Abstract
Background
There are now hundreds of systematic reviews on attention deficit hyperactivity disorder (ADHD) of variable quality. To help navigate this literature, we have reviewed systematic reviews on any topic on ADHD.
Methods
We searched MEDLINE, PubMed, PsycINFO, Cochrane Library, and Web of Science and performed quality assessment according to the Joanna Briggs Institute Manual for Evidence Synthesis. A total of 231 systematic reviews and meta-analyses met the eligibility criteria.
Results
The prevalence of ADHD was 7.2% for children and adolescents and 2.5% for adults, though with major uncertainty due to methodological variation in the existing literature. There is evidence for both biological and social risk factors for ADHD, but this evidence is mostly correlational rather than causal due to confounding and reverse causality. There is strong evidence for the efficacy of pharmacological treatment on symptom reduction in the short-term, particularly for stimulants. However, there is limited evidence for the efficacy of pharmacotherapy in mitigating adverse life trajectories such as educational attainment, employment, substance abuse, injuries, suicides, crime, and comorbid mental and somatic conditions. Pharmacotherapy is linked with side effects like disturbed sleep, reduced appetite, and increased blood pressure, but less is known about potential adverse effects after long-term use. Evidence of the efficacy of nonpharmacological treatments is mixed.
Conclusions
Despite hundreds of systematic reviews on ADHD, key questions are still unanswered. Evidence gaps remain as to a more accurate prevalence of ADHD, whether documented risk factors are causal, the efficacy of nonpharmacological treatments on any outcomes, and pharmacotherapy in mitigating the adverse outcomes associated with ADHD.
Keywords: Child and adolescent psychiatry, ADHD, Systematic reviews, Epidemiology, Public Health
Introduction
There are hundreds of systematic reviews on attention deficit hyperactivity disorder (ADHD) of variable quality and with partly or fully overlapping scope. This literature is increasingly difficult to navigate for clinicians, researchers, and policymakers. We aim to make this large literature on ADHD more available by systematically reviewing the published systematic reviews on ADHD and highlighting recent reviews of high quality where there are overlaps.
Methods
We performed a meta-review [1] to systematically appraise systematic reviews and meta-analyses published on ADHD-related topics by adopting Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [2] (Supplementary Table S1) and the Joanna Briggs Institute (JBI) methodology for umbrella review [3]. The study protocol was pre-registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020165638).
Search strategy and selection criteria Table 1
Table 1.
Selection criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Systematic reviews with or without meta-analyses on ADHD published in peer-reviewed journals 2. Search performed in more than one database including at least PubMed or Medline 3. Involvement of two or more reviewers at any stage of the systematic review |
1. Letter/Erratum/Protocols 2. No clear description of article selection process 3. Quality assessment of included studies not performed 4. Same article published in different journal 5. No full text available (after rejection from authors) |
We searched MEDLINE, PubMed, PsycINFO, Cochrane Library, and Web of Science for studies, using specific keywords (ADHD, systematic review, meta-analysis, see Supplementary 1 for a description of the search strategy) with no language restrictions. The search included all years and final search was completed in December 2021. Reference lists of included publications were also searched. All references from the literature search were imported to Endnote X7.2 [4] and then to Covidence [5]. Two reviewers (A.C. with I.L. or O.N.) independently performed title and abstract screening of all articles identified through database search and full-text screening of more than 95% of articles. Any discrepancies in assessments were resolved in consensus or by consulting the third reviewer or the last author (A.M.). To avoid overlap, when systematic reviews studied identical topics and included more than 50% of the same primary articles, we included only the latest reviews with more studies. The included latest systematic reviews and meta-analysis were of similar or high quality compared to the older reviews of same topics (Supplementary Table S2).
Quality appraisal and data extraction
The JBI guideline for quality appraisal and form for data extraction of systematic review and meta-analysis [6] was amended and piloted for the purpose of this meta-review (Supplementary 1). The quality appraisal checklist consists of nine items. Reviews that scored less than six on low risk of bias were categorized as low quality and excluded. Reviews scoring six to seven, and eight to nine were categorized as moderate and high quality reviews, respectively, and were included. Two reviewers (A.C., I.L.) independently assessed the quality of 35% of the systematic reviews to ensure consistency in the quality assessment rating. There was good agreement in quality assessment, and consequently, the remaining 65% of included studies were scored for quality by one author only. A similar process was followed for data extraction, where three reviewers were involved (A.C. with I.L. or O.N.). For each eligible article, pre-defined information was extracted, including topic studied, objective, timeframe of database search, main findings with key estimates, implications for clinical practice and future research, and conclusions. Further details are in Supplementary 1.
Data presentation
We present the objective, main findings, and conclusions of each included systematic reviews and meta-analyses in tables dividing the literature into nine topics of ADHD. In the text, we describe the literature in terms of a narrative synthesis, where for some reviews we have also presented effect estimates with 95% confidence intervals (CI) for some key findings. For overlapping reviews, we highlight results based on recency and quality. As the result section is dense, we have also included summary table that include major findings, limitations, and recommendation for future systematic reviews and meta-analyses for each topic.
Results
A total of 1,161 systematic reviews and meta-analyses were identified, where 231 were eligible for inclusion (Figure 1). The reasons for exclusions of each article selected for full-text review are presented in Supplementary Table S3.
Figure 1.
PRISMA flow diagram.
Characteristics and quality of included systematic reviews and meta-analyses
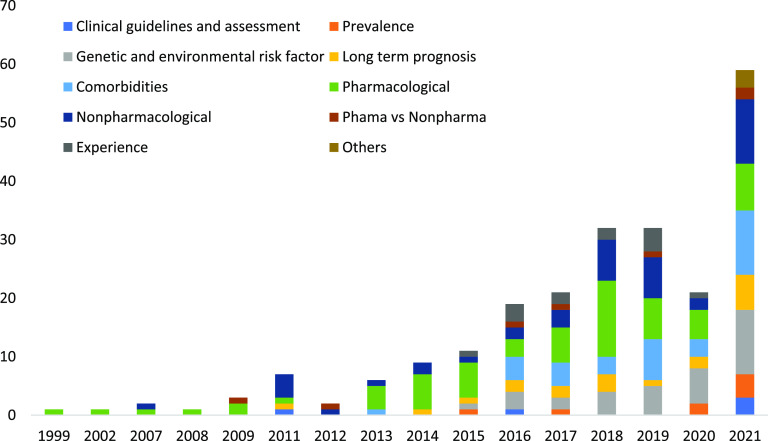
There has been an increase in published reviews annually with a very high number of reviews published in 2021. The most common topic for reviews was pharmacological interventions (28%) (Figure 2). Most of the studies included in reviews were conducted in Europe and North America (34 and 33%, respectively). Of the total included reviews, 59% were of high quality (Tables 3–11).
Figure 2.
Number of systematic reviews and meta-analyses published per year on different topics.
Table 3.
Clinical guidelines and assessment
| References | Objective | Keywords | Timeframe of database search | k | Sample size a | Analytical design | Main findings (Effect estimates, 95% CI) b | Conclusion and comments from the authors | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Razzak et al. [7] | Perform comprehensive review of clinical practice guidelines mainly emphasizing on diagnosis, evaluation and management recommendations for ADHD | Practice guidelines, Diagnosis | Not given | 5 | NA | Narrative synthesis | – The highest total score was achieved by the National Institute for Health and Care Excellence guidelines (91.4%) followed by the CPGs from the Scottish Intercollegiate Guidelines Network – Good agreement across guidelines about the conceptualization of ADHD, and for stimulants as the preferred first pharmacological choice of treatment – Emphasis on rating scales, psychoeducational assessment and laboratory tests varied across guidelines |
Improvements in the applicability of guidelines are needed to enhance its clinical use and relevance | Moderate (7/9) |
| Loyer Carbonneau et al. [8] | Identify sex differences among children and adolescents with ADHD on the primary symptoms of ADHD and on executive and attentional functioning | Sex difference, Symptoms, Cognitive deficits | 1997–October 2017 | 54 | Unclear | Random-effect meta-analysis | – Included studies were of moderate quality – Boys expressed more hyperactivity symptoms than girls did (g = −0.15, −0.33 to 0.03) and have more difficulties in terms of motor response inhibition and cognitive flexibility – Significant heterogeneity (I 2 > 80%) were observed across analyses |
Future research should refine the profile of girls with ADHD and develop diagnostic criteria adapted to each sex | Moderate (7/9) |
| Chang et al. [9] | Examine the diagnostic accuracy of ADHD rating scales CBCL-AP and CRS-R in children and adolescents | Rating scales, Children and adolescents | Not given | 25 | Ranged from 18 to 763 | Random-effect meta-analysis | – Of the total included studies,11 were of high quality, the rest of poor quality – CBCL-AP yielded moderate sensitivity and specificity of 0.77 (0.69–0.84) and 0.73 (0.64–0.81) respectively – Moderate sensitivities of 0.75 (0.64–0.84), 0.72 (0.63–0.79), and 0.83 (0.59–0.95) and moderate specificities of 0.75 (0.64–0.84), 0.84 (0.69–0.93), and 0.84 (0.68–0.93) were found for CPRS-R, CTRS-R and Conners ASQ, respectively – Moderate heterogeneity (I 2 > 50%) was observed across analysis |
Future meta-analyses comparing the diagnostic performance of two different tools should be conducted on the basis of studies that have directly compared the targeted tools by applying both tools to each participant or by randomizing each participant to undergo assessment by using one of the tool | High (9/9) |
| Staff et al. [10] | Determine the validity of teacher rating scales for assessing ADHD symptoms in the classroom | CTRS-R, SWAN, Clinical interview | 1980–January 2020 | 4: Clinical interviews scores, 18: Structured observation scores |
Clinical interviews scores: 1,744 children, Structured observation scores: 2,203 children | Random-effect meta-analysis | – Majority of studies were of high quality – Results showed convergent validity for rating scale scores, with the strongest correlations (r = 0.6, 0.5–0.7) for validation against interviews, and for hyperactive–impulsive behavior – Divergent validity was confirmed for teacher ratings validated against interviews, whereas validated against observations this was confirmed for inattention only – Significant heterogeneity (I 2 > 80%) were observed across analyses except for the meta-analytic correlations between rating scales and interview measures assessing inattention |
Further studies with psychometric properties are needed to confirm validity of teacher rating scales | Moderate (7/9) |
| Taylor et al. [11] | Describe and evaluate the properties of different ADHD diagnostic rating scales in adults | Rating scales, Adults | Database inception to June 2010 | 35 | Not given | Narrative synthesis | – Majority of studies were of poor quality – CAARS and WURS, and the WURS, short version had the best psychometric properties among 14 included scales |
Additional good quality research on CAARS and WURS including larger sample size are needed | High (9/9) |
Abbreviations: ASQ, Abbreviated Symptom Questionnaire; CAARS, The Conners Adult ADHD Rating scale; CBCL-AP, Child Behavior Checklist-Attention Problem; CPRS-R, Conners Parent Rating Scale – Revised; CRS-R, Conners Rating Scale – Revised; CTRS-R, Conners Teacher Rating Scale-Revised; k, Total number of primary studies included; NA, not applicable; SWAN, strengths and weaknesses of ADHD – Symptoms and Normal-Behaviors; WURS, Wender Utah Rating Scale.
Total participants included in the systematic review and meta-analysis unless otherwise indicated.
For findings from meta-analysis, if given effect estimates with 95% CI are presented unless otherwise indicated.
Table 11.
Patients’ and caregivers’ experience of ADHD beyond symptoms
| References | Objective | Keywords | Timeframe of database search | k | Sample size a | Analytical design | Main findings (Effect estimates, 95% CI) b | Conclusion and comments from the authors | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Impact on Quality of Life | |||||||||
| Lee et al. [226] | Compare children and parents rating for HRQOL for children with ADHD | Health related quality of life Informant agreement | 1970–August 2017 | 8 | 4,322 | Fixed and random-effect meta-analysis, meta-regression | – Included studies were of moderate to high quality – There was an small, but significant difference between parent–child rating of children and adolescents physical HRQOL (Hedge’s g = −0.23, −0.33 to −0.13), while moderate difference (g = −0.60, −0.71 to −0.48) for psychosocial HRQOL – Significant heterogeneity was observed across analysis |
Future meta-analyses may include studies that recruit children with ADHD from the community to reduce selection bias | High (8/9) |
| Lee et al. [227] | Assess quality of life in children and adolescents with ADHD | Health related quality of life, Parent–Child agreement | 1970–2014 | 9 | 8,020,867 | Random-effect meta-analysis | – Included studies were of moderate to high quality – ADHD severely impaired children’s all three aspects of HRQOL: physical, emotional, and social – For HRQOL of children and adolescents with ADHD, no significant difference was observed between parent’s and children’s rating – Significant heterogeneity (I 2 > 80%) was observed across analysis |
The mechanism through which HRQOL conceptualizations and subscales and family characteristics influence the parent–child agreement on ADHD children’s HRQOL, should be studied | High (8/9) |
| Experience with ADHD, pharmacological and nonpharmacological interventions | |||||||||
| Eccleston et al. [228] | Synthesize adolescents experiences of living with ADHD diagnosis | ADHD diagnosis, Adolescents experiences, Qualitative | Database inception – February 2017 | 11 | 166 | Narrative synthesis | – Included studies were of high quality – Interpersonal conflict, stigma and rejection lowered adolescents “self-esteem” and “identity” in youths with ADHD, although positive aspects of having ADHD were also recognized |
It is important to conduct research on diverse culture, ethnicity, religion and social class, to understand the experiences of different groups of people | High (8/9) |
| Wong et al. [229] | Synthesize perceptions of ADHD among children with ADHD and their parents | Illness perceptions, Common-sense model, Qualitative | Unclear | 101 | Not given | Narrative synthesis | The majority of children with ADHD displayed negative emotions, including shame, frustration, despair, and embarrassment, but some felt elated and joyful. Among parents, a much broader range of negative emotions was reported – frustration, stress, depression, guilt, helplessness, anger, loneliness; however, some of them felt relieved to find about the disorder | Research might be able to provide a more comprehensive understanding of perceptions of ADHD and their impact if they examine potential mediators and moderators and potential outcome of interactions between different level of ADHD | High (8/9) |
| Bjerrum et al. [230] | Synthesize adult experience of living with ADHD | Coping strategies Managing daily living, Qualitative | 1995–July 2015 | 10 | 159 | Narrative synthesis | – Included studies were of high quality – Adults with ADHD recognized the difference between themselves and others and hence struggle to fit into society Although adults with ADHD are creative and innovative, they found it difficult to arrange and complete daily life tasks. However, they find it to be rewarding and aimed to achieve a healthy balance in life through coping strategies |
Research on the experience of ADHD adults from non-western countries are required | High (9/9) |
| Rashid et al. [231] | Summarize medication-taking experiences of ADHD patients and caregivers | Medications, Adherence, Qualitative | 1987–October 2015 | 31 | Not given | Narrative synthesis | – Included studies were of moderate quality – As children and adolescents with ADHD transition into adulthood, they become more autonomous and self-directed toward all aspects of medications, and their decision-making process is framed by “trade-offs” where the advantage and disadvantages of medications are considered |
Future research should focus on family dynamics, including both siblings and parents, and how media portrayals impact ADHD perceptions and treatment | Moderate (7/9) |
| Moore et al. [232] | Synthesize attitude and experience toward school-based nonpharmacological interventions for ADHD | Nonpharmacological interventions, School, Qualitative | Unclear | 33 | 31 | Narrative synthesis | – Included studies were of high quality – Findings were categorized into four interrelated themes: “individualizing interventions”; “structure of interventions”; “barriers to effectiveness” and; “perceived moderators and impact of interventions.” In addition to ADHD symptoms, school-based interventions should consider the broader school context |
Future research should also study how school-based interventions influence attitudes toward ADHD and peer-relationships | High (8/9) |
| Laugesen et al. [233] | Summarize the experience of parents of ADHD children | Child, Parents, Qualitative | Databases from their inception – April 2015 | 21 | Not given | Narrative synthesis | – Included studies were of high quality – Having a child with ADHD can be an overwhelming and stressful experience, and one can feel guilty, hopeless, and frustrated. Parents were stigmatized and blamed. Their personal and family routines became chaotic and they had to fight to get professional support for their children’s at both school and health services. However, they felt that despite these challenges, living with ADHD child was not all bad |
Further research needs to explore how health professionals can support families and how future interventions can improve the competencies of health professionals and assist the families | High (8/9) |
| Martin et al. [234] | Assess the association between ADHD child sleep problems, parenting stress and parent mental health | Sleep problem, Parenting stress, Mental Health | Database inception – May 30, 2019 | 4 | Not given | Narrative synthesis | Sleep problems among children with ADHD led to higher parenting stress and negatively impacted parental mental health | Future research should control for potential confounders like child’s age, comorbidity to assess if the association is causal | Moderate (7/9) |
| Craig et al. [235] | Determine the coping strategies used by parents of ADHD children | Coping, Stress, Qualitative | Database inception – July 2018 | 14 | 3,024 | Narrative synthesis | Parents of children with ADHD often resort to an “avoidant-focused” coping strategy that is basically comprised of cognitive and behavioral activities aimed at avoiding direct contact with stressful demands, and is associated with distress and depression. In comparison to mothers of typically developing children, those with ADHD tended to seek more support and employ indirect methods | Future studies should study coping strategies in parents according to different subtypes of ADHD in children and also with other mental disorder | Moderate (7/9) |
| Societal and familial barriers to ADHD treatment | |||||||||
| French et al. [236] | To identify barriers and facilitators in understanding ADHD in primary care | Primary care, Qualitative | Database inception – January 2018 | 46 | Health professionals: 15,314 Parents: 134 |
Narrative synthesis | – Majority of the included studies were of high quality – Findings suggest that “need for education,” “misconception and stigma,” “constraint with recognition,” “management and treatment,” and “multidisciplinary approach,” are the main factors influencing the acknowledgment of ADHD by primary care practitioners. A considerable need for improved education about ADHD among primary care practitioners was also found – Significant heterogeneity were found across studies |
For more specific solutions, future research should study scarcity of resources, misperceptions, and multidisciplinary approaches in health care settings | High (9/9) |
| Gwernan-Jones et al. [237] | Explore the influence of school-context on ADHD symptoms | School-stigma, Attributions, Qualitative | 1980 – March 2013 | 34 | Not given | Narrative synthesis | – Majority of the included studies were of high quality – Teachers and students may be blind to the role that schools play in ADHD symptoms despite the fact that the potential for stigma-based discrimination in schools is known. This is because stigma-based discrimination criteria are implicit and seem normal and appropriate to those who belong to the group. The stigmatized individuals may experience emotional pain as a result of this lack of understanding, which may worsen the symptoms of ADHD. The implementation of school-based treatments may also be aided by awareness of these potential implications of the school context |
Future qualitative research could examine modifications to the school environment, routines, and expectations, including support for connections between students and their teachers and peers | Moderate (7/9) |
| Ogle et al. [238] | Assess efficacy of psychosocial treatment among ADHD children living in poverty | Poverty, Psychological interventions | Unclear | 5 | 461 | Narrative synthesis | Mixed evidence exists about the impact of poverty on psychosocial treatment for children with ADHD | Future research with more accuracy, precision, and quality in the reporting of income data are warranted | High (8/9) |
Abbreviations: HRQOL, health related quality of life; k, total number of included studies.
Total participants included in the systematic review and meta-analysis unless otherwise indicated.
For findings from meta-analysis, if given effect estimates with 95% CI are presented unless otherwise indicated.
Overview of findings
The included reviews were categorized into nine different topics and the major findings for each topic are presented in Table 2.
Table 2.
