Abstract
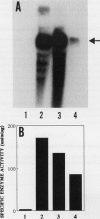
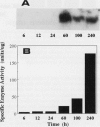
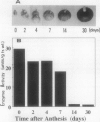
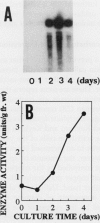
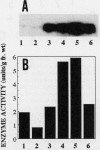
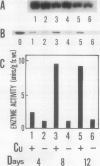
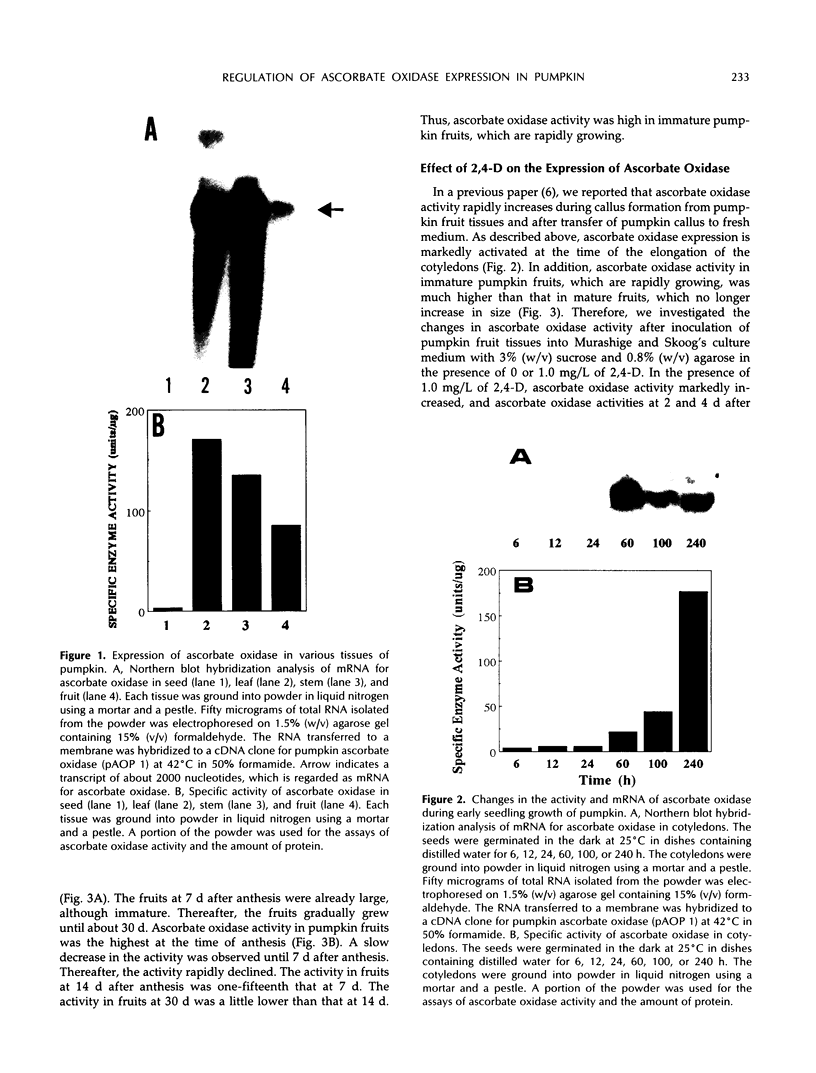
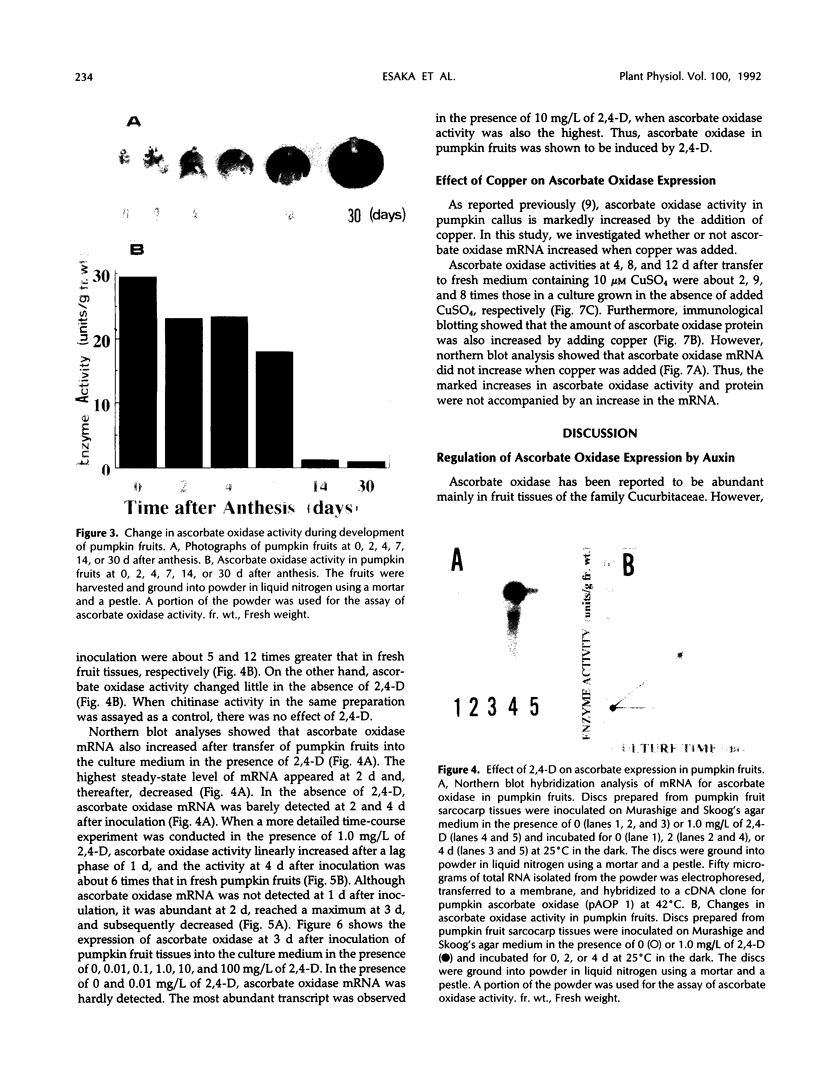
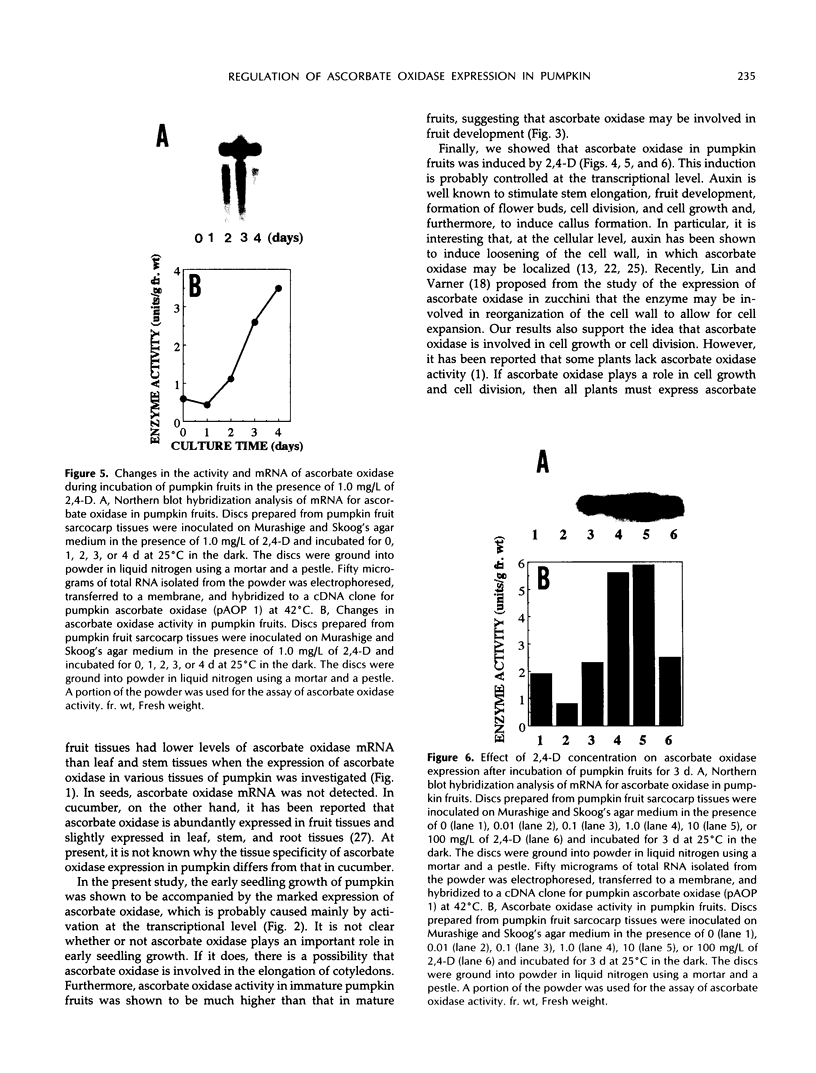
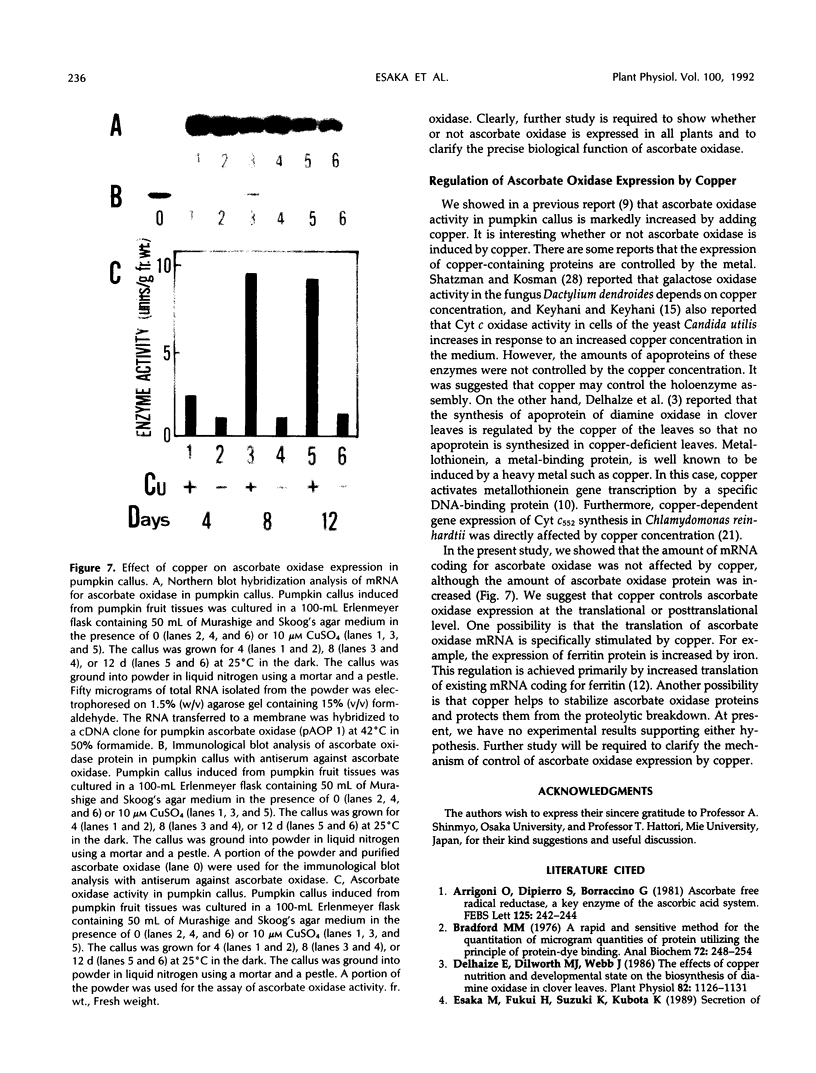
Ascorbate oxidase expression in pumpkin (Cucurbita spp.) tissues was studied. Specific ascorbate oxidase activities in pumpkin leaf and stem tissues were about 2 and 1.5 times that in the fruit tissues, respectively. In seeds, little ascorbate oxidase activity was detected. Northern blot analyses showed an abundant ascorbate oxidase mRNA in leaf and stem tissues. Fruit tissues had lower levels of ascorbate oxidase mRNA than leaf and stem tissues. Ascorbate oxidase mRNA was not detected in seeds. Specific ascorbate oxidase activity gradually increased during early seedling growth of pumpkin seeds. The increase was accompanied by an increase in ascorbate oxidase mRNA. When ascorbate oxidase activity in developing pumpkin fruits was investigated, the activities in immature fruits that are rapidly growing at 0, 2, 4, and 7 d after anthesis were much higher than those in mature fruits at 14 and 30 d after anthesis. The specific activity and mRNA of ascorbate oxidase markedly increased after inoculation of pumpkin fruit tissues into Murashige and Skoog's culture medium in the presence of an auxin such as 2,4-dichlorophenoxyacetic acid (2,4-D) but not in the absence of 2,4-D. In the presence of 10 mg/L of 2,4-D, ascorbate oxidase mRNA was the most abundant. Thus, ascorbate oxidase is induced by 2,4-D. These results indicate that ascorbate oxidase is involved in cell