %, which meant that the diet was inadequate(33).
Creating awareness about dietary diversity and increasing intake of the healthier food groups is a cost-effective way of improving diet quality(34). Nutrition education is a cost-effective strategy that has shown to increase the dietary diversity by 3 % within the household and 9 % increase in the dietary diversity in women in Africa(35). In an intervention study in Bangladesh, where interpersonal counselling and community awareness were utilised to improve diets, showed that the purchase of eggs, flesh foods increased in the intervention group (P < 0·01) while purchase of packaged juices, carbonated beverages reduced (P < 0·01) as compared to the baseline(36). Food synergy such as including Fe-rich foods along with vitamin C-rich foods in the diet will help to improve the bioavailability of micronutrients(37).
In the present study, the consumption of ultra-processed foods was majorly from food categories such as beverages, biscuits, pasta and noodles, ice creams and chips. Akin to our results, a study conducted in Sao Paulo using 24-h recall showed that commonly consumed ultra-processed foods were carbonated beverages (31 %), biscuits (20 %), packaged snacks (16 %), processed meat (13 %) and ice cream (10 %) among adults(38). Results from the present study showed that the intake of ultra-processed foods contributed to 17 % of total energy intake in the diets, which is lower compared with developed nations. Studies conducted using 24-h dietary recall showed that the intake of ultra-processed foods in developed nations contributed to 25 %, 42 % and 58 % of total energy intake in the diets in Korea, Australia and United States, respectively(39,40). A study using the Euromonitor database showed that the sale of ultra-processed food in India was projected to rise from 2000 to 2017 with a greater percentage share from carbonated beverages, biscuits, sweet and savoury snacks(41). Results showed that although nutrient intakes derived from ultra-processed foods were not in large amounts, for example, the total fat from ultra-processed food was 16 g, carbohydrate was 52 g, protein was 6·4 g, free sugar was 17 g (approximately 3 teaspoons) and Na was 266 mg; still, ultra-processed foods contributed to one-fifth of the total fat intake in the diet and around one-third of the free sugar and Na in the diets of Indian adults. Data from surveys conducted in urban India by the National Nutrition Monitoring Bureau in 2016 showed that ultra-processed foods contributed to 11·1 % of total energy intake, 10·3 % of protein, 10·7 % of carbohydrate and 11·6 % of total fat in the diet(10). Over the years, the contribution of ultra-processed foods in the diets of urban Indians has increased, which is evident in the present study as these foods contributed to 17 % of total energy intake, 12 % of protein, 17 % of carbohydrate, 29 % of added sugar, 20 % of total fat and 33 % of Na intake in the diets among adults. Nutrition transition in India is witnessing a huge shift in dietary pattern as seen by an increasing intake of ultra-processed foods that have replaced home-cooked foods. Appropriate interventions at the right time can help to slow down an increase in the consumption pattern trajectory in lower middle-income countries such as India that presently matches with that of developed nations(42). Overconsumption of ultra-processed food high in fat, sugar and salt is associated with obesity and the risk of developing many diet-related non-communicable diseases such as diabetes mellitus, CHD and cancers in adults(43). Owing to the harmful effects of ultra-processed foods and increased prevalence of diet-related non-communicable diseases in India, efforts should be made to monitor and decrease the daily consumption of ultra-processed foods among Indian adults. In the present study, there were positive significant associations between the percent energy contributed by ultra-processed food and the risk of niacin and folate inadequacies. This means that with an increase in intake of calories from ultra-processed food the risk of nutrient inadequacy also increased among participants. In another study conducted in Mexico, nutrient intakes of vitamin B6, Ca, Zn, niacin and folate from ultra-processed food component of the diet were lower (P < 0·01) when compared with the non-ultra-processed food intake(44). In the present study, some significant but weak negative correlations were observed in the case of Fe, vitamin A and riboflavin. This may be related to the kind of ultra-processed food consumed by participants. Some of the ultra-processed foods may be fortified and not necessarily high in fat, salt and sugar. A study conducted in Australia where fortified breads and breakfast cereals were excluded from the diet showed significant reductions (P < 0·05) in carbohydrate, fibre, thiamine, niacin, Fe and Zn intake in the diets of adults. Fulfilling these nutrient deficits may not be realistic from other cooked sources since there are barriers such as lack of time, lack of cooking skills and busy work schedules. Thus, healthfulness of ultra-processed foods should be assessed after considering the beneficial and harmful nutrients(45). Some of the ultra-processed foods such as beverages and instant pre-mixes contain added micronutrients and vegetables to enhance the healthfulness of the food product(46).