Summary table of major findings
| Topics | Major findings | Limitations | Further reviews needed |
|---|---|---|---|
| Clinical guidelines and assessment | Good agreement across guidelines for stimulants as first-line medication Psychosocial interventions was recommended despite lower degree of evidence |
Although guidelines recommend rating scales as part of the assessment, they vary in their emphasis and recommendation of which to use | Future reviews of rating scales should include head-to-head comparisons of different rating scales, sex-specific symptom profiles, and functional aspects of ADHD beyond core symptoms |
| Prevalence of ADHD | The pooled prevalence of ADHD was 7.2% in children and adolescents ADHD is twice as prevalent in boys The prevalence of ADHD is 2.5% in adults |
Estimates vary between studies, regions, and according to study design and methods | Meta-analyses addressing the effect of bias (for example effect of study design, geographical location, and assessment tools) on prevalence are needed |
| Genetic and environmental risk factors associated with ADHD | ADHD was associated with several biological and social factors including for example genes/polygenic risk scores, maternal health in pregnancy, nutrition, repeated general anesthesia in early childhood, age at school start | Despite a huge literature on risk factors, research designs are mostly correlational rather than allowing for causal inference | Reviews of risk factors of ADHD allowing for causal inference are called for |
| Long-term prognosis and life trajectories in ADHD | ADHD was associated with school performance and school dropout, work participation, welfare dependency, smoking, drug and alcohol addiction, injuries, suicidal spectrum behavior, crime, and comorbidities, in directions as expected | The literature describes a rather bleak prognosis in ADHD, but this may be exaggerated due to the lack of adjustment for potential and residual confounding factors. The described outcomes of ADHD may thus not be causally linked to ADHD | Reviews of studies of prognosis in ADHD properly accounting for confounding are needed. The degree of confounding in this literature may also be subject of a meta-analysis, comparing adjusted and un-adjusted studies |
| ADHD and comorbidities | ADHD was associated with a high degree of comorbid somatic conditions (e.g., obesity, asthma, headache/migraine, sleep problems) and psychiatric disorders (e.g., other neurodevelopmental-, affective-, anxiety-, and eating disorders) | Studies are mostly correlational and lack data on temporality. The reviews do not sufficiently distinguish comorbidities from side-effects of medication. The causal mechanisms in the comorbidities are not addressed | Reviews addressing these limitations are needed |
| Pharmacological treatment | Stimulants were recommended as first-line medication both for children (methylphenidate preferred) and adults (amphetamine preferred) Nonstimulants were also found effective in treating ADHD in children (atomoxetine, guanfacine) and adults (atomoxetine, bupropion), though less effective than stimulants Side-effects were common and include reduced appetite, sleep problems, headache, increased heart rate and increased blood pressure |
Most trials have short follow-up time (weeks and months rather than years) and focus mainly on core symptoms of ADHD as outcome There is no review evidence of pharmacological treatment effect on life-trajectory in ADHD over more than few months follow-up |
Reviews of pharmacological treatment effects in ADHD on long-term prognosis with real-life outcomes including educational attainment, welfare dependency and employment, criminality, injuries, and mortality are urgently needed |
| Nonpharmacological treatment | Behavioral interventions, parental training, dietary interventions (like omega-3), and mindfulness were found to have small but positive effects in children However, the evidence for nonpharmacological treatment effects was more mixed than for pharmacological treatment Further, there are also fewer original studies and fewer reviews In adults, cognitive behavioral therapy and meditation-based therapies have shown positive effects |
Most of the reviews address core ADHD symptoms and there were only few reviews that focuses on real-life outcomes and functional outcomes including for example social skills, peer relationships, school performance | Reviews of efficacy studies with outcomes beyond core ADHD symptoms are needed Reviews addressing effects of biases in this literature are needed. Biases include publication biases in favor of positive findings, the effect of nonblinded assessments should be subject for meta-analyses |
| Pharmacological versus nonpharmacological treatment in ADHD | Stimulants were superior to nonpharmacological treatment in reducing core symptoms among children and adolescents with ADHD While medications improved ADHD symptoms, psychosocial treatments were beneficial for academic and organizational skills in adolescents Pharmacological treatment was found to be cost-effective compared to nonpharmacological treatment or no treatment |
Reviews compared medication with groups of different nonpharmacological intervention rather than comparing medication with one particular nonpharmacological intervention There is no review evidence of long-term effect of pharmacological versus nonpharmacological treatment on ADHD |
Reviews comparing the long-term effects of pharmacological versus nonpharmacological treatment on ADHD are needed |
| Patients’ and caregivers’ experience of ADHD beyond symptoms | There was good evidence for a negative impact of ADHD on quality of life (QoL) both physically, emotionally and socially across the lifespan ADHD in children also increased parental stress. ADHD medications were chosen as a last resort and both patients and caregivers were concerned about its long-term side effects and financial costs |
Although some reviews include positive aspect of having ADHD, no reviews have addressed how treatment or other interventions may influence QoL over times | Reviews assessing the impact of pharmacological or nonpharmacological interventions on QoL of patients over time are needed Reviews of qualitative studies including in-depth experiences of patients and caregivers in long-term are needed |
Narrative synthesis
Clinical guidelines and assessment (number of studies, n = 5, Table 3)
In a recent systematic review of five clinical practice guidelines, all guidelines rated stimulants as the first-line pharmacological intervention and recommended the inclusion of psychosocial intervention in the treatment [7].
A meta-analysis of sex differences in ADHD symptoms showed that boys with ADHD are more hyperactive than girls and have more difficulties in terms of motor response inhibition and cognitive flexibility [8].
For screening for ADHD in children, the Child Behavior Checklist-Attention Problem and the Conner’s Rating Scale–Revised had moderate sensitivity and specificity [9]. Conner’s Rating Scale and Strengths and Weaknesses of ADHD – Symptoms and Normal-Behaviors were found as valid and time-efficient measures to assess ADHD symptoms in the classroom [10].
In adults, the Conners Adult ADHD Rating Scale and the Wender Utah Rating Scale short version showed the best screening properties [11].
Prevalence of ADHD (n = 8, Table 4)
Table 4.
Prevalence of ADHD
| References | Objective | Keywords | Timeframe of database search | k | Sample size a | Analytical design | Main findings (Effect estimates, 95% CI) b | Conclusion and comments from the authors | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Children and adolescents | |||||||||
| Thomas et al. [12] | Estimate worldwide prevalence of ADHD using DSM-criteria | Prevalence, DSM editions | 1977–2013 | 175 | 1,023,071 | Random-effect meta-analysis | – Majority of studies (75%) were of moderate or high quality – Pooled prevalence of ADHD was 7.2% (6.7–7.8) – In multivariate analyses, after adjusting for measurement and region, prevalence estimates for ADHD, was 2% point lower when DSM-III revised was applied as compared to DSM-IV – Significant heterogeneity (I 2 > 96%) were observed across analysis |
Underscore that the estimated prevalence can be considered a “benchmark” – that is, deviating prevalence rates indicates over-/under diagnosis | High (8/9) |
| Ayano et al. [13] | Assess the prevalence of ADHD in Africa | Prevalence, Africa | Database inception – (not mentioned last date of search) | 12 | 11,465 | Random-effect meta-analysis | – Majority of studies were of high quality – Pooled prevalence of ADHD was 7.47% (6.0–9.26), with a greater prevalence among boys than girls – The prevalence rate was highest for predominantly inattentive subtype (ADHD-I) in both boys (4.05%, 3.11–5.27) and girls (2.21%, 1.61–3.03) – Significant heterogeneity (I 2 > 90%) were observed across analyses |
Reasons for gender differences in ADHD needs to be explored | High (8/9) |
| Wang et al. [14] | Identify the prevalence of ADHD in China | Prevalence, China | Database inception – March 2016 | 67 | 275,502 | Random-effect meta-analysis | – Majority of studies were of moderate quality – The prevalence rate of ADHD was 6.26% (5.36–7.22), with ADHD-I being the most common subtype – Prevalence rate varied between studies due to “geographical location” and “information sources” differences – Significant heterogeneity (I 2 > 95%) were observed across analyses |
Estimated prevalence provides a “benchmark” to evaluate the disease burden of ADHD in China. However, a nation-wide research is needed to identify more “accurate estimate” | High (8/9) |
| Cénat et al. [15] | Estimate prevalence of ADHD among US Black individuals | Prevalence, US Black individuals | Database inception – October 2019 | 21 | 154,818 | Random-effect meta-analysis | – Included studies were of moderate to high quality – Overall prevalence of ADHD was 14.5% (10.6–19.6), for individuals less than 18 years 13.9% (9.6–19.6) – Significant heterogeneity (I 2 = 99.7% were observed across analyses |
Assessment and monitoring of ADHD among black individuals needs to be increased and research on ADHD prevalence across different ethnic groups in other western countries are needed | High (8/9) |
| Shooshtari et al. [16] | Provide an up to date prevalence of ADHD in Iran | Prevalence, Iran | January 1990–December 2018 | 36 | 33,621 children, adolescents, and adults | Narrative synthesis | – Prevalence estimates varied substantially across the studies and provided a range of heterogeneous data – Total prevalence of ADHD ranged between 11.0–25.8% among pre-school children; between 3.1–17.3% among school children, and between 3.9–25.1% among adults |
Comparing prevalence estimates across studies was difficult due to differences in assessment methods and samples. Therefore, prevalence studies need to apply well-defined diagnostic criteria | Moderate (7/9) |
| Kazda et al. [17] | Systematically evaluate, and synthesize the evidence on overdiagnosis of ADHD in children and adolescents utilizing published 5-question framework for detecting over diagnosis in noncancer conditions | Overdiagnosis | 1979–August 2020 | 334 | Not given | Narrative Synthesis | – One-third of the studies were of high quality – Substantial evidence of a reservoir of ADHD was reported by 104 studies, which suggest that number of diagnosis could increase in future – 45 studies provided an evidence that the actual ADHD diagnosis had increased – 25 studies reported that these additional cases may be on the milder end of the ADHD spectrum, and – 83 studies suggested that pharmacological treatment of ADHD was increasing |
High quality studies are needed to identify the long-term benefits and harms of diagnosing and treating ADHD in young people with milder symptoms | Moderate (7/9) |
| Adults | |||||||||
| Song et al. [18] | Assess the global prevalence of adult ADHD in the general population | Prevalence, Worldwide | January 2000–December 2019 | 40 | 107,282 for persistent adult ADHD and 50,098 for symptomatic adult ADHD |
Random-effect meta-analysis | – Majority of studies were of moderate to high quality – Based on data published from 2005 to 2019, the pooled prevalence was 4.61% (3.41–5.99) and 8.83% (7.23–10.57) for persistent and symptomatic adult ADHD respectively – The most common age group for adult ADHD cases was 18–24 – LMICs showed a higher prevalence of persistent adult ADHD than HICs, while WHO regions showed different rates of symptomatic adult ADHD – Significant heterogeneity (I 2 > 97%) were observed across analyses |
To better understand the global epidemiology of both persistent and symptomatic adult ADHD, there is still a need for well-defined diagnostic procedures and more large-scale international studies with minimal methodological heterogeneity | High (9/9) |
| Dobrosavljevic et al. [19] | Assess ADHD prevalence among older adults ≥50 according to different assessment methods | Prevalence, Older adults | Database inception – June 2020 | 20 | 20,999,871 | Random-effect meta-analysis | – Included studies were of moderate quality – The prevalence of ADHD was higher 2.18% (1.51–3.16) for validated scales in community sample compared to the prevalence assessed based on clinical diagnosis 0.23% (0.12–0.43), and treatment 0.09% (0.06–0.15) – Significant heterogeneity (I 2 > 87%) were observed across analyses |
Prevalence studies in community samples should use more comprehensive assessment tools to explore whether individuals with elevated ADHD symptoms meet established diagnostic criteria. They should examine the possible reasons behind high levels of ADHD symptoms reported via validated scales | Moderate (7/9) |
Abbreviations: DSM, diagnostic statistic model; HICs, high-income countries; k, total number of primary studies included; LMICs, lower and middle-income countries; WHO, World Health Organization.
Total participants included in the systematic review and meta-analysis or otherwise specified.
For findings from meta-analysis, if given effect estimates with 95% CI are presented or otherwise specified.
Prevalence of ADHD in children and adolescents
The prevalence of ADHD among children and adolescents was assessed in four different meta-analyses [12–15] and one systematic review [16]. Internationally, the pooled prevalence of ADHD in children and adolescents was estimated to be 7.2% (95% CI: 6.7–7.8) in a meta-analysis of 175 studies including more than 1 million participants [12]. Most of the included studies were conducted within school populations (74%), and few used a whole-population approach (10%). In a multi-variable analysis, the prevalence estimate was 2 percentage points lower in studies conducted in Europe compared to North America after adjusting for the edition of diagnostic manual and measurement tools which included clinical interviews, symptom-only criteria, and reports of ADHD diagnosis [12]. Further, a meta-analysis of prevalence studies from Africa reported a pooled prevalence of 7.47% (6.0–9.26). As expected, gender differences were found with a male: female ratio of 2.0:1.0 [13]. The prevalence in China was 6.26% (5.36–7.22) [14], while it was 13.87% (9.59–19.64) among black individuals in the USA [15]. All included reviews reported that significant heterogeneity in the prevalence attributed to the source of study population, geographical location, and source of data was found across included studies.
A systematic review found substantial evidence of overdiagnosis of ADHD. The authors reported that ADHD diagnoses have consistently increased between 1989 and 2017, and that the majority of new cases were on the milder end of the ADHD spectrum [17].
Prevalence of ADHD in adults
The worldwide pooled prevalence among adults was 4.61% for persistent adult ADHD and 8.83% for symptomatic adult ADHD [18]. By adjusting for the “global demographic structure,” the prevalence of persistent adult ADHD was 2.58% (95% CI:1.51–4.45) and symptomatic adult ADHD 6.76% (4.31–10.61), translating to 139.84 and 366.33 million affected adults in 2020 globally. The meta-analysis found that the prevalence of ADHD decreased with age [18]. The prevalence among adults aged ≥50 was 2.2% based on validated scales applied in the general population, and 0.2% when based on clinical diagnosis [19].
Genetic and environmental risk factors associated with ADHD (n = 41, Table 5)
Table 5.
Genetics and environmental risk factors associated with ADHD
| References | Objective | Keywords | Timeframe of database search | k | Sample size a | Analytical design | Main findings (Effect estimates, 95% CI) b | Conclusion and comments from the authors | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Ronald et al. [20] | Review if ADHD polygenic risk score is associated with ADHD and related traits | Genetics, Polygenic risk score | Not given | 44 | >14,000 | Narrative synthesis | – Majority (80%) of included studies were of high quality – Strong evidence was found for the of associations between ADHD PRS and ADHD, ADHD traits, brain structure, education, externalizing behaviors, neuropsychological constructs, physical health, and socioeconomic status – PRS associated with ADHD had an OR of 1.22 to 1.76%; variance explained in dimensional assessments of ADHD traits ranged from 0.7 to 3.3% |
The review suggest that the ADHD PRS is robust and reliable, associating not only with ADHD but many outcomes and challenges known to be linked to ADHD | High (8/9) |
| Prenatal factors | |||||||||
| Li et al. [21] | Clarify the association between maternal pre-pregnancy overweight/obesity and risk of ADHD in offspring | Obesity, Confounding | 1975–2018 | 14 (8 in meta-analysis) | 784, 804 mother–child pairs | Random-effect meta-analysis | – Included studies were of moderate to high quality – Maternal overweight (RR = 1.31,1.25–1.38) and obesity (RR = 1.92, 1.84–2.00) both increased the risk of ADHD in offspring – However, the association was significantly attributable to unmeasured familial confounding and was not a causal – No significant heterogeneity were observed across analysis |
Future studies using robust methodological design that considers unmeasured familial confounders, and genetic and environmental origin of such confounders and includes various populations are required | High (8/9) |
| Ai et al. [22] | Assess the association between antibiotic exposure and the risk of ADHD in childhood | Antibiotic ADHD, Microbiome | Database inception – January 2021 | 11 | 2,238,348 | Random effects meta-analysis | Included studies were of moderate to high quality – Maternal antibiotic exposure during pregnancy was associated with an increased risk of ADHD in offsprings (OR = 1.14; 1.10–1.18) – The included studies had insufficient adjustment for confounders – Moderate heterogeneity (I 2 = 64%) was observed across analysis |
Future research should examine whether different types, courses, and durations of antibiotic use affect ADHD risk and adjust for potential confounders | Moderate (7/9) |
| Gou et al. [23] | Evaluate the association between maternal acetaminophen use during pregnancy and the risk of ADHD in children | Acetaminophen, Pregnancy | Database inception – November 2018 | 8 | 244,940 | Random-effect meta-analysis | – Majority of included studies were of high quality – There was an association between maternal acetaminophen use during pregnancy and the risk of ADHD in offspring (Rrat = 1.25, 1.17–1.34) with a greater risk among offspring who were exposed to acetaminophen at the third trimester and for longer duration, that is, 28 days or more – No significant heterogeneity were observed across analyses |
Caution must be taken while interpreting the result as this observed association might be due to potentially unidentified or inadequately controlled confounders. Hence, further studies are required | Moderate (7/9) |
| Man et al. [24] | Assess the association between antidepressant exposure during pregnancy and ADHD in offspring | Antidepressant, Pregnancy | January 1946–July 2017 | 8 | 2,886,502 | Random-effect meta-analysis | – Included studies were of high quality – Increased risk of ADHD among those with prenatal exposure to antidepressants compared to nonexposure was observed (Rrat = 1.39, 1.21–1.64) – However, the meta-analysis result of three studies that used sibling-matched analyses yielded a nonsignificant association – No significant heterogeneity were observed across analyses |
Association between prenatal antidepressants exposure and ADHD in children is likely to be confounded by other factors | High (9/9) |
| Dan et al. [25] | Assess the relationship between maternal pregestational or gestational diabetes and occurrence of ADHD in children | Maternal diabetes, ADHD, offspring | Database inception – January 2019 | 7 | 3,169,529 out of which, 148,374 children exposed to maternal diabetes, and 3,021,155 belonging to the reference | Random-effect meta-analysis | – Majority of the studies were of moderate quality – Maternal pregestational diabetes increased the risk of ADHD in offspring by 44% (1.32–1.57) with no significant heterogeneity – -However, no association was observed between gestational diabetes and ADHD (RR = 1.19, 0.99–1.42) with moderate heterogeneity (I 2 = 68.7%) |
Given the limited availability of reliable information, further cohort studies are required to assess this relationship more comprehensively | Moderate (7/9) |
| Rowland et al. [26] | Explore the association between gestational diabetes and ADHD | Pregnancy, Gestational diabetes | Database inception – April 2021 | 15 | Children of 132,458 mothers with gestational diabetes, 857,623 control. 401 children with ADHD, 1,828 controls | Random effects meta-analysis | – Majority of included studies were of poor quality – No significant difference was observed between children of mothers with gestational diabetes and controls – Moderate heterogeneity (I 2 > 45%) was observed across analysis |
Not mention for ADHD | High (9/9) |
| Dong et al. [27] | Assess the association between prenatal exposure to MSDP and ADHD in offspring | Prenatal exposure, Maternal smoking during pregnancy | Database inception – June 2017 | 27 | 3,076,173 | Random-effect meta-analysis | – Included studies were of high quality – Significant association was observed between prenatal exposure to MSDP or maternal smoking cessation during the first trimester and ADHD in offspring, while there was no such association for maternal smoking cessation before pregnancy – Significant heterogeneity (I 2 > 80%) was observed across analysis for MSDP |
More studies with robust designs, more effective exposure assessment, and large sample size are needed to clarify the causal relationship between MSDP and ADHD in offspring | High (8/9) |
| Schwartz et al. [28] | Determine the association between POE and ADHD symptoms in children and adolescents | Prenatal opioid exposure | January 1950–October 2019 | 7 | 319 children with POE and 1,308 nonexposed children | Random-effect meta-analysis | – POE was associated with higher rates of hyperactivity (SMD:1.4, 0.49–2.31), inattention (SMD: 1.35, 0.69–2.01) and combined ADHD symptoms scores (SMD: 1.27, 0.79–1.75) – POE was associated with ADHD symptoms both at preschool and school age – Significant heterogeneity (I 2 > 87%) were observed across analyses |
Future research should clarify the relationship between biological, environmental, and social risk factors, respectively, and ADHD symptoms in children with POE | Moderate (7/9) |