growth. In pumpkin callus, ascorbate oxidase activity could be markedly increased by adding copper. Furthermore, immunological blotting showed that the amount of ascorbate oxidase protein was also increased by adding copper. However, northern blot analyses showed that ascorbate oxidase mRNA was not increased by adding copper. We suggest that copper may control ascorbate oxidase expression at translation or at a site after translation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Delhaize E., Dilworth M. J., Webb J. The effects of copper nutrition and developmental state on the biosynthesis of diamine oxidase in clover leaves. Plant Physiol. 1986 Dec;82(4):1126–1131. doi: 10.1104/pp.82.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaka M., Hattori T., Fujisawa K., Sakajo S., Asahi T. Molecular cloning and nucleotide sequence of full-length cDNA for ascorbate oxidase from cultured pumpkin cells. Eur J Biochem. 1990 Aug 17;191(3):537–541. doi: 10.1111/j.1432-1033.1990.tb19154.x. [DOI] [PubMed] [Google Scholar]
- Esaka M., Uchida M., Fukui H., Kubota K., Suzuki K. Marked increase in ascorbate oxidase protein in pumpkin callus by adding copper. Plant Physiol. 1988 Nov;88(3):656–660. doi: 10.1104/pp.88.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P., Hu S., Hackett R., Hamer D. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell. 1988 Nov 18;55(4):705–717. doi: 10.1016/0092-8674(88)90229-2. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Harrell C. M., McKenzie A. R., Patino M. M., Walden W. E., Theil E. C. Ferritin mRNA: interactions of iron regulatory element with translational regulator protein P-90 and the effect on base-paired flanking regions. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4166–4170. doi: 10.1073/pnas.88.10.