Advocating for reformulation of ultra-processed packaged foods to reduce the content of fat, sugar and salt in it will ensure healthier intakes among population(47). Application of front-of-pack labelling schemes on ultra-processed foods can be effective to help consumers judge the healthfulness of a food product(48). Policies such as taxation on ultra-processed food that are high in fat, sugar and salt should be implemented by governments to reduce their consumption(49). A study conducted in Chile showed that fiscal policies such as the combined effects of applying an 18 % tax on foods such as sweets and snacks along with subsidies on fruits and vegetables by removing the 19 % value-added tax is a way to improve dietary choices even among those in the highest income quintiles(50).
The strength of the present study is that the results can be used as baseline information before starting an intervention to improve diet quality. This study also measures the risk of nutrient inadequacy using the latest ICMR Nutrient requirements (2020) and links it with the proportion of total energy contributed by ultra-processed food in the diet. The limitation of the present study was that the study was conducted in Delhi on only upper middle-income and high-income group young adults, which limits generalisation of the findings to other regions of the country, income and age categories. In the case of collecting dietary intakes through 24-h dietary recall, a recall bias cannot be ruled out. However, an effort to reduce the bias was made by displaying three dimensional models of spoons and bowls, along with their respective picture cards, which helped by acting as memory cues.
Conclusion
Reduced dietary diversity and food group adequacy can still exist irrespective of the income status of an individual. To improve the micronutrient adequacy and dietary diversity, investment in behaviour change communication strategies can be rewarding. It is important to generate knowledge and understand the perceptions of the community regarding healthy eating to address the problem of micronutrient inadequacies in diets. Policymakers should monitor the consumption of ultra-processed foods in India and take appropriate measures to improve dietary habits. It is also important for the industry to reformulate ultra-processed foods to decrease fat, salt and sugar content and offer minimally processed, micronutrient-rich food products to the consumer.
Supporting information
Mediratta et al. supplementary material
Acknowledgements
The first author is grateful to University Grants Commission (India) for the award of Senior Research Fellowship to her.
Financial support
The first author received Junior and Senior Research Fellowship (Student ID: 1509/ NET-DEC. 2015) from the University Grants Commission (India). The University Grants Commission (India) had no role in the design, data collection, analysis or writing of this article.
Conflicts of interest
The authors report no conflict of interest towards production of the article. All authors have approved the final version of the article. The article is an original work of the authors and is not under consideration for publication elsewhere.
Authorship
The first author (S.M.) was involved in conducting literature review, conceptualisation, methodology, data curation, collecting resources, formal analysis, visualisation, interpretation and writing – original draft. The second author (S.G.) was involved in conceptualisation, formal analysis, interpretation of data and review of the draft. The third author (P.M.) was involved in providing critical suggestions for designing the study, conceptualisation, visualisation, interpretation, supervision and review of the draft. All authors have approved the final article.
Ethics of human subject participation
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Ethics Committee (IEC) of Lady Irwin College, New Delhi, India. Written informed consent was obtained from all subjects and participants were informed about the purpose of the study.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980023002112
References
- 1. International Institute for Population Sciences (2021) National Family Health Survey 5- Union Territory Fact Sheet NCT Delhi. New Delhi: IIPS, Ministry of Health and Family Welfare, Government of India. [Google Scholar]
- 2. World Health Organization (2020) Countdown to 2023. WHO Report on Global Trans-Fat Elimination 2020. Geneva: WHO. [Google Scholar]
- 3. Gopalan HS, Misra A & Jayawardena R (2018) Nutrition and diabetes in South Asia. Eur J Clin Nutr 79, 1267–1273. [DOI] [PubMed] [Google Scholar]
- 4. Monteiro CA, Cannon G, Lawrence M et al. (2019) Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System. Rome: FAO. [Google Scholar]