| Qu et al. [29] | Explore the relationship between maternal exposure to perfluoroalkyl substances and early ADHD in children | Perfluoroalkyl substances, Maternal exposure | Database inception – October 2020 | 15 (9 in meta-analysis | 17,565 | Narrative synthesis and random effects meta-analysis | – Majority of included studies were of high quality – No statistical significant differences was found for early ADHD and exposure to perfluoroalkyl substances (perfluorooctanoic acidperfluorooctane sulfonate, perfluorohexane sulfonate, perfluorononanoic acid, perfluorodecanoic acid) – Subgroup analysis showed a positive association between PFOS concentration in children’s blood and early ADHD (OR = 1.05, 1.02–1.08) – Subgroup analysis showed a positive association between perfluorononanoic acid level in maternal blood and ADHD in children (OR = 1.42, 1.04–1.81) – Subgroup analysis showed a positive association between perfluorooctane sulfonate level and ADHD in children in America (OR = 1.05, 1.02–1.08 – Moderate to significant (I 2 > 54.7–87.2) heterogeneity were observed across analyses before subgroup analysis |
To better understand the pathogenesis of ADHD, epidemiological studies are needed in several regions, particularly on PFAS exposure types | Moderate (7/9) |
| Perinatal factors | |||||||||
| Zhu et al. [30] | Evaluate the association between perinatal hypoxic–ischemic conditions and future ADHD | Hypoxia, ischemia, Risk factor | Database inception – before September 2015 | 10 | Cases: 45,821 Controls: 9 2,07,363 |
Fixed or random-effect meta-analysis | – Included studies were of moderate quality – Perinatal hypoxic–ischemic conditions like preeclampsia (OR = 1.31, 1.26–1.37), Apgar score < 7 at 5 minutes (OR = 1.31,1.12–1.54), breech/transverse presentations (OR = 1.14,1.06–1.23), and prolapsed nuchal cord (OR = 1.10,1.06 – 1.15) were all associated with increased risk of future ADHD – Moderate heterogeneity (I 2 = 63%) was observed for analysis of Apgar score < 7 at 5 minutes |
Given the limited number of studies, more well-designed studies are needed to confirm this association | High (8/9) |
| Franz et al. [31] | Determine the association between preterm and LBW and future ADHD, both for categorical diagnosis and dimensional symptomatology, compared with controls | Very Preterm, Very Low Birth Weight, Extreme Preterm, Extreme Low Birth Weight | Database inception – 2017 | 34 | 5,291 | Random-effect meta-analysis | – Included studies were of moderate quality – VP/VLBW and EP/ELBW individuals were at an increased risk of a categorical diagnosis of ADHD with highest OR for the most extreme cases – This was supported by a meta-analysis based on ADHD symptomatology showing a significant association for VP/VLBW and symptoms of inattention (SMD = 1.31,0.66–1.96), hyperactivity and impulsivity (SMD = 0.74, 0.35–1.13), and combined (SMD = 0.55, 0.42–0.68) as compared to controls – Significant heterogeneity (I 2 > 90%) was observed across analysis except for the combined dimension in the VP/VLBW group (moderate I 2 = 54%) |
Specific causal determinants associated with prematurity and LBW and subsequent ADHD needs to be studied | High (9/9) |
| Serati et al. [32] | Review the literature on obstetric and neonatal complications and future risk of ADHD | Perinatal complications, Child development | Database inception – December 2016 | 40 | 57–1,772,548 per study | Narrative synthesis | – Majority of included studies were of moderate quality – LBW (Cohen’s d effect size range = 0.31–1.64) and preterm birth (range d = 0.41–0.68) were identified as an important risk factors for future ADHD |
PB and LBW children should be carefully monitored for an early diagnosis of ADHD | Moderate (6/9) |
| Curran et al. [33] | Investigate the impact of CS compared to vaginal delivery and the odds of subsequent ADHD in children | ADHD, Cesarean section | Database inception – February 2014 | 4 | Not given | Random-effect meta-analysis | – Included studies were of poor to moderate quality – Unadjusted estimates showed a positive association between CS and ADHD in offspring – However, the only two studies reporting adjusted risk estimates showed that this relation was not significant (OR = 1.07,0.86–1.33) – No heterogeneity were observed across analyses |
Included studies were unable to provide a clear description of the possible association. Hence, future studies need to adjust for potential key confounders and effect modifiers (type of CS, sex of the child, maternal obesity, socioeconomic status, maternal age) to assess the relationship between CS delivery and ADHD | Moderate (7/9) |
| Xu et al. [34] | Examine the association between CS and ADHD in children | ADHD, Cesarean section, Confounders | Database inception – December 2018 | 9 | More than 2.5 million | Fixed or random‐effect meta-analysis | – Included studies were of high quality – CS was associated with a small increase in the later risk of ADHD in offspring (OR = 1.14, 1.11–1.17) – However, the meta-analysis result of data from sibling analyses showed the association as marginally significant (OR = 1.06, 1.00–1.13), suggesting the association was due to confounders – No heterogeneity were observed across analyses |
The observed association may have been overestimated as the included primary studies could not control for important confounding factors. Hence, more prospective cohort studies with large sample size and data on potential confounders/predictors are required | High (8/9) |
| Min et al. [35] | Explore the association between parental age at delivery and ADHD risk in offspring | Parental age; Children | Database inception – April 2021 | 11 | Cases: 111,101 Controls: 4,306,047 |
Random effects meta-analysis | – Majority of included studies were of moderate quality – Compared with the reference groups, the lowest parental age category was associated with an increased risk of ADHD in the offspring (OR = 1.49, 1.19–1.87) and (OR = 1.75,1.31–2.36) for mother and father, respectively – No significant association was found between the highest parental age and ADHD – Significant heterogeneity (I 2 > 95%) were observed across analyses |
A causal relationship and mechanisms between parental age and the risk of ADHD in offspring need to be explored in future research | High(8/9) |
| Postnatal factors | |||||||||
| Tseng et al. [36] | Examine the relationship between breastfeeding and ADHD in children, taking into account of important factors such as the duration and methods | Breastfeeding, Nutrition, Risk | Database inception – September 2017 | 11 | Cases: 4,107 Controls: 90,392 |
Random effects meta-analysis | – Majority of included studies were of moderate quality – Children with ADHD had significantly less breastfeeding duration than controls (Hedges’ g = −0.36, −0.61 to –0.11) – Association was found between nonbreastfeeding and ADHD children (ajOR = 3.71, 1.94–7.11) – Moderate heterogeneity (I 2 = 42.5%) was observed across analysis |
Additional longitudinal studies are needed to confirm/refute the association and to explore possible mechanisms underlying this association | Moderate (7/9) |
| Zeng et al. [37] | Investigate the association between maternal breastfeeding and ADHD in offspring | Breastfeeding, Nutrition, Protective factor | Unclear | 12 | ADHD Cases: 3,686 Controls: 106,907 | Random-effect meta-analysis | – Majority of included studies were of moderate quality – Maternal breastfeeding (of any duration) may reduce the risk of future ADHD in children (OR = 0.70, 0.52–0.93) compared to those who were never breastfed – Moderate heterogeneity (I 2 > 70%) was observed across analysis |
Prospective studies should adjust for potential confounders and apply standardized diagnostic methods to examine the causal relationship | Moderate (6/9) |
| Huang et al. [38] | Explore the association between postnatal exposure to SHS and future risk of ADHD | Second-hand smoking, Postnatal | Database inception – January 2020 | 9 | ADHD cases: 6,663 Controls: 93,825 | Random-effect meta-analysis | – Majority of included studies were of high quality – Children exposed to SHS were found to be at the increased risk of ADHD (OR = 1.60, 1.37–1.87) – Moderate heterogeneity (I 2 = 42.5%) was observed across analysis |
Causal relationship between SHS and ADHD needs to be determined by fully adjusting for potential confounders | Moderate (7/9) |
| Environmental factors | |||||||||
| Asarnow et al. [39] | Investigate ADHD diagnoses in children and adolescents following concussions and mild, moderate, or severe TBI | Injury, Traumatic Brain Injury | 1981–December 2019 | 24 | TBI cases: 12,374 Controls: 43,491 |
Random-effect meta-analysis | – Majority of included studies were of moderate quality – Children with TBI were at increased risk of getting ADHD compared with those with other injuries (1 year: OR = 4.81,CrI: 1.66–11.03) and (>1 year OR = 6.70,2.02–16.82) and noninjured controls (1 year OR = 2.6, 0.7–6.6), and (>1 year OR = 6.25, 2.06–15.04), as well as those with mild TBI (1 year OR = 5.69,1.46–15.67), (>1 year OR: 6.65, 2.14–16.44) – Of 5, 920 children with severe TBI, 35.5% had ADHD more than 1 year postinjury |
Further studies with large sample size particularly for concussion and other injuries are needed | Moderate (6/9) |
| Sun et al. [40] | Examine the evidence for a relationship between general anesthesia induced in childhood and the risk of ADHD | Attention, Behavior, Cognitive, Surgery, Anesthesia | Database inception – October 2020 | 7 | ADHD cases: 49,141 Controls: 251, 246 | Random effects meta-analysis | – Majority of included studies were of high quality – Exposure to general anesthesia in childhood was associated with a risk of ADHD in later life (RR = 1.24,1.11–1.38) – Subgroup analysis showed that a single anesthetic exposure was not associated with risk for ADHD – Two or more exposures was associated with risk for ADHD – Moderate heterogeneity (I 2 = 74.8%) was observed for two or more exposure |
To confirm these findings, more prospective cohort studies with larger sample sizes are needed | High (9/9) |
| Daneshparvar et al. [41] | Review relevant literature related to lead exposure and ADHD symptoms in children | Blood Lead Level, Lead Poisoning | Database inception – May 2014 | 18 | 12,195 | Narrative synthesis | – Majority of included studies were of high quality – Blood lead level of < 10 g/dL have a significant effect on at least one ADHD subtype |
Recommends revising the present threshold (less than 10 g/dL) for permissive blood levels and measuring the BLL in children to reduce the harm caused by prolonged exposure to lead | Moderate (6/9) |
| Kalantary et al. [42] | Examine the association between ADHD symptoms and exposure to PAH exposure during the prenatal and postnatal periods in children of nonsmoking mothers | PAHs, Children | Database inception – September 2018 | 6 | 2,799 | Fixed and random-effect meta-analysis | – Majority of included studies were of high quality – Although four of six studies (all by the same author) found a significant association between PAH exposure and later ADHD, no association was observed between prenatal and postnatal exposure to PAH and future ADHD in children when including adjusted analyses from all the six included studies (OR = 1.99,0.96–4.11) – No heterogeneity was observed across analysis |
Additional research needs to be conducted in different countries | Moderate (7/9) |
| Zhang et al. [43] | Determine the association between exposure to air pollutants and development of ADHD in children | PAHs, Nox, Particulate matter | Unclear | 9 | >98,000 | Random-effect meta-analysis | – Majority of included studies were of high quality – No significant association was found between exposure to PAHs (RR = 0.98, 0.82–1.17), NOx (RR = 1.04, 0.94–1.15, and PM (RR = 1.11, 0.93–1.33) and an increased risk of ADHD in children – Moderate heterogeneity (I 2 = 60.1%) was observed NOx and PM |
Further prospective studies to determine causal relationship between exposure to particles and ADHD are needed | High (8/9) |
| Aghaei et al. [44] | Synthesize relationship between exposure to air pollutants and risk of ADHD in children | Ambient air pollutants, particulate matter, ADHD | Database inception – 2018 | 28 | 140,159 | Narrative synthesis | – Due to the significant variation in methodology used in included studies, no firm conclusion can be drawn about the exposure to ambient gaseous and particulate matters and the risk of ADHD in children | Further studies need to apply accurate exposure and outcome assessment method and consider all possible confounders | High (8/9) |
| Lam et al. [45] | Assess association between developmental exposure to PBDE and ADHD | Polybrominated diphenyl ether, ADHD | Database inception – September 2016 | 9 | 62–622 mother–child pairs | Narrative synthesis | – Due to limited data, no firm conclusion can be drawn about the exposure to PBDE and the risk of ADHD in children | Further studies of good quality are needed | High (9/9) |
| Caye et al. [46] | Determine the relationship between age and ADHD diagnosis | Relative age, Immaturity | Database inception – December 2018 | 25 | 8,076,570 | Random-effect meta-analysis | – Majority of the included studies were of high quality – Children born in the last 4 months of the school calendar year were at higher risk of receiving ADHD diagnosis compared to their relatively older class peers (RR = 1.34,1.26–1.43) – Significant heterogeneity (I 2 = 96.7%) was observed across analysis |
Relative maturity and developmental age should be consistently considered while making an ADHD diagnosis | High (9/9) |
| Russell et al. [47] | Assess the association between SES and ADHD | Socioeconomic status, Health inequalities | 1999–2013 | 42 | 53 to 842 per study | Random-effect meta-analysis | – The association between socioeconomic disadvantage and ADHD was a consistent finding, but can be mediated by parental mental health, maternal smoking during pregnancy, or other risk factors that are more prevalent in families with low SES | Further research that considers possible mechanism between SES and ADHD is warranted | Moderate (7/9) |
| Langevin et al. [48] | Disentangle the association between CSA and ADHD | Child sexual abuse, Predictor | Database inception – January 2020 | 28 | 75,306 | Narrative synthesis | – Variation in quality of included studies and lack of longitudinal studies restricted from untangling the association between CSA and ADHD | Rigorous longitudinal studies including those that assess the confounding role of other maltreatment forms and trauma-related symptoms are needed | Moderate (7/9) |
| Del-Ponte et al. [49] | Examine the association between dietary patterns and ADHD | Diet, Dietary pattern | Unclear | 14 | 375–16,831 participants per study | Random-effect meta-analysis | – Majority of included studies were of moderate quality – Children and adolescents consuming healthy diets have lower odds of having ADHD (OR = 0.65, 0.44–0.97) compared to those consuming unhealthy diet (OR = 1.41, 1.15–1.74) – Moderate heterogeneity (I 2 > 73%) were observed across analysis |
Longitudinal studies are needed to strengthen the evidence about the relationship between diet and ADHD | Moderate (6/9) |
| Farsad-Naeimi et al. [50] | Determine relationship between sugar consumption and the development of ADHD symptoms | Sugar, Soft drink | Database inception – March 2020 | 7 | 25,945 | Fixed- and random-effect meta-analysis | – Majority of included studies were of high quality – A positive relationship was found between sugar and soft drink consumption and ADHD (d = 1.27, 1.02–1.42) – Significant heterogeneity (I 2 = 81.9%) was observed across analysis |
Longitudinal studies that examine a potential causal relationship between sugar and soft drink consumption, and ADHD are needed | High (8/9) |
| Khashbakht et al. [51] | Synthesize the available literature on the relation between vitamin D status and ADHD | Vitamin D, Children, Adolescents | Database inception – June 2017 | 13 | Cases: 33–1,331 Controls: 20–6,492; Cohort:3,733 |
Random-effect meta-analysis | – Majority of included studies were of moderate quality – Children and adolescents with ADHD had a lower mean concentration of serum 25 (OH)D than healthy controls – The studies which reported ORs also showed a significant association between lower vitamin D status and ADHD (OR = 2.57; 1.09–6.04) – Further, a meta-analysis from prospective studies also showed an inverse relationship between perinatal vitamin D and ADHD (RR = 1.40, 1.09–1.81) – Significant heterogeneity (I 2 > 80%) was observed across analysis |
To understand the causal association between vitamin D status and the risk of developing ADHD, prospective cohort studies with large sample size, including different population and even population-based intervention studies that consider the maximum number of possible confounders should be conducted | High (8/9) |
| La Chance et al. [52] | Estimate the relationship between blood ratio of Omega-6 and Omega-3 fatty acids (n6/n3) and AA/EPA, to ADHD symptoms | Omega-3 fatty acids, Omega-6 fatty acids | Database inception – April 2014 | 5 | Not given | Random-effect meta-analysis | – Included studies were of high quality – Children and youth with ADHD have higher n6/n3 fatty acid ratios (SMD = 1.97,0.90–3.0)4, and higher AA/EPA ratios (SMD = 8.25,5.94–10.56) than those in controls – Significant heterogeneity (I 2 = 83%) was observed for n6/n3 fatty acid ratios |
Future research could assess whether fatty acid ratios could be used as biomarkers to identify which children with ADHD who may specifically benefit from treatment with essential fatty acids to normalize the n6/n3 or AA/EPA ratios | High (8/9) |
| Huang et al. [53] | Determine the relationship between magnesium level and ADHD in children | Magnesium, Trace element, Nutrition | Database inception – October 2018 | 12 | Cases: 2,872 Controls: 2,838 |
Random-effect meta-analysis | – Included studies were of high quality – ADHD children have significantly lower peripheral blood magnesium level (Hedges’ g = −0.5,−0.8 to −0.2) and lower hair magnesium levels (Hedges’ g = −0.7,−1.3 to −0.1) compared to controls – Significant heterogeneity (I 2 = 85%) was observed for hair magnesium levels |
Prospective studies with a large sample size are required to determine the causal relationship between magnesium level and the pathophysiology of ADHD | High (8/9) |
| Effatpanah et al. [54] | Examine the relationship between magnesium status and ADHD | Magnesium, Trace element | Database inception – August 2018 | 7 | Cases: 9–1,331 Controls: 11–1,331 |
Random-effect meta-analysis | – Included studies were of moderate quality – Children and adolescents with ADHD have 0.10 mmol/l (−0.18−0.02) lower serum magnesium levels than controls indicating an inverse relationship between magnesium level and ADHD – Significant heterogeneity (I 2 > 95%) was observed across analysis |
Further observational studies using standard diagnostic measurement are required to draw meaningful conclusions about magnesium level and ADHD | High (9/9) |
| Shih et al. [55] | Assess the association between peripheral manganese level and ADHD | Manganese, Pediatric psychiatry | Database inception – March 2018 | 4 | Cases: 175 Controls: 999 |
Random-effect meta-analysis | – Included studies were of moderate quality – Meta-analysis found significantly higher peripheral manganese levels in ADHD children compared to controls when including studies with either blood or hair levels) (Hedges’ g = 0.30,0.02–0.58) – However, the association was no longer significant when only blood manganese level was analyzed separately (Hedges’ g = 0.32, −0.03 − 0.69) – Moderate heterogeneity (I 2 > 50%) was observed across analysis |
Additional primary studies are warranted to understand better the relationship between peripheral manganese level and ADHD | Moderate (7/9) |
| Degremont et al. [56] | Examine whether children with ADHD have lower serum and brain iron concentrations, compared with healthy control subjects | Brain iron, Serum iron, Iron status, Serum ferritin | 2000–June 2019 | 20 | Cases: 2,209 Controls: 2,982 |
Narrative synthesis | – Majority of included studies were of moderate quality – Serum ferritin concentrations varied between studies, with 10 of 18 studies finding higher concentration in patients with ADHD compared to healthy controls – For serum iron, 7 of 10 studies showed no difference, 2 studies showed lower concentrations in patients with ADHD, and 1 study showed higher concentration. – 3 studies reported lower brain iron in patients with ADHD – Study methods and participants were heterogeneous |
There is need for longitudinal studies and larger MRI studies using magnetic field correlation to measure brain iron concentration in different regions of the brain | Moderate (7/9) |
| Cortese et al. [57] | Summarize the evidence about iron level and ADHD | Iron, Ferritin, Trace element | Database inception – July 2012 | 22 | 500–2,000 | Narrative synthesis | – Most of the studies assessing iron status in children with ADHD used serum ferritin as a measure, with mixed findings (i.e., both significant and nonsignificant associations) | More research based on other measures for assessment than serum ferritin are required to elucidate the relationship between iron status and ADHD | Moderate (7/9) |
| Tseng et al. [58] | Identify the association between iron status and ADHD | Iron, Ferritin | Database inception – August 2017 | 17 | >10,000 | Random-effect meta-analysis | – Majority of included studies were of high quality – Children with ADHD have lower peripheral serum ferritin levels (Hedges’ g = −0.24,−0.44 to −0.05), but not iron or transferrin levels compared to healthy controls – Children with iron deficiency have more severe ADHD symptoms than ADHD children without ID (Hedges’ g = 0.88, 0.32–1.45) – Significant heterogeneity (I 2 = 82.9%) was observed for peripheral serum ferritin level |
Prospective studies are required to provide better insight into the relationships between iron status and ADHD symptoms and to clarify the potential pathophysiological mechanisms | High (8/9) |
| Ghoreisy et al. [59] | Estimate the association between hair and serum/plasma zinc levels and ADHD | Zinc, Trace elements | Database inception – October 2020 | 22 | Cases: 1,280 Controls: 1,200 | Random effects meta-analysis | – Majority of included studies were of high quality – Serum or plasma zinc levels in subjects with ADHD were not statistically different compared to controls (WMD = − 1.26 μmol/L,−3.72–1.20) – After removing one study which showed a significant higher levels of serum/plasma zinc in subjects with ADHD compared to the controls, zinc levels were lower in ADHD patients (WMD = − 2.49 μmol/L,−4.29 to −0.69) – Further, hair zinc levels in cases with ADHD were not statistically different compared to controls (WMD: − 24.19 μg/g; −61.80 – 13.42) – Significant heterogeneity (I 2 > 98%) were observed across analyses |
Further well-designed studies are needed to clarify the role of zinc in the etiology of ADHD | High (8/9) |
| Luo et al. [60] | Explore the available evidence on the correlation between zinc and ADHD | Zinc, ADHD | Database inception – April 2019 | 11 | 1,517 | Random-effect meta-analysis | – Majority of the included studies were of good quality – No significant difference in zinc level in blood (SMD = −0.91,−1.88–0.07) or hair (SMD = 1.4,−4.49–7.33) was found between children and adolescents with and without ADHD – Significant heterogeneity (I 2 > 95%) were observed across analysis |
Additional studies with a large sample size and robust methodology are required | Moderate (7/9) |
Abbreviations: AA, arachidonic acid; CrI, credible interval; CS, cesarean section; CSA, child sexual abuse; d, effect size; EA, eicosapentaenoic acid; ID, iron deficiency; k, total number of included studies; MSDP, maternal smoking during pregnancy; Nox, nitrogen oxides; OR, odds ratio; PAH, polycyclic aromatic hydrocarbon; PB, preterm birth; PBDE, polybrominated diphenyl ether; PM, particulate matter; POE, prenatal opioid exposure; RR, relative risk; Rrat, risk ratio; SES, socioeconomic status; SHS, second-hand smoke; SMD, standardized mean difference; TBI, traumatic brain injury; VLBW/ELBW, very/extreme low birth weight; VP/EP, very and extreme preterm; WMD, weighted mean difference.