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S. I. Ascorbic Acid Oxidase in Barley Roots. Plant Physiol. 1955 Mar;30(2):174–181. doi: 10.1104/pp.30.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Carbonell J. Regulation of the synthesis of barley aleurone alpha-amylase by gibberellic Acid and calcium ions. Plant Physiol. 1984 Sep;76(1):213–218. doi: 10.1104/pp.76.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhani E., Keyhani J. Cytochrome c oxidase biosynthesis and assembly in Candida utilis yeast cells. Function of copper in the assembly of active cytochrome c oxidase. Arch Biochem Biophys. 1975 Apr;167(2):596–602. doi: 10.1016/0003-9861(75)90503-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Dawson C. R. Ascorbate oxidase. Further studies on the purification of the enzyme. J Biol Chem. 1973 Oct 10;248(19):6596–6602. [PubMed] [Google Scholar]
- Lin L. S., Varner J. E. Expression of Ascorbic Acid Oxidase in Zucchini Squash (Cucurbita pepo L.). Plant Physiol. 1991 May;96(1):159–165. doi: 10.1104/pp.96.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Yamada K., Osajima Y. Ascorbate electrode for determination of L-ascorbic acid in food. Anal Chem. 1981 Nov;53(13):1974–1979. doi: 10.1021/ac00236a006. [DOI] [PubMed] [Google Scholar]
- Merchant S., Bogorad L. Metal ion regulated gene expression: use of a plastocyanin-less mutant of Chlamydomonas reinhardtii to study the Cu(II)-dependent expression of cytochrome c-552. EMBO J. 1987 Sep;6(9):2531–2535. doi: 10.1002/j.1460-2075.1987.tb02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. L., Miller S. C., Torti S. V., Tsuji Y., Torti F. M. Iron-independent induction of ferritin H chain by tumor necrosis factor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4946–4950. doi: 10.1073/pnas.88.11.