- 5. Olatona FA, Onabanjo OO, Ugbaja RN et al. (2018) Dietary habits and metabolic risk factors for non-communicable diseases in a university undergraduate population. J Health Popul Nutr 37, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jain A & Mathur P (2020) Intake of ultra-processed foods among adolescents from low-and middle-income families in Delhi. Indian Pediatr 57, 712–714. [PubMed] [Google Scholar]
- 7. Pinho MGM, Mackenbach JD, Charreire H et al. (2018) Exploring the relationship between perceived barriers to healthy eating and dietary behaviours in European adults. Eur J Nutr 57, 1761–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shankar B, Agrawal S, Beaudreault AR et al. (2017) Dietary and nutritional change in India: implications for strategies, policies, and interventions. Ann N Y Acad Sci 1395, 49–59. [DOI] [PubMed] [Google Scholar]
- 9. Kennedy G, Ballard T & Dop MC (2013) Guidelines for Measuring Household and Individual Dietary Diversity. Rome: Nutrition and Consumer Protection Division, Food and Agriculture Organization of the United Nations. [Google Scholar]
- 10. Indian Council of Medical Research & National Institute of Nutrition (2018) What India Eats- A Report of the Expert Group. Hyderabad: ICMR-NIN, Ministry of Health and Family Welfare, Government of India. [Google Scholar]
- 11. Ritchie H, Reay DS & Higgins P (2018) Quantifying, projecting, and addressing India’s hidden hunger. Front Sustain Food Syst 2, 1–13. [Google Scholar]
- 12. National Nutrition Monitoring Bureau (2017) Diet and Nutritional Status of Urban Population in India and Prevalence of Obesity, Hypertension, Diabetes and Hyperlipidemia in Urban Men and Women. NNMB Technical Report no. Hyderabad: National Institute of Nutrition. [Google Scholar]
- 13. Magee PJ & McCann MT (2019) Micronutrient deficiencies: current issues. Proc Nutr Soc 78, 147–149. [DOI] [PubMed] [Google Scholar]
- 14. Municipal Corporation of Delhi (2014) Circle Rate Calculator – Delhi Online Registration Information System. Government of National Capital Territory of Delhi. https://eval.delhigovt.nic.in/ (accessed March 2019).
- 15. Singh T, Sharma S & Nagesh S (2017) Socio-economic status scales updated for 2017. Int J Res Med Sci 5, 3264–3267. [Google Scholar]
- 16. World Health Organization (2020) Healthy Diet Factsheet. https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed January 2021).
- 17. Hall JN, Moore S, Harper SB et al. (2009) Global variability in fruit and vegetable consumption. Am J Prev Med 36, 402–409. [DOI] [PubMed] [Google Scholar]
- 18. Raina U, Kashyap S, Narula V et al. (2008) Basic Food Preparation. A Complete Manual, 3rd ed. New Delhi: Orient Blackswan. [Google Scholar]
- 19. Raj R & Kurpad A (2015) Nutritive Value of Common Indian Cooked Foods. Nutrition in Obesity and Diabetes, 1st ed. New Delhi: Jaypee Brothers Medical Publishers. [Google Scholar]
- 20. Longvah T, Ananthan R, Bhaskarachary K et al. (2017) Indian Food Composition Tables (IFCT). Hyderabad: Department of Health Research, National Institute of Nutrition, Indian Council of Medical Research. [Google Scholar]
- 21. Indian Council of Medical Research & National Institute of Nutrition (2020) Recommended Dietary Allowances and Estimated Average Requirements -Nutrient Requirements for Indians. A Report of the Expert Group of the Indian Council of Medical Research- National Institute of Nutrition. Hyderabad: ICMR-NIN. [Google Scholar]
- 22. Kurpad AV, Ghosh S, Thomas T et al. (2021) When the cure might become the malady: the layering of multiple interventions with mandatory micronutrient fortification of foods in India. Am J Clin Nutr 114, 1261–1266. [DOI] [PubMed] [Google Scholar]
- 23. Affrifah NS, Uebersax MA & Amin S (2023) Nutritional significance, value-added applications, and consumer perceptions of food legumes: a review. Legume Sci 192, 1–16. [Google Scholar]
- 24. Mukherjee A, Paul S, Saha I et al. (2018), Dietary diversity and its determinants: a community-based study among adult population of Durgapur, West Bengal. Med J DY Patil Vidyapeeth 11, 296–301. [Google Scholar]
- 25. Zhang Q, Chen X, Liu Z et al. (2017) Diet diversity and nutritional status among adults in southwest China. PLOS ONE 12, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ganpule-Rao AV, Bhat D, Yajnik CS et al. (2021) Dietary diversity scores, nutrient intakes and biomarkers vitamin B12, folate and Hb in rural youth from the Pune Maternal Nutrition Study. Br J Nutr 126, 236–243. [DOI] [PubMed] [Google Scholar]
- 27. Siegrist M & Hartmann C (2019) Impact of sustainability perception on consumption of organic meat and meat substitutes. Appetite 132, 196–202. [DOI] [PubMed] [Google Scholar]