Total participants included in the systematic review and meta-analysis or otherwise specified.
For findings from meta-analysis, if given effect estimates with 95% CI are presented or otherwise specified.
In this section, we use terms like risk, correlation, protective, and association according to reports in the systematic reviews, without indicating causality. Generally, this literature did not bring evidence for conclusions on causality, which will be discussed later.
Genetic factors
One systematic review found strong evidence that the common genetic variants underlying ADHD, as measured by the ADHD polygenic risk score, were associated not only with diagnosed ADHD but also with more dimensional ADHD traits [20].
Maternal factors
Maternal pre-pregnancy overweight [21], use of antibiotics [22], acetaminophen [23], and antidepressants [24] during pregnancy, and maternal pregestational diabetes [25], but not gestational diabetes [26], were associated with increased rates of ADHD in offspring. However, the authors stated that associations may be due to unmeasured confounding and thus not causal (e.g., the association with maternal overweight was explained by familial confounding). Maternal smoking during pregnancy (odds ratio (OR) = 1.56, 95% CI: 1.41–1.72) or smoking cessation during the first trimester was associated with ADHD in offspring [27]. Similarly, prenatal opioid exposure was associated with higher ADHD symptom scores (standardized mean difference (SMD) =1.27, 0.79–1.75) [28]. Maternal exposure to perfluoroalkyl substances was not associated with ADHD in their children [29].
Perinatal complications like maternal preeclampsia [30], very preterm birth/very low birth weight (OR = 3.04, 95% CI: 2.19–4.21) [31], or low birth weight [32] were associated with offspring ADHD. Likewise, the caesarian section was reported to be associated with later ADHD diagnosis in unadjusted analyses [33], but a later review reported this association to be partly or entirely accounted for by residual confounding [34].
A nonlinear relation between parental age and risk for ADHD in the offspring was found with the highest risk for parents below 20 years and lowest risk for parents in the mid-thirties [35].
While maternal breastfeeding was associated with a reduced risk of ADHD in children [36, 37], postnatal exposure to second-hand smoking was associated with increased risk (OR = 1.60, 95% CI: 1.30–1.80) [38].
Compared to children with other injuries or without injuries, children with severe traumatic brain injuries had an increased risk of being diagnosed with ADHD at less or more than 1 year, respectively, after the injuries [39]. Likewise, two or more exposures to general anesthesia were associated with an increased risk of ADHD in later life (relative risk RR = 1.84, 1.14–2.97) [40]. Blood lead level was associated with higher ADHD rates in children and adolescents [41]. No significant association was found between polycyclic aromatic hydrocarbon exposure and ADHD in children [42, 43], and there were inconclusive evidence for an association between exposure to air pollution [44] or polybrominated diphenyl ethers [45] and ADHD.
According to two included reviews, children being relatively younger than classmates had higher rates of ADHD diagnosis [17, 46], with one reporting the relative risk of 1.34 (95% CI: 1.26–1.43) for the youngest children [46]. Further, two reviews suggested an association between socioeconomic disadvantage and risk of ADHD [15, 47], with one suggesting it to be mediated by factors such as parental mental health and maternal smoking during pregnancy [47]. The evidence regarding child sexual abuse as a predictor for ADHD was unclear [48].
Dietary pattern, nutrition, and trace elements
Children and adolescents consuming healthy diet had lower risk of having ADHD compared to those consuming unhealthy diet [49]. Positive relationship was indicated between total sugar intake from soft drinks and dietary sources and ADHD symptoms in children and adolescents [50]. Other nutritional factors associated with higher rates of ADHD among children and adolescents were low serum concentration of 25-hydroxyvitamin D, lower perinatal and childhood vitamin D status [51], elevated ratios of both blood omega-6 to omega-3 and arachidonic acid to eicosapentaenoic acid fatty acids [52]. Two systematic reviews reported significantly lower serum manganese levels in children with ADHD [53, 54]. Another review revealed higher peripheral manganese levels in both blood and hair in children and adolescents with ADHD compared to healthy controls [55].
A review from 2021 suggested that brain iron concentrations, specifically in the thalamus, were lower in children with ADHD than in healthy controls [56]. However, mixed results were reported for systemic iron level [56, 57]. In contrast, a review from 2018 concluded that low serum iron levels were associated with ADHD [58]. There was no difference in zinc levels in blood, serum, plasma [59] or hair between children and adolescents with ADHD and healthy controls [60].
Long-term prognosis and life trajectories in ADHD (n = 19, Table 6)
Table 6.
Long-term prognosis and life-trajectories in ADHD
| References | Objective | Keywords | Timeframe of database search | k | Sample size a | Analytical design | Main findings (Effect estimates; 95% CI) b | Conclusion and comments from the authors | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Christiansen et al. [61] | Synthesize evidence of an association between ADHD diagnosis in childhood and later education, earnings and employment, compared to children without an ADHD diagnosis | Life-course, Occupation, Work, Employment | Database inception – November 2020 | 6 | Cases: 1,380 Controls: 888 |
Narrative synthesis | – Majority of the included studies were of moderate quality-ADHD was associated with lower quality employment, lower lifetime income, and more part time and unskilled work and with lower educational attainment measured in several ways | There is a need for further high-quality research evaluating factors and interventions that reduce the long-term vocational impacts of childhood ADHD, especially ADD, which is not addressed in the present literature | High (9/9) |
| Erskine et al. [62] | Explore the potential outcomes associated with ADHD diagnosis | ADHD, Long-term prognosis | 1980–March 2015 | 101 | 71 to slightly less than 2 million | Random-effect meta-analysis | – Included studies were of moderate to high quality – ADHD was associated with a range of negative life outcomes, including failure to complete high school (OR = 3.70, 1.96–6.99), use of education services (OR = 6.37, 2.58–15.73), dismissal from work (OR = 3.92, 2.68–5.74), substance dependence (OR = 2.45,1.44–4.17), violence – related arrest (OR = 3.63, 2.31–5.70) – Significant heterogeneity (I 2 = 83.1%) was observed for substance dependence |
A better comprehension of the underlying mechanism for the association between ADHD and several long-term outcomes are required to prevent or reduce these long-term adverse outcomes | High (9/9) |
| Tosto et al. [63] | Determine the relationship between ADHD and mathematics | Mathematical ability, Mathematics achievement | Database inception – February 2015 | 34 | 2,000–10,000 | Narrative synthesis | – Majority of the included studies were of high quality – There was clear evidence for a negative relationship between ADHD and mathematical ability, which was stronger for inattentive compared to hyperactivity-impulsivity symptoms |
More longitudinal research is required to gain a better understanding of the mechanism behind this association | Moderate (6/9) |
| Di Lorenzo et al. [64] | Present the clinical and social outcomes among adults who suffered from ADHD in childhood or adolescence | Alcohol-related disorders, Antisocial personality disorder, Problem behavior | 2015–2020 | 27 | Unclear | Weighted Mean Difference | – Majority of included studies were of high quality – ADHD persisted into adulthood with a mean rate of 43% – ADHD before adulthood was associated with substance or alcohol use disorders and antisocial behavior in adulthood, and, to a lesser degree, with anxiety and depressive disorders |
New studies with uniform diagnostic criteria and more studies on nonwestern region are needed | High (9/9) |
| Charach et al. [65] | Quantify the association between childhood ADHD and future risk of alcohol, nicotine and substance use | Substance use disorder, Alcohol use disorder, Nicotine use | Database inception – October 2009 | 13 | 2,000–10,000 | Random-effect meta-analysis | – Majority of included studies were of high quality – Children with ADHD were more likely to develop alcohol use disorder in their early adulthood (OR = 1.35, 1.11–1.64) and nicotine use in their middle adolescence (OR = 2.36, 1.71–3.27) compared to controls – However, the magnitude of the association with future psychoactive SUD and nonalcohol drug use disorder was unclear due to the influence of a single study – No heterogeneity were observed across analyses |
For better evidence, multiple cohort studies that use survival analysis to assess time to initial substance use outcomes are warranted as it might help to increase power and to determine baseline risk factors | Moderate (6/9) |
| Fond et al. [66] | Compare the smoking behavior of adult smokers with a childhood history of ADHD (CH) to adult smokers without CH | Smoking, Nicotine Dependence | Database inception – 2013 | 9 | 365 smokers with CH and 1,708 smokers without | Random-effect meta-analysis | – Included studies were of high quality – Adolescents with CH were found to consume more cigarettes (SMD = 0.15, 0.01–0.28) during adolescence and started smoking at earlier age (SMD: −0.28, −0.49 to −0.07) – However, such associations were not found among adults – Moderate heterogeneity (I 2 > 60%) were observed across analyses |
Additional studies from different countries and in adolescents are needed for better clarification. Research should also investigate the smoking behavior pattern in adulthood |
High (9/9) |
| Oliva et al. [67] | Determine the prevalence of cocaine use and cocaine use disorder among ADHD adults | Cocaine, Cocaine use disorder | Database inception – July 2019 | 12 | 3,329 | Random-effect meta-analysis | – Majority of the included studies were of high quality – Prevalence of cocaine use was 26.0% (18.0–35.0) and for cocaine use disorder 10.0% (8.0–13.0) – Significant heterogeneity (I 2 > 95%) were observed across analyses |
Studies on effects of stimulants and other treatment for ADHD on cocaine use disorder among ADHD adults are required | High(8/9) |
| Amiri et al. [68] | Estimate the association between ADHD and injuries | Injuries, Accidents | 2000–2014 | 35 | 49,200 | Random-effect meta-analysis | – Included studies were of moderate quality – Meta-analysis results from all comparative studies showed that individuals with ADHD were nearly two times more likely to be injured (OR = 1.96, 1.63–2.37) – Likewise the odds ratio from cohort studies was (OR = 2.18, 1.46–3.24) – Significant heterogeneity (I 2 > 95%) were observed across analyses |
Individuals with ADHD and caregivers should be informed about the potential risk of injuries, and necessary measures aimed to reduce such risk at the community level should be taken | Moderate (7/9) |
| Seens et al. [69] | Assess the prevalence of bone fractures among children and adolescents with ADHD | Bone fracture, Injury | Database inception – (date of last search not given) | 5 | 53,849 | Random-effect meta-analysis | – Prevalence of bone fractures among children and adolescents with ADHD was 4.83% (3.07–6.58) | Prospective study designs that can follow children and adolescents with ADHD over time to determine the type of fractures, source of injuries and age of fracture are needed | High (8/9) |
| Ruiz-Goikoetxea et al. [70] | Examine the association between ADHD and risk of unintentional physical injuries in children/adolescents | Unintentional physical injuries, Child Psychiatry | Database inception – June 2017 | 14 | Cases: 371,301 Controls:4,957,511 |
Random-effect meta-analysis | – Majority of the included studies were of moderate quality – For unintentional injuries, OR was 1.53 (1.40–1.67), while the HR based on the result of four studies was 1.39 (1.06–1.83) – Significant heterogeneity (I 2 > 82%) were observed across analyses |
Further studies should assess the association between different subtypes of ADHD and unintentional injuries | High (9/9) |
| Cook et al. [71] | Explore the association between ADHD and clinical outcome from sport-related concussion | Brain trauma, Outcome research | Database inception – February 2019 | 14 | Cases: 359 Controls: 3,264 |
Narrative synthesis | – Methodological weakness of the included studies restricted from making any firm conclusion about the ADHD and the risk of sport related concussion | Longitudinal studies that include a large sample size and adjust for potential confounders are required | Moderate (7/9) |
| Ruiz-Goikoetxea et al. [72] | Determine the risk of poisoning and compare the magnitude of the risk of unintentional physical injuries and poisoning | Poisoning, Unintentional injuries, Children and adolescents | Database inception – November 2017 | 35 | Cases:84,756 Controls: 1,398,946 |
Random-effect meta-analysis | – Included studies were of poor quality – Children and adolescents with ADHD have more than three times greater risk of poisoning compared to the control group (RR = 3.14, 2.23–4.42), which was significantly higher than that of unintentional physical injuries (Beta coefficient = 0.68, 0.16–1.20) – Significant heterogeneity (I 2 > 88%) were observed across analyses |
Studies should examine the influence of medication and comorbidities on the risk of poisoning | High(9/9) |
| Septier et al. [73] | Examine the association between ADHD and SSB | Suicide, Children, Adults | Database inception – April 6, 2018 | 57 | 90,805 participants with ADHD and 239,778 without ADHD | Random-effect meta-analysis | – Included studies were of poor quality – An overall significant association between ADHD and SSB was found; suicidal attempt (OR = 2.37,1.64–3.43); suicidal ideations (OR = 3.53, 2.94–4.25); suicidal plans (OR = 4.54, 2.46–8.37), and completed suicide (OR = 6.69, 3.24–17.37) – Significant heterogeneity (I 2 > 87%) were observed for suicidal attempts and complete suicide, while moderate heterogeneity (I 2 = 73.73%) for suicidal ideations |
Recommend screening for SSB in ADHD patients by clinicians at each visit | Moderate (7/9) |
| Nourredine et al. [74] | Assess the association between ADHD and the risk of subsequent PD | Childhood ADHD, Psychotic Disorder | Database inception – July 7, 2020 | 15 (12 in meta-analysis) | 1.85 million | Meta-regression using random-effect model | – Majority of the included studies were of high quality – Significant association was found between childhood ADHD and subsequent PD (OR = 4.74, 4.11–5.46) – No significant between-group differences were found for subgroup analyses according to PD (OR = 5.04, 4.36–5.83) or schizophrenia (OR = 4.59, 3.83–5.50) outcomes, cohort (OR = 4.64, 4.04–5.34) or adjusted (OR = 4.72, 4.11–5.46) or unadjusted (OR = 3.81, 1.39–10.49) estimates – No heterogeneity were observed across analyses |
Future studies need to determine the mechanisms linking these common conditions | High (9/9) |
| Baggio et al. [75] | Determine the prevalence of ADHD in detention settings | Prevalence, Prisons | Database inception to January 2, 2018 | 102 | 69,997 | Meta-regression using random-effect model | – Majority of included studies were of high quality – The prevalence of ADHD among adults was 26.2% (22.7–29.6), and the prevalence of childhood ADHD was 41.1% (34.9–47.2) while measured retrospectively – No significant difference was found between prevalence assessed by screening and those by clinical interviews |
Adequate screening, diagnosis, and treatment of ADHD among those living in prisons are warranted | High(8/9) |
| Mohr-Jensen et al. [76] | Examine the long-term effect of ADHD on criminal outcomes | ADHD, Crime, Longitudinal studies | Database inception – August 2015 | 11 | 15,442 | Random-effect meta-analysis | – Majority of the included studies were of high quality – Childhood ADHD was associated with a long-term higher risk of convictions (RR = 3.3, 2.1–5.2) followed by incarcerations (RR = 2.9, 1.9–4.3) and arrest (RR = 2.2, 1.3–3.5) – Potential predictors for antisocial outcomes were early behavior problems, childhood maltreatment, sex, and IQ |
Prospective studies with a large sample size that clarifies the role of sex, IQ, SES, other mental disorder and childhood maltreatment while assessing the long-term risk of ADHD on criminal activities are needed | High (8/9) |
| Buitelaar et al. [77] | Summarize the evidence for ADHD and risk of being perpetrator of DV and IPV | Domestic violence, Intimate partner violence | Database inception – January 2015 | 7 | 64–11,238 participants per study | Narrative synthesis | Heterogeneity in methodology and outcome measures across studies restricted from making any firm conclusion about the association between ADHD and IPV or DV | Longitudinal studies should include a large sample size and assess the role of potential confounders | High (8/9) |
| Kittel-Schneider et al. [78] | Analyze current evidence for association between maternal ADHD including stimuland treatment and pregnancy risk, birth outcomes, health behavior in pregnancy, and early parent–child interaction and early child development in the first 3 years | Mother, Father, Pregnancy, Lactation, Breastfeeding, Methylphenidate, Atomoxetine, Guanfacine, Lisdexamfetamine, Amphetamine, Modafinil | Database inception – November 2021 | 32 (4 to 12 for each of four research questions) | Several different subgroups and methods, including 1,951,940 participants in studies of healthy behavior in pregnancy, 1,266 families or child–parent dyads, and 29,282 exposed and 11,452,476 controls for six medication exposures | Narrative synthesis | – Majority of included studies were of moderate quality – ADHD in girls was associated with teenage and unintended pregnancies, and with pregnancy and birth complications – ADHD in parents was associated with increased parenting stress, risk of ADHD in children, increased aggression in children, and reduced effects of parent training – Maternal ADHD was associated with parenting warmth in some studies – Use of ADHD medications was associated with increased risk for some birth defects, but the increase was low |
Further studies with a higher quality are needed to assess the causal mechanism | Moderate (6/9) |
| Chhibber et al. [79] | Synthesize current evidence on the economic burden of ADHD on the global scale, focusing on describing the methodological variations of studies estimating this burden | Economic burden, High income countries | Database inception – December 2020 | 44 | NA | Narrative synthesis | – Included studies were of high quality – ADHD leads to a substantial economic burden on society – Estimates based on marginal costs ranged from $US244.15 to 18,751.00 for per person estimates and from $US12.18 million to 141.33 billion for national estimates – Studies that calculated economic burden across multiple domains of direct, indirect, and education and justice system costs for both children and adults with ADHD reported higher costs and translated gross domestic product than did studies that captured only a single domain or age group |
There is an urgent need to conduct cost-of-illness research in LMICs | High (8/9) |
Abbreviations: DV, domestic violence; HR, hazard ratio; IPV, intimate partner violence; k, total number of included studies; LMICs, low and middle-income countries; OR, odds ratio; PD, psychotic disorder; RR, relative risk; SSB, suicidal spectrum behaviors.
Total participants included in the systematic review and meta-analysis unless otherwise indicated.
For findings from meta-analysis, if given effect estimates with 95% CI are presented unless otherwise indicated.
Education and employment
ADHD was associated with lower educational attainment [61], including failure to complete high school (OR = 3.7, 95% CI: 2.0–7.0) and failure to attend tertiary education (OR = 6.47, 4.58–9.14) [62]. Further, a negative association was found between ADHD and mathematical ability [63]. Individuals with ADHD were more prone to experience occupational challenges [61], for example, they were more often dismissed from work (OR = 3.92, 2.68–5.74), unemployed (OR = 1.97, 1.01–3.85) [62], and more likely to receive public welfare payments [61].
Alcohol, smoking, and substance use
Childhood ADHD was significantly associated with alcohol use disorder [64], including the development of alcohol use disorder by early adulthood (OR = 1.35, 95% CI: 1.11–1.64) and nicotine use by middle adolescence (OR = 2.36, 1.71–3.27) [65]. Smokers with ADHD in childhood smoked significantly more cigarettes as adolescents than smokers without childhood ADHD [66]. There was an association between ADHD and substance use disorder [64], with an earlier meta-analysis reporting an odds ratio of 1.73(1.24–2.41) [62]. The estimated average prevalence of cocaine use in adults with ADHD was 26.0% (18.0–35.0) [67].
Injuries, poisoning, and suicidal spectrum behavior
Both children and adults with ADHD were at higher risk of injuries (OR = 1.96, 95% CI: 1.63–2.37) [68], including bone fracture [69] and unintentional physical injuries (OR = 1.53, 1.40–1.67) [70], but not for sports-related concussions [71]. Children and adolescents with ADHD also had a higher risk of poisoning than controls (RR = 3.14, 2.23–4.42) [72]. Suicidal spectrum behavior was higher in ADHD, including suicidal ideations (OR = 3.53, 2.94–4.25), suicidal plans, attempts (OR = 2.37, 1.64–3.43), and completed suicide [73].
Psychotic disorders
Childhood ADHD was associated with an increased risk of subsequent psychotic disorders (OR = 4.74, 95% CI: 4.11–5.46) [74].
Criminal offenses and domestic violence
The pooled prevalence of ADHD among individuals in detention settings was 26.2% (95% CI: 22.7–29.6) [75]. There was a significant association between childhood ADHD and adolescent and adulthood arrests (RR = 2.2, 1.3–3.5), convictions (RR = 3.3, 2.1–5.2), and incarcerations (RR = 2.9, 1.9–4.3) with a younger age of onset of antisocial involvement and an increased risk of criminal recidivism [76]. However, there was no conclusive evidence for an association between ADHD and domestic violence [77].
Pregnancies and postpartum risk
Adolescent girls with ADHD had an increased risk of teenage and unintended pregnancies, and women with ADHD had a higher risk of pregnancy and birth complications, such as pre-eclampsia, infection, and cesarean section [78].
Global economic burden of ADHD
The per person annual economic burden of ADHD ranged from $US832 to 20,539 for patients with ADHD, and from $US2,670 to 4,120 for family members of patients with ADHD [79].