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWCOMB E. H. Effect of auxin on ascorbic oxidase activity in tobacco pith cells. Proc Soc Exp Biol Med. 1951 Mar;76(3):504–509. doi: 10.3181/00379727-76-18538. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Makino N., Ogura Y. Purification and properties of ascorbate oxidase from cucumber. J Biochem. 1968 Aug;64(2):189–195. doi: 10.1093/oxfordjournals.jbchem.a128879. [DOI] [PubMed] [Google Scholar]
- OBERBACHER M. F., VINES H. M. Spectrophotometric assay of ascorbic acid oxidase. Nature. 1963 Mar 23;197:1203–1204. doi: 10.1038/1971203a0. [DOI] [PubMed] [Google Scholar]
- Ohkawa J., Okada N., Shinmyo A., Takano M. Primary structure of cucumber (Cucumis sativus) ascorbate oxidase deduced from cDNA sequence: homology with blue copper proteins and tissue-specific expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1239–1243. doi: 10.1073/pnas.86.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARK G. R., DAWSON C. R. On the accessibility of sulfhydryl groups in ascorbic acid oxidase. J Biol Chem. 1962 Mar;237:712–716. [PubMed] [Google Scholar]
- Shatzman A. R., Kosman D. J. The utilization of copper and its role in the biosynthesis of copper-containing proteins in the fungus, Dactylium dendroides. Biochim Biophys Acta. 1978 Nov 15;544(1):163–179. doi: 10.1016/0304-4165(78)90220-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines H. M., Oberbacher M. F. Citrus Fruit Enzymes. I. Ascorbic Acid Oxidase in Oranges. Plant Physiol. 1963 May;38(3):333–337. doi: 10.1104/pp.38.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W. Ascorbic acid and biological systems. Ascorbic acid and electron transport. Ann N Y Acad Sci. 1975 Sep 30;258:190–200. doi: 10.1111/j.1749-6632.1975.tb29279.x. [DOI] [PubMed] [Google Scholar]