- 28. Angeles AI, Lenighan YM, Jacquier EF et al. (2019) The impact of wealth status on food intake patterns in Filipino school-aged children and adolescents. Nutrients 11, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chae W, Ju YJ, Shin J et al. (2018) Association between eating behaviour and diet quality: eating alone v. eating with others. Nutr J 17, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Young MF, Nguyen P, Tran LM et al. (2020) A double edged sword? Improvements in economic conditions over a decade in India led to declines in undernutrition as well as increases in overweight among adolescents and women. J Nutr 150, 364–372. [DOI] [PubMed] [Google Scholar]
- 31. Ghosh S, Thomas T, Pullakhandam R et al. (2023) A proposed method for defining the required fortification level of micronutrients in foods: an example using iron. Eur J Clin Nutr 77, 436–446. [DOI] [PubMed] [Google Scholar]
- 32. DiBaise M & Tarleton SM (2019) Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract 34, 490–503. [DOI] [PubMed] [Google Scholar]
- 33. Shalini T, Sivaprasad M, Balakrishna N et al. (2019) Micronutrient intakes and status assessed by probability approach among the urban adult population of Hyderabad city in South India. Eur J Nutr 58, 3147–3159. [DOI] [PubMed] [Google Scholar]
- 34. Gupta S, Sunder N & Pingali PL (2020) Market access, production diversity, and diet diversity: evidence from India. Food Nutr Bull 41, 167–185. [DOI] [PubMed] [Google Scholar]
- 35. Murendo C, Nhau B, Mazvimavi K et al. (2018) Nutrition education, farm production diversity, and commercialization on household and individual dietary diversity in Zimbabwe. Food Nutr Res 62, 1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Warren AM, Frongillo EA, Nguyen PH et al. (2020) Nutrition intervention using behavioral change communication without additional material inputs increased expenditures on key food groups in Bangladesh. J Nutr 150, 1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nair KM & Augustine LF (2018) Food fortification and dietary diversification as effective interventions to improve iron status of Indian population. Proc Indian Natn Sci Acad 84, 891–897. [Google Scholar]
- 38. Costa CD, Faria FR, Gabe KT et al. (2021) Nova score for the consumption of ultra-processed foods: description and performance evaluation in Brazil. Rev Saúde Pública 55, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Machado PP, Steele EM, Levy RB et al. (2019) Ultra-processed foods and recommended intake levels of nutrients linked to non-communicable diseases in Australia: evidence from a nationally representative cross-sectional study. BMJ Open 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shim JS, Shim SY, Cha HJ et al. (2022) Association between ultra-processed food consumption and dietary intake and diet quality in Korean adults. J Acad Nutr Diet 122, 583–594. [DOI] [PubMed] [Google Scholar]
- 41. Baker P & Friel S (2016) Food systems transformations, ultra-processed food markets and the nutrition transition in Asia. Glob Health 12, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Popkin BM & Ng SW (2022) The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes Rev 23, 13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elizabeth L, Machado P, Zinöcker M et al. (2020) Ultra-processed foods and health outcomes: a narrative review. Nutrients 12, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marrón-Ponce JA, Sánchez-Pimienta TG, Rodríguez-Ramírez S et al. (2023) Ultra-processed foods consumption reduces dietary diversity and micronutrient intake in the Mexican population. J Hum Nutr Diet 36, 241–251. [DOI] [PubMed] [Google Scholar]
- 45. Estell ML, Barrett EM, Kissock KR et al. (2022) Fortification of grain foods and NOVA: the potential for altered nutrient intakes while avoiding ultra-processed foods. Eur J Nutr 61, 935–945. [DOI] [PubMed] [Google Scholar]
- 46. Kroker-Lobos MF, Mazariegos M, Guamuch M et al. (2022) Ultra processed products as food fortification alternatives: a critical appraisal from Latin America. Nutrients 14, 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gupta P & Sachdev HS (2022) The escalating health threats from ultra-processed and high fat, salt, and sugar foods: urgent need for tailoring policy. Indian Pediatr 59, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vargas-Meza J, Jáuregui A, Pacheco-Miranda S et al. (2019) Front-of-pack nutritional labels: understanding by low-and middle-income Mexican consumers. PLOS ONE 14, 0225268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sánchez-Romero LM, Canto-Osorio F, González-Morales R et al. (2020) Association between tax on sugar sweetened beverages and soft drink consumption in adults in Mexico: open cohort longitudinal analysis of Health Workers Cohort Study. BMJ 369, 1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caro JC, Valizadeh P, Correa A et al. (2020) Combined fiscal policies to promote healthier diets: effects on purchases and consumer welfare. PLOS ONE 15, 0226731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mediratta et al. supplementary material