ADHD and comorbidities (n = 33, Table 7)
Table 7.
ADHD and comorbidities
| References | Objective | Keywords | Timeframe of database search | k | Sample size a | Analytical design | Main findings (Effect estimates, 95% CI) b | Conclusion and comments from the authors | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Holingdale et al. [80] | Estimate the prevalence of ASD among children and adolescents with ADHD | Autism Spectrum Disorder, Comorbidity | 2007–2017 | 28 | 61,985 | Random-effect meta-analysis | – Majority of the included studies were of moderate quality −21% of children and adolescents with ADHD also fulfill criteria for ASD – Children with ADHD also had more dimensionally measured ASD traits compared to controls. – There were no significant differences between community and clinical samples or the US and non-US studies – Significant heterogeneity (I 2 > 87%) were observed across analyses |
Future studies should include a large sample size and standard methods. Research assessing the effect of medication on ADHD should also consider the presence of ASD among ADHD patients | High (8/9) |
| Carruthers et al. [81] | Compare the pragmatic language profiles of children with ADHD to those of TD children and those with autism | Communication, Pragmatic language | Database inception – October 2019 | 34 | Cases: 1,407; Controls: 1,058 Autism: 38 |
Narrative Synthesis | – Majority of the included studies were of moderate quality – Children with ADHD were found to have higher rates of pragmatic difficulties than their TD peers with specific difficulties linked to inappropriate initiation, presupposition, social discourse, and narrative coherence – Children with ADHD appear to differ from those with autism in the degree of their pragmatic language impairments |
Good quality research that assess the differences in profile of pragmatic language impairments in the children with ADHD and those with autism are required | High (8/9) |
| Jacobsson et al. [82] | Review the associations between adult ADHD and personality traits, organized within a maladaptive five factor framework | Personality disorder, Personality traits | January 2000 – date of last search not given | 13 | Cases: 2,023 Controls: 16,835 |
Random-effect meta-analysis | – Majority of the included studies were of moderate quality – Effect size for Negative Emotionality and Conscientious Inhibition were greater and significant (d = 1.11 and −0.89, respectively) – Effect sizes for Agreeable Inhibition and Positive Emotionality were moderate and significant (d = −0.39 and −0.43, respectively) – Significant heterogeneity (I 2 > 84%) were observed across analyses |
Further research that more specifically delineate how ADHD can be fit into the personality/psychopathology hierarchy are needed | Moderate (6/9) |
| Schiweck et al. [83] | Investigate the comorbidity of ADHD and BD in adults | Adult bipolar disorder, Prevalence, Comorbidity | Database inception – October 2020 | 71 | 646,766 | Random-effect meta-analysis | – Prevalence of BD among ADHD patients was 7.95% (5.31–11.06), with earlier age of onset of BD than for persons without ADHD – There was however significant variation observed across the different diagnostic systems, geographic locations, sample size – Significant heterogeneity (I 2 > 94%) was observed across analyses |
The effect of different versions of the specific diagnostic systems requires further clarification, and clinicians should consider the potential presence of BD among adults with ADHD | High (8/9) |
| Nazar et al. [84] | Assess the comorbidity of ADHD and ED | Eating disorder, ADHD | Not given | 22 | >45,753 | Random-effect meta-analysis | – ADHD patients were nearly four times more likely to have ED compared to controls (OR = 3.82, 2.34–6.24), with significant variation observed across assessment methods – Clinical assessment yielded significantly higher risk (OR = 5.89, 4.32–8.04) than self-report instrument (OR = 2.23, 1.23–4.03) – Significant heterogeneity (I 2 > 94%) was observed across analysis |
There is a need to address whether ADHD patients with a comorbid ED have a different prognosis, course and treatment response when compared to patients with either disorder alone | Moderate (6/9) |
| Kaisari et al. [85] | Examine the association between ADHD and DEB | Eating disorders, Disordered eating behavior | Database inception – May 2016 | 72 | 15,418 | Narrative synthesis | – Majority of the included studies were of moderate quality – Existing literature showed moderate evidence for the positive association between ADHD and DEB, particularly overeating behavior |
Further studies should include a large representative sample, use uniform assessment techniques and also control for health characteristics, stimulant medication and ADHD-related comorbidities to clarify the nature of the relationship between ADHD and disordered eating behavior | Moderate (7/9) |
| Curtin et al. [86] | Summarize the evidence for association between ADHD and ED among youth | Adolescents, Eating Disorders, Eating Pathology | 1971–2012 | 8 | ADHD: 2,032 Control: 5,173 |
Narrative synthesis | – Included studies were of moderate quality – The result from available few primary studies showed a positive association between ADHD and ED |
Additional studies are required to confirm this finding | Moderate (7/9) |
| Dullur et al. [87] | Review the association between ADHD and GD | Gaming disorder, Mental health comorbidity | Not given | 29 | 56,650 | Narrative synthesis | – Included studies were of moderate quality – ADHD symptoms were consistently associated with GD, with more frequent associations displayed with inattention than other ADHD subscales – No conclusive findings were available regarding the type of game on severity of either condition, or on completion of treatment |
Future studies needs to elucidate the direction of the relationship between ADHD and GD | Moderate (6/9) |
| Wang et al. [88] | Determine the association between IA and ADHD | Internet addiction, ADHD | Database inception – June 2016 | 15 | 12,774 | Random-effect meta-analysis | – Quality of included studies ranged from poor to moderate – Those with IA were 3.76 times more likely to be diagnosed with ADHD and had more severe symptoms of ADHD compared to those without IA – Moderate heterogeneity (I 2 > 60%) was observed across analysis |
The causal relation between IA and ADHD needs to be studied | High (9/9) |
| Keenan et al. [89] | Explore potential overlaps and distinctions in objective measures of sleep between PTD and ADHD | Objective sleep measures, Polysomnography | Database inception – September 2020 | ADHD only (N = 16) ADHD +PTD (N = 3) |
ADHD-only (N = 316) PTD + ADHD (N = 79) |
Random-effect meta-analysis | – Majority of the included studies were of high quality – Compared to controls, no significant effect was found for. Sleep efficiency, sleep onset latency in ADHD-only populations – A small effect size was found in PTD + ADHD populations with significantly lower sleep efficiency compared to controls (SMD = −0.25,−0.46 to −0.04) and increased sleep onset latency (SMD = 0.33, 0.01–0.66) – Moderate heterogeneity (I 2 > 40%) was observed across analyses for ADHD only |
Greater emphasis should be placed on confounding variables including treatment, symptom severity, cooccurring disorders, and age. In particular, more highly controlled studies with unmedicated ADHD populations are warranted | Moderate (7/9) |
| Lee et al. [90] | Assess the association between short or long sleep duration and the risk for ADHD | Short sleep duration, Long sleep duration | 1971–2019 | 10 | 5,963 | Random-effect meta-analysis | – Majority of the included studies were of high quality – Short sleep duration was associated with ADHD and particularly with hyperactivity (OR/RR = 1.60,1.18–2.17) – Significant heterogeneity (I 2 > 90%) was observed across analysis |
Prospective studies with adequate power that control for potential confounders are needed to clarify the association | High (8/9) |
| Diaz-Roman et al. [91] | Examine the association between sleep physiology and ADHD in some sleep parameters | Sleep, ADHD | 1987–March 2014 | 11 | 500–2,000 | Fixed-effect meta-analysis | – Included studies were of high quality – By using polysomnography, it was found that children with ADHD had a lighter sleep than those without ADHD (SMD = 0.32, 0.08–0.55) – No heterogeneity was observed across analysis |
Additional studies are needed to confirm this finding | High (8/9) |
| DeCrescenzo et al. [92] | Determine the role of actigraphy in monitoring of changes in motor activity and in sleep patterns in ADHD | Actigraphy, Sleep | Database inception – July 2014 | 24 | 2,179 | Random-effect meta-analysis | – Included studies were of high quality – Although sleep duration did not differ, children with ADHD had moderate mean motor activity (SMD = 0.65, 0.45–0.84), higher sleep latency (SMD = 0.51, 0.10–0.92) and lower sleep efficiency (SMD = −0.69,−1.32 to −0.05) compared to typically developing controls – No heterogeneity was observed across analysis |
Actigraphy may be a clinically useful tool to monitor motor activity and sleep patterns in children with ADHD, particularly to monitor the effect of medical treatment | High (9/9) |
| Diaz-Roman et al. [93] | Understand the sleep parameters among adults with ADHD | Sleep, Adults, Actigraphy | Database inception – August 2017 | 13 | Cases: 652 Controls: 769 |
Random-effect meta-analysis | – Included studies were of high quality – Adults with ADHD had longer sleep onset latency (SMD = 0.67, 0.41–0.92), lower sleep efficiency (SMD = −0.55, −0.83 to −0.27), and higher daytime sleepiness (SMD = 0.75, 0.29–1.21) – Actigraphic measures also showed similar results – Significant heterogeneity (I 2 = 86%) was observed for daytime sleepiness, while moderate (I 2 = 54%) for sleep onset latency |
Further studies should assess the impact of ADHD subtype and comorbid psychiatric disorders | High (8/9) |
| Instanes et al. [94] | Review evidence about ADHD and several somatic condition | Obesity, Asthma, Migraine | 1994–2015 | 126 | >17,007,576 | Narrative synthesis | – Majority of the included studies were of moderate quality – Evidence for an association between adult ADHD and somatic conditions like obesity, sleep disorders, and asthma was found, while preliminary evidence existed for migraine and celiac disease – No association was found for ADHD and cardiovascular disease |
Registry-based studies will help to provide better insight into the association by adjusting for familial and unfamilial confounders. Future studies should also use uniform diagnostic assessment | Moderate (7/9) |
| Lugo et al. [95] | Review sleep disturbances in adults with ADHD | Adults, Sleep | 1994–February 2019 | 39 | 3,382 | Narrative synthesis and random-effect meta-analysis | – Included studies were of high quality – Sleeping problems like large sleep onset latency, poor sleep efficiency, great number of night awakenings, and decreased sleep quality were found more often in ADHD adults than in controls – Significant heterogeneity (I 2 > 80%) was observed across analysis |
Additional studies with a large sample size and with adequate controlling for other covariates like medication and comorbid mental disorder are needed | Moderate (7/9) |
| Souto-Souza et al. [96] | Examine the relationship between ADHD and bruxism | Bruxism, ADHD | Database inception – April 2019 | 32 | ADHD: 2,629 Bruxism: 1,739 |
Random-effect meta-analysis | – Majority of the included studies were of moderate quality – Children and adolescents with ADHD were nearly three times more likely to have both sleep bruxism (OR = 2.77, 1.90–4.03), and awake bruxism was 10.64 (2.41–47.03) – Moderate heterogeneity (I 2 > 60%) was observed across analysis |
High quality studies with robust methodology are warranted | High (8/9) |
| Li et al. [97] | Estimate the global prevalence of obesity, overweight and underweight among ADHD patients | ADHD, Obesity, Prevalence | Database inception – June 2020 | 48 | Not given | Random-effect meta-analysis | – Majority of the included studies were of moderate quality – A high prevalence of overweight (20.9% (18.5–23.3)) and obesity (14.7% (12.9–16.4)) was found among individuals with ADHD |
Well-designed studies assessing causality between ADHD and obesity and overweight are required | High (9/9) |
| Cortese et al. [98] | Examine the association between obesity/overweight and ADHD | Obesity, Overweight, ADHD | Databases inception – August 2015 | 42 | Cases: 48,161 Controls: 679,975 |
Random-effect meta-analysis | – Majority of the included studies were of moderate quality – A significant association exists between ADHD and obesity among children/adolescents and adults with ADHD – Moderate heterogeneity (I 2 > 50%) was observed across analyses |
The underlying mechanism for the relationship between ADHD and obesity needs to be established | High (9/9) |
| Mocanu et al. [99] | Assess the impact of ADHD on outcomes of bariatric surgery | Bariatric surgery, Obesity, ADHD | 1946–August 2018 | 5 | 492 | Random-effect meta-analysis | – Included studies were of moderate quality – Patients with ADHD did not have a statistically significant mean BMI difference compared to patients without ADHD following bariatric surgery – However, they had a statistically significant reduction in postoperative follow-up time (months) versus non-ADHD patients (MD = −7.28, −13.83 to −0.73). – Significant heterogeneity (I 2 = 84%) was observed for mean BMI change |
Studies that elucidate the neurophysiologic mechanisms of obesity and their impact on associated mental health disorders are needed | Moderate (6/9) |
| Pan et al. [100] | Investigated the cooccurrence of headache in children with ADHD | Headache, Comorbidity | Database inception – June 2020 | 13 | Cases: 267,556 Controls: 2,464,878 |
Random-effect meta-analysis | – Majority of the included studies were of high quality – The pooled prevalence of headaches in children with ADHD was 26.6% – Significant heterogeneity (I 2 > 93%) was observed across analyses |
For example, no eligible longitudinal studies aiming to disentangle the temporal relationship between the emerging of attention problems and headache symptoms were identified | High (8/9) |
| Kaas et al. [101] | Investigate the association between asthma and ADHD | ADHD, asthma | Database inception – March 2019 | 25 | Not given | Random-effect meta-analysis | – Majority of the included studies were of moderate quality – Significant association was found between asthma and ADHD (OR = 1.52, 1.42–1.63) – Moderate heterogeneity (I 2 = 60%) was observed |
Clinicians should be aware of such association to aid an early diagnosis and treatment of such comorbidity | Moderate (6/9) |
| Cortese et al. [102] | Assess the association between ADHD and asthma | ADHD, asthma | Not given | 84 | Cases: 210,363 Controls: 3,115,168 |
Random-effect meta-analysis | – Included studies were of high quality – Significant association found between asthma and ADHD (unadjusted OR = 1.66, 1.22–2.26), and (adjusted OR = 1.53, 1.41–1.65) – Significant heterogeneity (I 2 = 99.47%) was observed for unadjusted analysis, while moderate heterogeneity (I 2 = 50.76%) was observed for adjusted analysis |
Further cohort studies are required to examine the causal relationship between ADHD and asthma | High (8/9) |
| Schans et al. [103] | Investigate the association between atopic disorders and ADHD in children and adolescents | Asthma, atopic eczema, allergic rhinitis | Database inception – September 2015 | 28 | 166,375 | Narrative synthesis and random-effect meta-analysis | – Majority of the included studies were of moderate quality – ADHD was significantly associated with atopic disorders like asthma (OR = 1.34, 1.24–1.44), atopic eczema (OR = 1.32, 1.20–1.45), and allergic rhinitis (OR = 1.52,1.43–1.63) – Significant heterogeneity (I 2 = 82%) was observed for allergic rhinitis, while for other analysis no heterogeneity was observed |
Cohort studies that perform multiple measurements for atopic disorder symptoms among ADHD individuals are warranted to clarify the association | High (8/9) |
| Miyazaki et al. [104] | Examine the association between ADHD and allergic diseases in children | Allergic diseases, ADHD | Database inception – November 2015 | 5 | 61,811 | Random-effect meta-analysis | – Majority of the included studies were of moderate quality – Children with ADHD were nearly two times more likely to have asthma (OR = 1.80, 1.57–2.07) than control – Significant associations were found between ADHD and allergic rhinitis (OR = 1.59, 1.13–2.32), atopic dermatitis (OR = 1.43, 1.09–1.88), and allergic conjunctivitis (OR = 1.69, 1.04–2.76) compared to controls – No significant difference was found between ADHD and control group for food allergies (OR = 1.13, 0.88–1.47) – Significant heterogeneity (I 2 > 86%) was observed for allergic rhinitis, atopic dermatitis, allergic conjunctivitis, while moderate heterogeneity (I 2 = 60%)was observed for asthma analysis |
Longitudinal studies that adjust for potential confounders are needed | High (9/9) |
| Robe et al. [105] | Estimate the effect of ADHD on HRV | Heart rate variability, Vagally-mediated HRV | Database inception – January 2018 | 13 | Cases:856 Controls: 905 |
Random-effect meta-analysis | – Majority of the included studies were of high quality – Low vagally-mediated HRV was noted among individuals with ADHD (Hedge’s g = 0.20, 0.01–0.40) compared to healthy subjects – Moderate heterogeneity (I 2 = 77%)was observed across analysis |
Additional studies to further clarify this association are required | High (8/9) |
| Oliveira et al. [106] | Assess the cooccurrence of ADHD and nocturnal enuresis | Nocturnal enuresis, Enuresis | Database inception – June 2020 | 25 (13 in meta-analysis) | Cases: 16,000 Controls: 59,600 |
Random-effect meta-analysis | – Included studies were of moderate quality – ADHD children had more than two times higher risk of having enuresis than controls (OR = 2.49, 2.12–2.93) – Children with enuresis had two times higher risk of having ADHD than those without enuresis (OR = 2.03, 1.20–3.42) – Moderate heterogeneity (I 2 = 75%)was observed for the association between enuresis and ADHD |
Additional studies with larger sample and with uniform terminology, are required | Moderate (6/9) |
| Athanasiadou et al. [107] |
Investigate early motor signs of ADHD | Early motor signs, Infancy, ADHD | Database inception – January 2017 | 9 | 7,383 | Narrative synthesis | – Included studies were of high quality – Small sample size and studies focusing on group reports rather than individuals limited power to show association between early motor signs and later ADHD |
Cohort studies with large sample size and that use more precise measurements are required | High (8/9) |
| Havmoeller et al. [108] | Assess relationship between early motor development before 3 years of age in children and later ADHD | ADHD, Early motor development | Database inception – February 2018 | 5 | 65,016 | Narrative synthesis | – Included studies were of high quality – Variation in results from individual studies restricted from drawing any firm conclusion about early motor development and ADHD |
Cohort studies with a large population and clinically diagnosed children, that consider clinical features of ADHD and other potential covariates like sex, comorbid mental disorder need to be conducted | Moderate (7/9) |
| Bishop et al. [109] | Assess the association between anxiety and social functioning (social problems, peer status, and social skills/ competence) in children and adolescents with ADHD | ADHD, Anxiety, Social functioning | Database inception – August 2018 | 31 | 5,116 | Narrative synthesis | – Included studies were of high quality – The association between anxiety and social functioning among individuals with ADHD varied substantially across individual studies |
Future research that controls for potential confounders are needed to elucidate the association between anxiety and social functioning among individuals with ADHD | Moderate (6/9) |
| Li et al. [110] | Examine the relationship between ADHD and associated neuro retinal features (i.e., RNFL thickness and GCL thickness) | Ocular biomarker, Retinal Nerve Fiber Layer, Ganglion Cell Layers | Unclear | 4 | Cases:164 Controls: 150 |
Random-effect meta-analysis | – Included studies were of moderate quality – Meta-analysis revealed that ADHD in children was associated with a reduction in global RNFL thickness (SMD = − 0.23, −0.46 to −0.01) – The global GCL thickness between ADHD children and controls was not statistically significant – Significant heterogeneity (I 2 > 75%) were observed across analysis |
Further larger-scale, multi-ethnic, and longitudinal studies are needed to confirm this association | Moderate (7/9) |
| Chamorro et al. [111] | Assess the effects reported in studies comparing oculomotor inhibition in ADHD patients and healthy control subjects | Biological markers, Oculomotor inhibition | Not given | 31 | Cases: 766 Controls: 801 |
Random-effect meta-analysis | – Majority of included studies were of moderate quality – Among inhibitory outcomes, direction errors in AS, showed a moderate effect (g = 0.57, 0.27–0.88), while anticipatory saccades in memory-guided saccade and saccades during prolonged fixation showed a large effect (g = 0.86, 0.64–1.08) and (g = 1.11, 0.56–1.65) respectively – Significant heterogeneity (I 2 > 74%) were observed across analysis |
Further research to integrate findings across different pathologies would help to identify what mechanisms are specific for each condition | Moderate (7/9) |
| Bellato et al. [112] | Investigate electrophysiological correlates of performance monitoring (error-related negativity, ERN; error positivity, Pe; feedback-related negativity, FRN; feedback-P3) in ADHD individuals | Electroencephalography, Electrophysiological correlates | Database inception – February 2021 | 46 (28 in meta-analysis) | Cases: 1,050 Controls: 1,076 |
Random-effect meta-analysis | – Majority of the included studies were of poor quality – ERN and Pe amplitude were significantly reduced in ADHD compared to controls (g = −0.47, −0.67 to −0.26) and (g = −0.50, −0.69 to −0.32) respectively |
Larger samples, collecting, and processing EEG data under conditions suitable for data pooling across studies will help to systematically investigate the cross-study heterogeneity | High (9/9) |
Abbreviations: ASD, autism spectrum disorder; BD, bipolar disorder; BMI, body mass index; DEB, disorder eating behavior; ED, eating disorder; ERN, error-related negativity; FRN, feedback-related negativity; GCL, ganglion cell layers; GD, gaming disorder; HRV, heart rate variability; IA, internet addiction; k, total number of included studies; OR, odds ratio; PTD, persistent tic disorder; RNFL, retinal nerve fiber layer thickness; RR, relative risk; SMD, standardized mean difference; TD, typically developing.
Total participants included in the systematic review and meta-analysis unless otherwise indicated.
For findings from meta-analysis, if given effect estimates with 95% CI are presented unless otherwise indicated.
ADHD and other mental and neurological disorders
Children and adolescents with ADHD had significantly higher rates of autism spectrum disorder (ASD) (SMD = 1.23, 95% CI: 0.94–1.51) [80] and pragmatic language difficulties than healthy controls [81]. ADHD in adults was strongly related to negative emotionality and low conscientious inhibition [82]. Similarly, the prevalence of bipolar disorder among adults with ADHD was 7.95% (5.31–11.06), and with 4 years earlier age of onset than in bipolar disorder without ADHD [83]. ADHD was associated with eating disorders (OR = 3.82, 2.34–6.24) [84], with similar findings reported in several systematic reviews [85, 86]. Reviews have also shown a link between ADHD and gaming disorder [87] and internet addiction (OR = 3.76, 2.75–5.15) in adolescents and young adults [88].
Sleep
While a recent meta-analysis found no significant difference in sleep parameters between individuals with ADHD and healthy controls as measured by polysomnography [89], previous meta-analyses have reported impaired sleep among children with ADHD [90], including those measured by polysomnography [91] or actigraphy [92]. In adults, a meta-analysis showed longer sleep onset latency and lower sleep efficiency among adults with ADHD than without ADHD [93]. Clinically, adults with ADHD had an increased risk of nearly all types of sleep disorders, including insomnia and circadian rhythm disorders [94, 95] and sleep bruxism [96].
Obesity
Associations between ADHD and obesity have been found in children, adolescents, and adults [94, 97], with one review reporting the association also after adjustments for possible confounders (children OR = 1.20, 95% CI: 1.05–1.37; adults OR = 1.55, 1.32–1.81) [98]. A review on bariatric surgery showed that patients with ADHD responded equally well as patients without ADHD in terms of change in body mass index after surgery [99].
Somatic conditions
Associations with ADHD were reported for headache (OR = 1.98, 95% CI: 1.60–2.45) [100], asthma (OR = 1.52, 1.42–1.63) [101] and (OR = 1.53, 1.41–1.65) [102], eczema (OR = 1.32, 1.20–1.45), and allergic rhinitis (OR = 1.52, 1.43–1.63) [103]. Associations between ADHD and other atopic conditions have also been found [94, 104]. ADHD patients had reduced vagally-mediated heart rate variability (Hedge’s g = 0.20, 0.01–0.40) [105], but no associations between adult ADHD and diseases of the circulatory system were found [94].
Other associated conditions
Children with ADHD had two times higher risks of having nocturnal enuresis than healthy controls. [106]. Reviews suggested that the findings were inconsistent for the relation between early signs of deviating motor functioning or development and later ADHD [107, 108] and on anxiety and social functioning in children and adolescents with ADHD [109].
ADHD in children was associated with a reduction in global retinal nerve fiber layer thickness (SMD = −0.23, 95% CI: −0.46 to −0.01) [110] and individuals with ADHD showed more oculomotor inhibition failure than control groups [111]. Similarly, altered electrophysiological performance monitoring (i.e., reduced error-related negativity and the error positivity amplitude) during cognitive tasks, indicative of difficulties in evaluating errors in performance, have been reported both in children and adults with ADHD [112].
Pharmacological treatment (n = 65, Table 8)
Table 8.
Pharmacological treatment
| References | Objective | Keywords | Timeframe of database search | k | Sample size a | Analytical design | Main findings (Effect estimates, 95% CI) b | Conclusion and comments from the authors | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Efficacy, acceptability, and tolerability in children and adolescents | |||||||||
| Children and adolescents | |||||||||
| Stimulants | |||||||||
| Cerrillo-Urbina et al. [113] | Examine the efficacy and safety of stimulant and nonstimulant medication | Children and adolescents, Stimulants, Nonstimulant | Database inception – August 31, 2017 | 15 | 4,648 | Random-effect meta-analysis |
|
Future research should assess the long-term efficacy of these medications and consider the effect of dosage, frequency, use of different outcome measurement | High (9/9) |
| Riera et al. [114] | Assess all-cause treatment discontinuation and efficacy of pharmacological treatment | Efficacy, Discontinuation, Children and adolescents | Not given | 63 | 11,788 | Random-effect meta-analysis and meta-regression |
|
Effects of several covariates like age, gender, ADHD subtype, comorbidity, types of drug, dosage, frequency, nonpharmacological interventions, and sponsorship should be considered by future multi-treatment meta-analysis and RCTs | High (8/9) |
| Cortese et al. [115] | Compare efficacy and tolerability of oral medications for ADHD | Oral medication, ADHD, Children and adolescents |
1987–2017 | 133 of which 82 in children and adolescents | 14,346 | Network meta-analysis |
|
Further, research on the long-term effects of medications is urgently needed | High (8/9) |
| Maia et al. [116] | Determine the long-term effects (>12 weeks) of MPH-IR | Children and adolescents, Methylphenidate immediate release | Database inception – April 2014 | 7 | 500–2,000 | Random-effect meta-analysis and meta-regression |
|
Additional comparative meta-analyses should be performed to determine the pooled head-to-head long-term efficacy of available ADHD medications | Moderate (7/9) |
| Rezaei et al. [117] | Compare the efficacy of ATX and MPH | Children and adolescents, Atomoxetine, Methylphenidate | Database inception – 2015 | 11 | 2,772 | Random-effect meta-analysis |
|
Studies with long-term follow-up are needed | Moderate (7/9) |
| Liu et al. [118] | Compare the efficacy and safety of atomoxetine (ATX) and methylphenidate (MPH) | Children and adolescents, ATX, MPH | Database inception – April 2016 | 11 | 3,317 | Random-effect meta-analysis |
|
Additional clinical trials with a large sample size and long-term follow-up are required | Moderate (7/9) |
| Maneton et al. [119] | Examine the efficacy, acceptability, and tolerability of d-MPH in children and adolescents with ADHD | Children and adolescents, dex-methylphediate | January 2002–February 2015 | 12 | 1,124 | Random-effect meta-analysis |
|
Suggest caution in interpreting the findings due to significant heterogeneity, industry sponsor studies, variation in study settings and recommends further study to address these limitations | High (9/9) |
| Punja et al. [120] | Summarize the efficacy and safety of long-acting versus short-acting MPH | Children and adolescents, MPH | Database inception – 2012 | 13 | 882 | Random-effect meta-analysis |
|
Apart from assessing risk to benefit ratio, future clinical trials should also assess the cost-to-benefit ratio of these medications and perform analysis according to ADHD subtype | Moderate (7/9) |
| Stuhec et al. [121] | Compare the efficacy and acceptability of bupropion, ATX, LDX, and MPH | Children and adolescents, Bupropion, Atomoxetine, Lisdexamfetamine, MPH | Database inception – April 2014 | 28 | 4,699 | Random-effect meta-analysis |
|
Research using high-quality methodology is required to assess the efficacy of bupropion | High (8/9) |
| Manetton et al. [122] | Assess the efficacy, acceptability, and tolerability of LDX | Children and adolescents, Lisdexamfetamine | January 2007–September 2014 | 5 | 1,016 | Random-effect meta-analysis |
|
The small number of evidence in primary studies limits the relevance of findings from this review, and hence, further clinical trials are needed to provide conclusive evidence | High (9/9) |
| Storebø et al. [123] | Assess the effect of MPH in treating ADHD | Children and adolescents, Methylphenidate | Database inception – March 2015 | 185 | 5,111 | Random-effect meta-analysis |
|
Recommends conducting “nocebo trials” as MPH was found to be associated with serious adverse events making it difficult for blinding of the participants and outcome assessors | High (9/9) |
| Punja et al. [124] | Review the evidence for efficacy and safety of amphetamines | Children and adolescents, Amphetamines | Database inception – August 2015 | 23 | 2,675 | Random-effect meta-analysis | The presence of a high risk of bias in most of the studies, low to very low quality of evidence restricted from making any firm conclusion about the efficacy and safety of amphetamine | Studies that assess the long-term effect of amphetamines and perform subgroup analysis for important factors like age, gender, ADHD subtype, dose, and frequency are needed | High (8/9) |
| Nonstimulants | |||||||||
| Ruggiero et al. [125] | Examine the efficacy and safety of guanfacine | Children and adolescents, Guanfacine | Database inception – May 2014 | 7 | 1,752 | Random-effect meta-analysis, Sensitivity analysis using fixed-effect model |
|
Head-to-head trials are needed to identify the efficacy of guanfacine compared to other ADHD medications. Such trials should consider the efficacy of medication against comorbidities for ADHD | Moderate (7/9) |
| Cheng et al. [126] | Assess the efficacy and safety of atomoxetine | Atomoxetine, Efficacy, Children and adolescents | January 1985–September 2006 | 9 | 1,828 | Random-effect meta-analysis |
|
ATX is efficacious in reducing ADHD symptoms | High (8/9) |
| Connor et al. [127] | Assess the efficacy of clonidine | Clonidine, Children and adolescents | Database inception – 1999 | 11 | 209 | Random-effect meta-analysis |
|
Further high-quality randomized controlled trial are needed to understand the efficacy and safety of clonidine in reducing ADHD symptoms and comorbid conditions | Moderate (7/9) |
| Manetton et al. [128] | Compare efficacy, acceptability, and tolerability of bupropion and MPH | Children and adolescents, Bupropion, Methylphenidate | Database inception – January 2014 | 2 | 62 | Random-effect meta-analysis | Insufficient data from the small number of studies and the small sample size restricted from making any firm conclusion about the possible differences in efficacy, acceptability, and tolerability between these medications | Further clinical trials should be conducted to provide firm conclusion | High (8/9) |
| Otasowie et al. [129] | Evaluate the efficacy of Tricyclic antidepressant | Children and adolescents, Tricyclic antidepressant | Database inception – September 2013 | 6 | 216 | Random-effect meta-analysis | Less number of trials, small sample size, and heterogeneity in outcome measures restricted from making any firm conclusion about the efficacy of desipramine | Longitudinal multicenter observational studies are needed as their result can also be generalized to clinical population | High (9/9) |
| Matsui et al. [130] | Summarize the beneficial effect of azapirones (buspirone) compared to other medications | Children and adolescents | Database inception – October 2015 | 8 | 499 | Narrative synthesis and random-effect meta-analysis | Limited data restricted from making any firm conclusion about the efficacy of buspirone in the treatment of ADHD | Studies assessing the long-term efficacy and safety of azapirone, including larger sample, are needed | High (8/9) |
| Adults | |||||||||
| Stimulants | |||||||||
| Cortese et al. [115] | Compare efficacy and tolerability of oral medications for ADHD | Oral medication, Adult | 1987–2017 | 52 | 10,296 | Network meta-analysis |
|
Future network meta-analysis should include individual patient data for a more reliable estimation of predictors of individual response. Further, research on the long-term effects of medications is urgently needed | High (8/9) |
| Stuhec et al. [131] | Assess the efficacy, acceptability, and tolerability of lisdexamfetamine, mixed amphetamine salts, modafinil, and methylphenidate with placebo in adults | ADHD, Adults, Psychostimulants | Database inception – May 2016 | 20 (19 in quantitative synthesis) | 5,528 | Random-effect meta-analysis |
|
Meta-analyses of head-to-head trials of stimulants and nonstimulants are required. Additional clinical trials are needed to assess the efficacy of modafinil | High (9/9) |
| Maneeton et al. [132] | Evaluate the efficacy, acceptability, and tolerability of lisdexamfetamine in adults | Lisdexamfetamine, Placebo | Database inception – January 2014 | 5 | 822 | Random-effect meta-analysis |
|
Further clinical trials on LDX are warranted | High (9/9) |
| Castells et al. [133] | Compare all-cause treatment discontinuation rate between MPH and placebo in adults | Methylphenidate, All-cause treatment discontinuation | Database inception – January 2012 | 12 | 2,496 | Random-effect meta-analysis |
|
Long-term follow-up studies with large sample size are needed | High (8/9) |
| Peterson et al. [134] | Assess the effectiveness and safety of nonstimulants and longer-acting stimulants compared to conventional shorter-acting stimulants | Stimulants, Nonstimulants, Adults | Database inception – March 2007 | 22 | 2,203 | Fixed and random-effect meta-analysis |
|
Additional high-quality, head-to-head trials are urgently needed to confirm the findings | High (9/9) |
| Cândido et al. [135] | Determine efficacy and adverse events of IR-MPH in adults | Immediate release methylphenidate, Adults | Database inception – January 2020 | 12 | 497 | Narrative synthesis and random-effect meta-analysis |
|
The long-term efficacy and risks of IR methylphenidate needs to be studied | High (9/9) |
| Castells et al. [136] | Assess the efficacy and safety of AMP in adults | Amphetamines, Placebo | Database inception – August 2017 | 19 | 2,521 | Random-effect meta-analysis | High risk of bias, and significant heterogeneity restricted from making any firm conclusion about the efficacy and safety of AMP | In addition to studies that assess the long-term effect of amphetamines, head-to-head trials of AMP versus other ADHD medications in adults are also warranted | High (9/9) |
| Nonstimulants | |||||||||
| Cunill et al. [137] | Compare the efficacy and all-cause treatment discontinuation rate of ATX and placebo in adults | Atomoxetine, Placebo | Database inception – June 2012 | 12 | 3,375 | Fixed and random-effect meta-analysis and meta-regression |
|
Long-term follow-up studies with enough statistical power are warranted | High (8/9) |
| Verbeeck et al. [138] | Assess the efficacy and safety of bupropion for the treatment of adults with ADHD | Bupropion, Placebo | Database inception – February 2017 | 6 | 438 | Fixed effect meta-analysis |
|
Further research is needed to reach more definite conclusions as well as to assess the long-term outcome | High (9/9) |
| Manetton et al. [128] | Compare efficacy, acceptability, and tolerability of bupropion MPH | Bupropion, Methylphenidate | Database inception – January 2014 | 2 | 20 | Random-effect meta-analysis | Insufficient data from the small number of studies and small sample size restricted from making any firm conclusion about possible differences in efficacy, acceptability, and tolerability between these medications | Further clinical trials should be conducted to provide firm conclusion | High (8/9) |
| ADHD pharmacotherapy as a class | |||||||||
| Elliott et al. [139] | Evaluate the relative effects of pharmacologic treatments for ADHD in adults | ADHD pharmacotherapy, Adults | Not clear | 81 | 12,423 | Network meta-analysis |
|
Observational studies that assesss the long-term effects of ADHD medications in adults are needed | Moderate (7/9) |
| Adverse effect of ADHD medication | |||||||||
| Liang et al. [140] | Analyze the effects of ATX and MPH on HR, SBP, and a number of adverse cardiac events in children and adults | Atomoxetine, Methylphenidate, Heart rate; Systolic blood pressure | Database inception – May 31, 2016 | 22 | 46,107 | Random-effect meta-analysis |
|
Additional research is needed that compare the long-term effects of these medications on heart rate and blood pressure | High (8/9) |
| Liu et al. [141] | Assess the association between ADHD medications and the risk of CVD cardiovascular diseases and mortality | Myocardial infarction, Stroke, Arrhythmia | Database inception – May 2018 | 8 | 4,221,929 | Random-effect meta-analysis |
|
Further studies using robust methodology that controls for all potential confounders and estimate dose-response relationship are required | Moderate (7/9) |
| Kidwell et al. [142] | Determine the effects of stimulant medications on sleep in youth with ADHD using objective measurement | Stimulant medications, sleep, ADHD youths | Database inception – March 2015 | 9 | 171 | Random-effect and mixed-effect meta-analysis |
|
Future studies should estimate the effects of stimulants on different sleep stages | High (8/9) |
| DeCrescenzo et al. [143] | Evaluate the effect of MPH on sleep characteristics using actigraphy | Methylphenidate, Actigraphy, Children | Database inception – June 2013. | 8 | Not given | Random-effect meta-analysis |
|
Measures that focus on reducing sleep problems among children using MPH should be considered | High (9/9) |
| Pan et al. [100] | Assess the effects of ADHD medications on headache | ADHD medications Headache |
Database inception – June 2020 | 58 | 12,341 | Random-effect meta-analysis |
|
Clinical trials with large sample size using standardized method to detect treatment-related headaches, such as the Side Effects Rating Scale for stimulants are required | High (8/9) |
| Holmskov et al. [144] | Evaluate the effect of MPH on the risk of gastrointestinal adverse event | Methylphenidate, Gastrointestinal adverse event, Children and adolescents | Database inception – February 2015 | 61 | 5,983 | Random-effect meta-analysis |
|
Further clinical trials assessing the long-term effect of MPH on the gastrointestinal adverse events are needed | High (9/9) |
| Chierrito de Oliveira et al. [145] | Evaluate the safety profile of ADHD medications | ADHD treatment, Adult | Database inception – September 2017 | 10 | 3,006 | Pair-wise and network meta-analysis |
|
Apart from assessing the efficacy and safety of ADHD medications, research should also conduct a cost-effectiveness analysis of these medications | Moderate (7/9) |
| Kittel-Schneider et al. [78] | Synthesize recent evidence on the risks of stimulant and nonstimulant treatment for ADHD in pregnancy and lactation | Pregnancy, Lactation, ADHD medications |
Database inception – November 2021 | 25 | 29,282 exposed and 11,452,476 controls for six medication exposures | Narrative synthesis |
|
Longitudinal large studies using robust design, for example, example sibling designs and including as many as possible confounders and variables are needed to strengthen the evidence | Moderate (6/9) |
| Li et al. [146] | Examine the potential effect of exposure to ADHD medication during pregnancy on pregnancy and offspring related outcome | ADHD medications, Pregnancy, Offspring | Database inception – July 2019 | 8 | More than 2 million | Narrative synthesis |
|
Future studies should account for all potential confounders and assess the effect of ADHD medication during different stages of pregnancy and on long-term outcomes in offspring | High (8/9) |
| Jiang et al. [147] | Identify association between exposure to ADHD medication during pregnancy and adverse outcome | Pharmacoepidemiology, Prenatal |
Database inception – May 2018 | 8 | Not given | Fixed and random-effect meta-analysis |
|
Larger cohort studies that adjust for potential confounders are needed to gain better insight | Moderate (7/9) |
| Adverse events of MPH treatment | |||||||||
| Storebø et al. [148] | Identify the adverse effect of MPH in nonrandomised studies | Methylphenidate, Adverse events, Children and adolescents | Database inception – January 2016 | 260 | >2,207,751 | Random-effect meta-analysis | Very low-quality of evidence and low reliability of the evidence existed for the association between MPH use and risk of adverse events in children and adolescent | High quality, large-scale RCTs along with studies that are aimed towards identifying individuals who responds to treatment versus those who do not respond are needed | High (9/9) |
| Efficacy of medication on ADHD symptoms in patients with comorbid conditions | |||||||||
| Rodrigues et al. [149] | Assess the efficacy and safety of pharmacological treatment on ADHD symptoms among patients with ASD | Pharmacotherapy, ASD, Children and youth | 1992–April 2018 | 25 | 2,606 | Narrative synthesis and random-effect meta-analysis |
|
Further research on long-term effect of medication on clinical symptoms including real-life outcomes (education, employment, cognitive) are needed | Moderate (6/9) |
| Sun et al. [150] | Examine effects of MPH for ADHD among children with ID or borderline intellectual functioning | Methylphenidate, ADHD, Intellectual disability | Database inception – August 2018 | 8 | 423 | Random-effect meta-analysis and meta-regression |
|
Large-scale randomized controlled trials assessing the efficacy and tolerability of MPH in children with comorbid ADHD and ID or borderline intellectual function are needed | High (8/9) |
| Tarrant et al. [151] | Examine the beneficial effect of methylphenidate in reducing ADHD symptoms among individuals with intellectual disabilities | Methylphenidate, Intellectual disabilities, Children and adolescents | Database inception – August 5, 2017 | 15 | Not given | Narrative synthesis | Small sample size and high risk of bias across studies restricted from making any firm conclusion about the effect of MPH on reducing ADHD symptoms among those with intellectual disabilities | Clinical trials with sound methodological design that are not sponsored by pharmaceutical companies and that includes both children and adult population with ID and ADHD are needed | Moderate (6/9) |
| Froehlich et al. [152] | Asssess the effectiveness of FDA-approved ADHD medications on ADHD and comorbid reading disorder | Methylphenidate, Atomoxetine, Pediatric |
Not clear | 14 | 371 | Narrative synthesis |
|
Studies assessing the long-term effect of medications are needed | Moderate (7/9) |
| Osland et al. [153] | Examine the effects of medications for ADHD in children with comorbid tic disorder | Medications, ADHD, Tic disorder | Database inception – September 2017 | 8 | 510 | Narrative synthesis | Small number of studies, small sample size, and high risk of bias across included studies restricted from making any firm conclusion about the effects of MPH, clonidine, gunfacine, despiramine, and ATX in treating ADHD symtopms among those with comorbid tic disorder | Further studies with robust methodology including larger sample are needed | High (8/9) |
| Woon et al. [154] | Evaluate the effectiveness of medication on comorbid ADHD and stimulant dependence | Pharmacotherapy, Stimulant dependence, Adult | Database inception – June 2017 | 5 | 358 | Narrative synthesis | Evidence about the effectiveness of pharmacotherapy for comorbid ADHD and stimulant dependence were preliminary and promising, but mixed | High-quality research with sufficient statistical power is needed to draw firm conclusion | High (8/9) |
| Anand et al. [155] | Evaluate the quality of evidence about the efficacy, safety, and tolerability of medications used in treating behavioral insomnia among ADHD children | ADHD, Behavioral insomnia, Pharmacological treatment | Database inception – February 2017 | 12 | Not given | Narrative synthesis |
|
Well-designed clinical trials with adequate statistical power are required | Moderate (7/9) |
| Tsujii et al. [156] | Summarize the efficacy and safety of treatments for children and adolescents with ADHD and common comorbidities | Comorbidities Pharmacotherapy |
Database inception – October 2019 | 69 | 500–1,000 | Narrative synthesis |
|
Further studies are required to support evidence-based drug selection for these populations | Moderate (6/9) |
| Thomson et al. [157] | Examine the effectiveness of amphetamine for the treatment of ADHD among those with intellectual disabilities | Amphetamine, Intellectual Disabilities | Database inception – August 2007 | 1 | 15 | Narrative synthesis |
|
–Further high-quality RCTs are urgently needed | High (9/9) |
| Thomson et al. [158] | Examine the effectiveness of risperidone for the treatment of ADHD among those with intellectual disabilities (ID) | Risperidone, Intellectual Disabilities | Database inception – February 2009 | 11 considered but none were suitable for inclusion | NA | Narrative synthesis | There is no evidence from RCTs that risperidone is effective for the treatment of ADHD in people with ID | Research into effectiveness and tolerability is urgently needed | High (9/9) |
| Efficacy of medication on comorbidity and other consequences of ADHD | |||||||||
| Fluyau et al. [159] | Evaluate the beneficial effect of pharmacotherapy to treat SUD in ADHD patients | Pharmaceutical intervention, SUD, ADHD | 1971–2020 | 17 | 2,155 | Random-effect meta-analysis |
|
Further clinical trials that apply uniform outcome measurement scale and diagnostic criteria are required, in addition to focusing on the neurobiological mechanism that links or differentiate both disorders | High (8/9) |
| Man et al. [160] | Estimate the association between ADHD medications and physical injury | Pharmacological treatment, ADHD, Physical injuries | Database inception – May 2017 | 10 | 751,602,319 | Random-effect meta-analysis |
|
Further studies need to assess the causal effect of ADHD medication on risk of physical injuries | Moderate (6/9) |
| Zhang et al. [161] | Assess effect of ADHD medication and the risk of fracture in ADHD patients | Stimulants, Nonstimulants, Fracture | Database inception – December 2020 | 10 | >10,000 | Random-effect meta-analysis |
|
Future research should comprise analyses based on an accurate definition of ADHD and fracture | High (8/9) |
| Schoenfelder et al. [162] | Evaluate the beneficial effect of stimulant medication for ADHD on smoking | ADHD, Psychostimulant medication, Cigarette smoking | Database inception – July 2013 | 17 | 2,804 | Fixed-effect and random-effect meta-analysis and meta-regression |
|
Research that assess causal relationship between ADHD stimulants and smoking behavior is needed | High (9/9) |
| Liu et al. [163] | Evaluate association between ADHD medication and risk of suicidal attempt | Pharmacoepidemiology, Central stimulants, Suicidal behavior |
Database inception – February 2020 | 7 | 4,790,600 | Random-effect meta-analysis |
|
Multinational studies using uniform assessment methods that adjust for all potential confounders are needed
|
Moderate (7/9) |
| Prasad et al. [164] | Evaluate the effectivenss of medication on improving ADHD children task-behavior and academic performance | Medication, Education, Achievement | Database inception – June 2010 | 43 | 2,110 | Narrative synthesis and random-effect meta-analysis |
|
Head-to-head trials of medications are required to compare the superiority of one medication over another in improving academic achievement among ADHD individuals | High (9/9) |
| Hulsbosch et al. [165] | Assess the effects of medication, on instrumental learning in children with ADHD | Instrumental learning, ADHD |
Database inception – March 2020 | 3 | Not given | Narrative synthesis |
|
Further studies with large sample size and high statistical power are required | High (8/9) |
| Pievsky et al. [166] | Assess the beneficial effect of MPH on neurocognitive performance | ADHD adults, Methylphenidate, Neurocognition | Database inception – November 2016 | 21 | 832 | Random-effect meta-analysis and meta-regression |
|
Further research with high statistical power is needed | High (8/9) |
| Effect modification of comorbid conditions and drug effects on cooccurring symptoms | |||||||||
| Villas-Boas et al. [167] | Assess the effectiveness of pharmacotherapy on ADHD and comorbid AD | Drug therapy, Safety, Children and adolescents, Adults | Database inception – July 2018 | 5 | Not given | Narrative synthesis |
|
Larger multi-center clinical trials with long-term follow-up are needed to clarify the present findings | High (8/9) |
| Lenzi et al. [168] | Evaluate the effects of medications on ED in adults with ADHD | Stimulants, Emotional dysregulation, ADHD | Database inception – April 1, 2017 | 21 | 4,940 | Random-effect meta-analysis |
|
Further studies assessing the effect of ADHD medications on ED are required | High (9/9) |
| Connor et al. [169] | Assess the efficacy of stimulants on overt and covert aggression-related behaviors in children with ADHD | ADHD, Aggression | 1970–2001 | 28 | 683 | Random-effect meta-analysis |
|
Additional research into the effectiveness of medications for aggression-related behaviors in clinically referred youths are needed | High (8/9) |
| Pringsheim et al. [170] | Assess the therapeutic effects of antipsychotics and traditional mood stabilizers for aggression and conduct problems in youth with ADHD, ODD, and CD | Antipsychotics, Mood stabilizers, Youth | Database inception – October 2013 | 11 | 500–2,000 | Random-effect meta-analysis |
|
Long-term follow-up studies are needed to assess the long-term safety and efficacy of medications | High (9/9) |
| Pharmacogenetics and animal models | |||||||||
| Yuan et al. [171] | Evaluate whether noradrenergic gene polymorphisms impact the efficacy of MPH in children with ADHD | Methylphenidate, Noradrenergic gene polymorphism | Database inception – March 2020 | 15 | 1,382 | Random-effect meta-analysis and meta-regression |
|
Further investigations with larger samples will be needed to confirm these results | Moderate (7/9) |
| Soleimani et al. [172] | Evaluate the potential role of SLC6A3 polymorphisms in response to MPH in children with ADHD | MPH, Genetic, Child and adolescent psychiatry | Database inception – May 1, 2017 | 16 | 1,357 | Random-effect meta-analysis and meta-regression |
|
Further research that studies gene–gene interaction and elucidate the role of potential confounders or effect modifiers are required | High (9/9) |
| Leffa et al. [173] | Assess the behavioral effect of MPH in spontaneously hypertensive rat (SHR) | MPH, Animal model, SHR | Database inception – February 2017 | 36 | 657 | Random-effect meta-analysis and meta-regression |
|
For human treatment to be more accurate, further research on animal models will need to be conducted. Such studies will include doses analogous to those commonly used in humans | High (9/9) |
| Pharmacological treatment rate | |||||||||
| Massuti et al. [174] | Estimate the rate of ADHD pharmacological treatment in both diagnosed and undiagnosed children and adolescents | Prevalence, Treatment, Pharmacological interventions |
Database inception – April 2020 | 36 | 104,305 | Random-effect meta-analysis and metaregression |
|
Further medical and parental education on ADHD is needed worldwide, as part of public health policies | High (8/9) |
Abbreviations: AMP, amphetamine; ASD, autism spectrum disorder; ATX, atomoxetine; CVD, cardiovascular disease; d-MPH, dex-methylphenidate; dex-AMP, dex-amphetamine; ED, eating disorder; HR, heart rate; IR-MPH, immediate-release methylphenidate; k, total number of included studies; LDX, lisdexamphetamine; MPH, methylphenidate; NA, not applicable; OR, odds ratio; OROS, osmotic release oral system; SBP, systolic blood pressure; SMD, standardized mean difference; SUD, substance use disorder; WMD, weighted mean difference.
Total participants included in the systematic review and meta-analysis unless otherwise indicated.
For findings from meta-analysis, if given effect estimates with 95% CI are presented or otherwise specified.
Efficacy, acceptability, and tolerability of ADHD medication
Children and adolescents
Systematic reviews have shown efficacy for pharmacological treatment of ADHD, with SMDs between 0.6 and 0.8 and consistently stronger efficacy for stimulants than nonstimulants [113, 114]. All-cause treatment discontinuation was lower with pharmacological treatment than placebo (OR = 0.68, 95% CI: 0.58–0.79) [114].
Stimulants
In terms of efficacy, acceptability, and tolerability, evidence exists for methylphenidate (MPH) as the preferred first choice for pharmacological treatment of ADHD [115–120]. For instance, in a network meta-analysis of 82 randomized controlled trials (RCTs) including more than 14,000 children and adolescents, at time point closest to 12 weeks, with clinician’s rating, MPH was found to be efficacious in reducing ADHD core symptoms as compared to placebo (SMD = –0.78,–0.93 to −0.62) [115]. MPH was the only drug that had better acceptability than placebo, and with similar tolerability as placebo [115]. A systematic review of four prospective or naturalistic studies and three RCTs showed MPH immediate-release (MPH-IR) as efficacious also for periods longer than 12 weeks (parent ratings for inattention and hyperactivity/impulsivity: SMD = 0.96, 0.60–1.32 and 1.12, 0.85–1.39, respectively) [116].
Lisdexamfetamine (LDX) was also efficacious in reducing ADHD symptoms compared to placebo [121, 122], for example, as reported in a meta-analysis of 28 double-blind, placebo-controlled RCTs with around 4,700 participants (SMD = –1.28, −1.84 to −0.71) [121].
However, according to the earlier Cochrane review and meta-analyses, the efficacy of stimulants like MPH [123] and amphetamine [124] cannot be unequivocally established, due to methodological limitations. The authors point to small number of trials, short follow-up time, low-quality data, high risk of bias in several domains, including lack of sufficient blinding and selective outcome reporting, and heterogeneity between studies. This quite divergent conclusion is probably not due to different evidence included in the systematic reviews, but subjectively different interpretation of the evidence by the authors of different reviews.
Nonstimulants
Guanfacine was reported as safe and efficacious in treating ADHD compared to placebo, pooled (OR = 3.18, 95% CI: 2.44–4.13) [125]. Compared to placebo, atomoxetine (ATX) was also reported as safe and efficacious in reducing ADHD symptoms. [121, 126]. A meta-analysis from 1999 also suggested similar findings for clonidine [127]. However, as for stimulants, meta-analyses and Cochrane review stated that the efficacy of some of the nonstimulants cannot be established due to short follow-up time, low quality, limited number of studies, sample sizes, and heterogeneity [128–130]. There is thus disagreement on how to interpret the literature also for nonstimulants.
Adults
Stimulants
A network meta-analysis of 52 RCTs, including more than 10,000 adults, supported amphetamines as the preferred first pharmacological choice for treatment of ADHD both regarding efficacy (clinicians’ ratings, SMD = –0.79, 95% CI: −0.99 to −0.58) and tolerability (OR = 3.26, 1.54–6.92) [115]. Other reviews have also shown the efficacy of LDX, with one meta-analysis of 19 RCTs with more than 5,500 participants, reporting an SMD of −0.89 (−1.09 to −0.70) [131] and another suggesting an SMD of −0.97 (−1.15 to −0.78) [132] for short-term treatment, compared to placebo. MPH also showed efficacy in reducing ADHD symptoms as compared to placebo (OR = 2.66, 2.12–3.33), and in terms of treatment discontinuation no significant difference was found between MPH and placebo (OR = 1.19, 0.82–1.74) [133]. An indirect comparison meta-analysis of placebo-controlled trials from 2008 suggested shorter-acting stimulants, specifically IR-MPH as efficacious in reducing ADHD symptoms in adults [134]. There was disagreement on how to interpret the original efficacy studies for adults as well. Cochrane reviews suggested that due to high risk of bias, limited sample sizes and number of studies, and heterogeneity in findings, the quality of the evidence for the efficacy of immediate-release MPH (compared to placebo or lithium) [135] and amphetamine (compared to placebo) [136] was low to very-low.
Nonstimulants
In a meta-analysis of 12 RCTs with around 3,400 participants, ATX was found to have small efficacy in reducing the severity of ADHD symptoms (SMD = –0.33, 95% CI: −0.43 to −0.23) and increased rates of discontinuation compared to placebo (OR = 1.39, 1.17–1.64) [137]. Cochrane review found low-quality evidence to conclude about the efficacy of bupropion in reducing ADHD symptoms severity and its tolerability compared to placebo [138]. In another review, bupropion was found to be as efficacious as MPH, but due to limited number of studies, the authors suggested the findings as preliminary [128].
ADHD pharmacotherapy as a class
A network meta-analysis suggested that, despite a positive class effect of ADHD medication relative to placebo in improving clinical response, the quality of evidence was low to very low about the efficacy of different ADHD drugs on treating ADHD symptoms in adults [139].
Adverse effect of ADHD medication
Cardiovascular
Patients in all age groups showed significant increases in heart rate and systolic blood pressure (SBP) from pre- to post-treatment when treated with MPH as compared to placebo (for SBP, SMD = 1.61, 95% CI: 0.81–2.41 for children and adolescents and SMD = 1.40, 0.62–2.18 for adults) [140]. Furthermore, children and adolescents treated with ATX had a significant increase in SBP compared to those treated with MPH [140]. However, no association was found between ADHD medication and the number of serious adverse medical events including sudden death, arrhythmia, stroke, myocardial infarction, and all-cause mortality [141].
Sleep
Longer sleep latency (effect size = 0.54, 95% CI: 0.28–0.81) and shorter sleep duration (effect size = −0.59, −0.84 to –0.35) were noted in children and adolescents that used stimulants [142]. Similar findings were noted for children treated with MPH as measured by actigraphy for both longer sleep latency and shorter sleep duration [143].
Headache
Children and adolescents that used ADHD drugs were at increased risk of having headache during treatment period compared to placebo (for guanfacine, OR = 1.43, 95% CI: 1.12–1.82; for MPH, OR = 1.33, 1.09–1.63) [100].
Appetite
Short-term MPH treatment decreased appetite relative to placebo (RR = 3.66, 95% CI: 2.56–5.23) [144]. This was also found for extended-release mixed amphetamine salts and ATX [145].
Pregnancy and postpartum-related side effects
No evidence of adverse offspring consequences of ADHD medication during pregnancy was found [78, 146, 147]. MPH was reported as safe to use during breastfeeding, while the reported stimulating effect of guanfacine on prolactin secretion was considered to affect breastfeeding negatively [78].
Adverse events of MPH treatment
A meta-analysis showed low quality and reliability of evidence for adverse events of both short and long-term MPH treatment in children and adolescents and young adults [148].
Efficacy of medication on ADHD symptoms in patients with comorbid conditions
Seven systematic reviews reported about the efficacy of medication on ADHD symptoms in patients with comorbid disorders [149–156]. For example, both MPH and ATX were efficacious in reducing ADHD symptoms also in youth with ASD [149]. MPH was further found to decrease ADHD symptoms compared to placebo in children and adolescents with borderline intellectual functioning or intellectual disability (ID) (Hedges’ g = 0.87, 95% CI: 0.61–1.14) [150]. However, another review stated that the quality of evidence to conclude MPH as efficacious in treating ADHD symptoms in ID as poor [151]. No sufficient evidence was found to conclude about the effect of either amphetamine [157] or risperidone [158] in the treatment of ADHD symptoms in people with ID.
Efficacy of medication on comorbidity and other consequences of ADHD
In general, pharmacotherapy for ADHD was found to be efficacious (SMD = 0.40, 95% CI: 0.25–0.55) in reducing both ADHD symptoms and substance use in patients with both ADHD and substance use disorder [159].
ADHD medications generally lowered the risk of injury (rate ratio = 0.76, 0.61–0.93) in individuals with ADHD, based on within-individual analysis [160]. However, a recent meta-analysis revealed higher risk of bone fracture among individuals treated with nonstimulants for ADHD (OR = 1.37, 1.20–1.58) whereas lower, but nonsignificant risk was observed for individuals treated with stimulants [161].
Youths treated with stimulants had lower smoking rates than untreated youths with ADHD (OR = 0.51, 0.32–0.80) [162]. Further, long-term use (>90 days) of stimulants among ADHD individuals was associated with lower risk of suicide attempt (RR = 0.75, 0.66–0.84), based on within-individual analysis [163].
In-school assignment was increased by 15% and on-task behavior by 14% or more among ADHD children treated with stimulants [164]. Likewise, MPH improved instrumental learning in children with ADHD [165]. Compared to placebo adult ADHD patients using MPH scored better on several neurocognitive measures and tests of driving ability (Hedge’s g = 0.17, 0.05–0.28) [166].
Effect modification of comorbid conditions and drug effects on cooccurring symptoms
Comorbid anxiety did not change the efficacy of ATX on ADHD in children, adolescents, and adults [167]. While ADHD medications like MPH and ATX showed smaller effects, LDX was moderately efficacious in treating emotional dysregulation in adults with ADHD (SMD = –0.50; 95% CI: −0.80–0.21) [168]. Compared to placebo, stimulants were efficacious in reducing both overt and covert aggression in children and adolescents with ADHD [169]. Similarly, risperidone reduced aggressive behavior in youth with ADHD [170].
Pharmacogenetics and animal models
While noradrenergic gene polymorphisms were associated with improved MPH response in children with ADHD [171], low-quality evidence exists regarding the impact of SLC6A3 polymorphisms in response to MPH in children with ADHD [172]. No effect was found for MPH on hypertensive rats as an animal model of ADHD [173].
Pharmacological treatment rate
The first systematic review on undertreatment, overtreatment, and misuse of ADHD medication reported that 19.1% (95% CI: 11.5–29.9) of school-aged children and adolescents having ADHD were treated with medication for the disorder, and 0.9% (0.5–1.7) of individuals without the diagnosis used medication for ADHD. Their findings indicated both overtreatment and misuse of drugs in individuals without ADHD, and undertreatment of ADHD drugs in youths with the disorder [174].
Nonpharmacological treatment (n = 42, Table 9)
Table 9.
Nonpharmacological treatment
| References | Objective | Keywords | Timeframe of database search | k | Sample size a | Analytical design | Main findings (Effect estimates, 95% CI) b | Conclusion and comments from the authors | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Children and adolescents | |||||||||
| Daley et al. [175] | Assess effectiveness of behavioral interventions (behavioral, social, academic skills training, CBT) in ADHD | ADHD, Parenting, Intervention | Database inception – February 2013 | 32 | 500–2,000 | Random-effect meta-analysis | – Majority of included studies were of moderate quality – Behavioral interventions showed small beneficial efficacy in reducing child ADHD symptoms (SMD = 0.35, 0.19–0.50), social skills (SMD = 0.47, 0.15–0.78), academic performance (SMD = 0.28, 0.06–0.50), and positive parenting (SMD = 0.68, 0.28–1.09) – With probably blinded assessments, significant moderate effects persisted for parenting (SMD for positive parenting 0.63, SMD for negative parenting 0.43) – Significant heterogeneity (I 2 > 80%) was observed across analysis |
There is a need for further analysis of the moderating effects of child’s age on intervention outcomes, and proximal outcomes such as academic performance and social skills should be confirmed by blinded assessment | Moderate (7/9) |
| Rimestad et al. [176] | Assess the effectiveness of parent training for preschool children with ADHD or ADHD symptoms | Parent training, early intervention | Database inception – May 2015 | 16; | 1,003 | Random-effect meta-analysis | – Included studies were of moderate quality – Based on parental report of ADHD, parental training was moderately efficacious in reducing ADHD symptoms (Hedges’s g = 0.51, 0.33–0.65) and negative parenting (Hedges’s g = 0.63, 0.32–0.93) – In depended-rated outcomes revealed small beneficial effect only for negative parenting (Hedges g = 0.33, 0.13–0.53) – Significant heterogeneity (I 2 > 70%) was observed across analysis |
Future studies should assess the long-term effect of such interventions on ADHD | High (8/9) |
| Moore et al. [177] | Determine the effectiveness of school-based non -pharmacological interventions for ADHD | School-based, Children | 1980–January 2018 | 30 | 1,807 | Narrative synthesis and random-effect meta-analysis | – Most of the included studies were of low quality – Combined intervention (social skills training, behavior modification technique, etc.) showed greater efficacy in ADHD combined symptoms rated by both teachers (Hedges’ g = 0.79, 0.45–1.12) and parents (g = 0.97, 0.45–1.12) – School-based combined interventions showed small beneficial effect in improving both teacher-rated (Hedges’ g = 0.30, 0.12–0.7) and parent-rated (Hedges’ g = 0.37, 0.19–0.55) academic outcomes – Moderate heterogeneity (I 2 > 60%) was observed across analysis |
Interventions should be evaluated across different age groups and degrees of ADHD severity | Moderate (7/9) |
| Iznardo et al. [178] | Synthesize the effectiveness of DBRC for ADHD children | School-based intervention, Daily Behavior Report Cards, Teachers | Database inception- November 2015 | 24 | 272 | Narrative synthesis and random-effect meta-analysis | – DBRCs showed small beneficial effect in reducing ADHD symptoms in classroom (teacher-rated Hedges’s g = 0.36, 0.12–0.60) – No heterogeneity was observed across analysis |
Methodologically rigorous studies investing mediator and moderator variables that may influence the effectiveness of this intervention need to be conducted | Moderate (6/9) |
| Cordier et al. [179] | Determine the impact of peer inclusion in interventions for ADHD children | Peer inclusion | Database inception – 2016 | 17 | 2,567 | Random-effect meta-analysis | – Included studies were of moderate to low quality – Peer inclusion (peer involvement or peer proximity) interventions in addition to pharmacological treatment was found to be beneficial in improving social competence and peer relation compared to treatment as usual or no treatment – Moderate heterogeneity (I 2 > 45%) was observed across analysis |
Trials that use peer-mediated interventions, and control for medication as potential confounder are needed | High (8/9) |
| Chen et al. [180] | Assess the effect of cognitive intervention on symptoms and executive function behaviors of children with ADHD | Cognitive training, Executive function | Not given | 17 | 1,075 (904 for effect estimates) | Random-effect meta-analysis | – Majority of included studies were of low quality – When all of the training are considered together, cognitive training can improve the presentation of inattention symptoms [SMD = −0.39, (−0.67, −0.10) and executive function behaviors (SMD = −0.31, (−0.52, −0.11)] – Effects of working memory training on both presentations were not statistically significant – Significant heterogeneity (I 2 > 75%) were observed across analyses |
Further research is needed to confirm and extend these psychology fields | High (8/9) |
| Pauli-Pott et al. [181] | Assess the effectiveness of cognitive interventions on executive function and ADHD in pre-school children | Executive function, Cognitive interventions | Database inception- April 2018 | 35 (29 in meta-analysis) | 3,068 | Narrative synthesis and random-effect meta-analysis | – Included studies were of moderate quality – Cognitive scaffolding interventions were most effective in terms of reducing ADHD symptoms |
Additional well-controlled clinical trials with large sample size are required | Moderate (6/9) |
| Cortese et al. [182] | Investigate the effectiveness of cognitive training on ADHD | Working memory, Executive functions | Database inception – May 2015 | 15 | 826 | Random-effect meta-analysis | – Included studies were of high to moderate quality – According to blinded assessment, cognitive training improved working memory but did not seem to reduce ADHD symptoms – No significant heterogeneity was observed across analysis |
Long-term studies with wider range of functional outcomes are required | Moderate (7/9) |
| Romero-Ayuso et al. [183] | Determine the effectiveness of virtual reality-based interventions (VR based interventions) on cognitive deficits in children with ADHD | Virtual reality, Rehabilitation | Database inception – October 2020 | 4 | 125 | Random-effect meta-analysis | – Included studies were of moderate to low quality – The magnitude of the effect was large for omissions (SMD = −1.38, p = 0.009), correct hits (SMD = −1.50, p = 0.004), and perceptual sensitivity (SMD = −1.07, p = 0.01); and moderate for commissions (SMD = −0.62, p = 0.002) and reaction time (SMD = −0.67, p = 0.03) – Significant heterogeneity (I 2 > 80%) were observed across analyses |
Additional well-controlled clinical trials with large sample size are required | High (8/9) |
| Barranco-Ruiz et al. [184] | Synthesize the effectiveness of mind–body therapies interventions on ADHD | Mind–body therapies, Relaxation therapies, Children and adolescents | 2000–2018 | 12 | 311 | Narrative synthesis | – Most of the included studies were of low quality – Most of the included studies found that mind–body therapies were effective in reducing ADHD symptoms in children and adolescents – Considerable conceptual heterogeneity was observed across studies |
To confirm the existing findings, RCTs should include representative samples with greater statistical power | High (8/9) |
| Pelsser et al. [185] | Determine the effectiveness of diet on ADHD | Dietary-based interventions, Nonpharmacological treatment, Children and adolescents | Database inception-December 2015 | 6 | 1,937 | Random-effect meta-analysis | – Most of the included studies were of moderate quality – The average FFD showed greater efficacy (Parent-rated SMD = 0.80, 0.41–1.19 in reducing ADHD symptoms – The effectiveness of polyunsaturated fatty acid supplementation and artificial food color elimination on ADHD was negligible – Moderate heterogeneity (I 2 > 60%) was observed across analysis |
Further FFD research should focus on establishing the underlying mechanisms of food (e.g., incrimination of gut microbiota) to simplify the FFD approach in children with ADHD | High (8/9) |
| Heirs et al. [186] | Assess the efficacy and safety of homeopathy | ADHD, Homeopathy | Database inception – March 2006 | 4 | 168 | Random-effect meta-analysis | – Included studies were of low quality – No evidence exist about the effectiveness for homeopathy for the global symptoms, core symptoms or related outcomes of ADHD |
Optimal treatment protocols needs to be developed before conducting further randomized controlled trials | High (9/9) |
| Oliva et al. [187] | Assess the effects of MBIs on either ADHD and associated features, associated clinical conditions, neurocognitive impairments, mindfulness skills, global functioning and quality of life | Mindfulness-based interventions | Database inception- June 2020 | 15 | 412 | Narrative synthesis | Smaller number of studies, sample size, poor quality of included studies and lack of active-control studies restricted from making any firm conclusion about the efficacy of MBIs on reducing ADHD core symptoms and other concerning outcomes | The low general methodological quality highlights the need to conduct more active-controlled studies, on larger sample sizes with measurement at follow-up | High (9/9) |
| Evans et al. [188] | Assess the effect of meditation-based interventions on ADHD | Meditation, Children and adolescents, Parents | Database inception – March 2017 | 16 | >416 | Narrative synthesis | Low quality of included studies and high heterogeneity restricted from making any firm conclusion about the efficacy of symptoms meditation-based interventions in reducing ADHD symptoms | RCTs with adequate power are needed | Moderate (6/9) |
| Zhang et al. [189] | Address the effectiveness of meditation-based interventions on ADHD patients | Meditation-based interventions, Children and adolescents, Adults | Database inception – May 5, 2018 | 13 | 270 | Random-effect meta-analysis | Small number of RCTs, low quality of included studies and high heterogeneity across studies restricted from making any firm conclusion about the efficacy of meditation-based interventions on ADHD | Future RCTs should be of high quality, with larger sample size, have uniform definition of control group and with long-term follow-up period | High (9/9) |
| SampedroBaena et al. [190] | Analyze the effects of NF interventions in children with ADHD | Neurofeedback, Treatment | 2017–June 2021 | 9 | 620 | Narrative synthesis | – NF showed a significant improvement of the symptoms in children with ADHD | Additional randomized controlled trials are needed to determine the significant effects | Moderate (6/9) |
| Goode et al. [191] | Compare the effectiveness of nonpharmacologic treatments for ADHD | Nonpharmacological interventions, Children and adolescents | January 2009–November 2016 | 54 | 353 | Narrative synthesis | Low quality of included studies and significant heterogeneity restricted from making any firm conclusion about the efficacy of different nonpharmacological interventions (CBT; child or parent training, neurofeedback, herbal or dietary approach) on ADHD | Pragmatic RCTs with long-term follow up and adequate sample size are required | Moderate (6/9) |
| Cortese et al. [192] | Assess the effectiveness of NF on ADHD symptoms | Neurofeedback, Nonpharmacological treatment, Children and adolescents | Database inception – August 2015 | 13 | 520 | Random-effect meta-analysis | Meta-analysis of blinded outcomes assessment showed no significant effect of NF on ADHD symptoms or other cognition covariates | Research should determine the most suitable electro-physiological treatment targets, develop standardized EEG and learning protocols, and identify predictors of treatment response for individual patients or group of patients | High (8/9) |
| Sonuga-Barke et al. [193] | Assess the impact of nonpharmacological interventions on ADHD | Dietary-based interventions, Psychological treatment, Children and adolescents | Database inception – April 2012 | 54 | 3,215 | Random-effect meta-analysis | Based on probably blinded assessment, free fatty acid supplementation was found to have a significant but small effect on ADHD symptoms (SMD = 0.17, 0.01–0.34), while evidence for other nonpharmacolgical interventions (blinded assessments is required for behavioral interventions, neurofeedback, cognitive training, and restricted elimination diet) were limited | Further clinical trials using blinded assessment are required to assess the efficacy of nonpharmacological interventions like behavioral interventions, neurofeedback, cognitive training, and restricted elimination diet on ADHD symptoms | High (9/9) |
| Händel et al. [194] | Assess the efficacy of PUFAs in the treatment for ADHD | Fatty acids; omega 3; polyunsaturated | Database inception – June 2020 | 31 | 1,755 | Random-effect meta-analysis | – Majority of included studies were of poor quality – PUFAs did not showed any effect The results showed no effect on ADHD core symptoms rated by parents (SMD = −0.17, −0.32 to −0.02) or teachers (SMD = −0.06, −0.31 to −0.19) – Moderate heterogeneity (I 2 > 50%) was observed across analysis |
Further high quality RCTs are required to make firm conclusion | High (8/9) |
| Gillies et al. [195] | Evaluate the effectiveness of PUFA in treating ADHD symptoms | Polyunsaturated fatty acid, Nonpharmacological treatment, Children and adolescents | Database inception – August 2011 | 13 | 1,011 | Fixed and random-effect meta-analysis | PUFA supplementation did not appear to be beneficial in most studies, although some studies showed some benefits with a combination of omega-3 and omega-6 supplementation | Future trials should include larger sample size and use a robust methodology to lower the risk of bias | High (8/9) |
| Bloch et al. [196] | Evaluate the efficacy of omega-3 fatty acid supplementation in ADHD | Polyunsaturated fatty acids, Omega-3 fatty acids, Children and adolescents | Database inception – December 2010 | 10 | 699 | Random-effect meta-analysis | Omega-3 fatty acid showed a small, but a significant effect on ADHD symptoms (SMD = 0.31, 0.13–0.47). However, small sample size and low quality of included studies restricted from making any firm conclusion about its efficacy in reducing ADHD symptoms | Future trials should include larger sample size and supplements with a high concentration of EPA, an omega-3 fatty acid, to determine the dose–response relationship | Moderate (7/9) |
| Abdullah et al. [197] | Evaluate the efficacy of omega-3 fatty acid supplementation in ADHD | Polyunsaturated fatty acids, Omega-3 fatty acids, Children and adolescents | Database inception – February 2018 | 7 | 926 | Narrative synthesis | Small number of RCTs and small sample size restricted from making any firm conclusion about the efficacy of omega-3 fatty acid supplementation in treating ADHD | Future research should include larger sample size and be based on long-term follow-up period | High (9/9) |
| Cooper et al. [198] | Assess the effectiveness of omega-3 polyunsaturated fatty acid supplementation on ED, oppositional behavior, conduct problems and aggression in children with ADHD | Omega-3, Emotional liability, Oppositional behavior | Database inception – 2014 | 12 | 500–2,000 | Random-effect meta-analysis | PUFA supplementation did not show improvements in measures of ED, oppositional behavior, conduct problems or aggression. in children with ADHD | Future studies need to include larger sample size and high concentration of n − 3 PUFA supplements to identify dose–response relationship | High (9/9) |
| Talebi et al. [199] | Determine the efficacy of zinc supplementation on ADHD | Zinc, Clinical trials | Database inception – January 2021 | 6 | 489 | Random-effect meta-analysis | – Included studies were of high quality – Zinc supplementation showed greater and significant effect on ADHD total score (SMD = −0.62, −1.24 to −0.002) but not in hyperactivity and inattention scores – The certainty of evidence was rated moderate to very low |
Well-designed, large-scale randomized controlled trials are needed to establish the effect of zinc supplementation on ADHD | High (9/9) |
| Granero et al. [200] | Determine the effect of iron and zinc in the treatment of ADHD | Zinc, Iron, Treatment | January 2000–July 2021 | 9 | Not given | Narrative synthesis | – Included studies were of moderate quality – The specific role of dietary nutrients with zinc and iron still seems controversial for the treatment of ADHD |
Further investigations including large sample size and high quality clinical trials are needed to confirm the effects of these diet interventions | Moderate (6/9) |
| Gan et al. [201] | Quantify the effect of vitamin D supplementation on ADHD | Vitamin D, Supplementation | Not clear | 4 | 256 | Random-effect meta-analysis | – Most of the included studies were of moderate quality – Vitamin D supplementation showed a small, but a significant effect on ADHD symptoms – Moderate heterogeneity (I 2 > 50%) was observed across analysis |
More high quality clinical trials are needed to make a definitive conclusion | High (9/9) |
| Bruton et al. [202] | Assess efficacy of phosphatidylserine for symptoms of ADHD | Integrative medicine | Not clear | 4 (3 for meta-analysis) | 344 | Random-effect meta-analysis | Due to low quality of evidence no firm conclusion can be made about the efficacy of phosphatidylserine for treatment of ADHD | Additional rigorous research is warranted to investigate phosphatidylserine as a low-cost and likely low-risk intervention for children with ADHD | High (8/9) |
| Anheyer et al. [203] | Evaluate the impact of herbal medicines on ADHD | Herbal medicines, Nonpharmacological treatment, Children and adolescents | Database inception – July 2016 | 9 | 464 | Narrative synthesis | Small number of RCTs restricted from making any firm conclusion about the efficacy of different herbal medicines on treating ADHD symptoms | Methodologically rigorous studies are needed | Moderate (7/9) |
| Zwi et al. [204] | Assess the effectiveness of parent training interventions for ADHD in children and adolescents | Parent training interventions, Nonpharmacological interventions, Children and adolescents | Database inception-September 2010 | 5 | 284 | Narrative synthesis and random-effect meta-analysis | Small sample size and low quality of included studies restricted from making any firm conclusion about the efficacy of parental training for ADHD | Further well-designed, randomized controlled trials within this population are needed and should be reported clearly following the principles set out in the CONSORT 2010 Statement | High (9/9) |
| Montoya et al. [205] | Evaluate the impact of psychoeducation interventions for parents and teachers in ADHD children | Psychoeducation, Parents, Teachers | Database inception – 2010 | 7 | 2,034 | Narrative synthesis | – Few studies showed positive results for improvement in patients’ behavior, parents’ and children’s satisfaction, child’s knowledge about ADHD, including their positive opinion and adherence to medical treatment – However, availability of limited data, low sample size, and variability in the concept of psychoeducation restricted from making any firm conclusion about this intervention |
Future studies should include adequate sample size and use the clear concept of psychoeducation | High (8/9) |
| Storebø et al. [206] | Evaluate the effect of social skills training on ADHD | Social skills training, Nonpharmacological treatment, Children and adolescents | Database inception – July 2018 | 25 | 2,690 | Narrative synthesis and fixed and random-effect meta-analysis | Low quality of included studies, lack of clinical significance, high heterogeneity, and low certainty restricted from making any firm conclusion about the efficacy of social skills training in reducing ADHD symptoms | Methodologically rigorous clinical trials with a large sample size are needed | High (9/9) |
| Richardson et al. [207] | Evaluate the effectiveness of school-based nonpharmacological interventions on ADHD | Nonpharmacological interventions, School-based settings, Children and adolescents | 1980 February–August 2013 | 54 | Not given | Narrative synthesis | Low quality of included studies and substantial heterogeneity in effect size restricted from making any definitive conclusion about the efficacy of school-based nonpharmacological interventions on ADHD | The lack of standardized tools and outcome measures for assessing ADHD behavior, as well as the lack of studies assessing possible moderators in combinations with interventions, present opportunities for future research | High (8/9) |
| Helmer et al. [208] | Evaluate the effectiveness of EAS in children with ADHD | Equine-assisted services and therapies, Environment | Database inception-December 2020 | 12 | 184 | Narrative synthesis | – Included studies were of moderate quality – EAS may be beneficial in promoting the physiological functions of body systems for children with ADHD |
Further controlled studies, with larger sample sizes, are needed to understand the specific effects of different EAS on the core symptoms and consequence of ADHD | High (8/9) |
| Perez -Gomez et al. [209] | Synthesize the effectiveness of equine assisted activities in children with ADHD | Animal- assisted therapy, Equine-assisted therapy, Nonpharmacological interventions | Database inception to November 2019 | 9 | 181 | Narrative synthesis | Low quality of included studies restricted from making any firm conclusion about the efficacy of equine assisted activities in children with ADHD | Studies with robust methodology are needed | Moderate (6/9) |
| Wilkes-Gillan et al. [210] | Synthesize the evidence for video-modeling interventions for individuals with ADHD | Video-modeling | Not given | 11 | 1–35 participants | Narrative synthesis | – Included studies were of high quality – Small number of clinical trials and sample size restricts from making any firm conclusion about the effectiveness of video-modeling interventions |
Future studies need to lower the risk of bias and use larger sample sizes before the efficacy of video-modeling interventions can be fully investigated | Moderate (7/9) |
| Lee et al. [211] | Determine the effect of acupuncture on ADHD | Acupuncture, Nonpharmacological treatment, Children and adolescents | Database inception- October 2010 | 3 | Not given | Random-effect meta-analysis | Small number of RCTs and low quality of included studies restricted from making any firm conclusion about the efficacy of acupuncture on treating ADHD symptoms | Future studies should include robust methodology to avoid or lower the risk of bias | High (8/9) |
| Cerrillo-Urbina et al. [212] | Examine the evidence for the effectiveness of exercise interventions on ADHD | Exercise, Children and adolescents | Database inception- November 2014 | 8 | 249 | Random-effect meta-analysis | Small number of RCTs, low quality of included studies and heterogeneity in the outcome measures restricted from making any firm conclusion about the efficacy of physical exercise like aerobics, yoga on treating ADHD symptoms | Additional studies are needed to obtain consistent clinically relevant conclusions | High (9/9) |
| Adults | |||||||||
| Lopez -Pinar et al. [213] | Assess the long-term efficacy of psychosocial treatments (CBT, dialectical-behavior therapy, mindfulness-based cognitive therapy) for ADHD | Adult ADHD treatments, Psychosocial treatments | Database inception-September 2017 | 12 | 1, 073 | Random-effect meta-analysis and meta-regression | – Included studies were of low quality – Compared to the control group, the treatment group as a whole reported better improvement in terms of total ADHD symptoms, inattention, and hyperactivity/impulsivity, as well as for clinical global assessment and global functioning for at least 12 months after ended treatment – Significant heterogeneity (I 2 > 75%) was observed across analysis |
Further research should examine whether psychosocial interventions works on different ADHD symptom dimensions or not | High (9/9) |
| Lopez et al. [214] | Examine the effectiveness of cognitive-behavioral-based therapy for ADHD | Cognitive-behavioral therapy, Adults | Database inception – June 2017 | 14 | 700 | Random-effect meta-analysis | Imprecision (i.e., inaccurate results), inconsistency (i.e., results differ across trials) and methodological limitations restricted from making any firm conclusion about the efficacy of CBT in treating ADHD symptoms | Multi-center long-term studies are needed to determine the effectiveness of CBT for adults with ADHD. Such studies should also include cost-effectiveness analyses | High(9/9) |
| Lopez-Pinar et al. [215] | Determine the efficacy of psychotherapies on comorbid internalizing symptoms in ADHD adults | Nonpharmaceutical intervention, Internalizing comorbidity, Adult ADHD treatment | Database inception – October 2018 | 35 in quantitative analysis and 6 in qualitative synthesis | 1,389 | Random-effect meta-analysis | – Included studies were of low quality – CBT was efficacious in improving comorbid anxiety and depression symptoms, quality of life, and emotional dysregulation, particularly at 3–12 months follow-up period – Moderate to significant heterogeneity (I 2 : 60–82%) was observed across analysis |
Findings should be further strengthened using a large sample and comparison group, address issues of randomization and attrition bias, perform intention to treat analysis | High(9/9) |
| Poissant et al. [216] | Examine the effectiveness of MBI in adults with ADHD | Mindfulness-based interventions, Behavioral and cognitive impact, Adults | Database inception – July 2018 | 13 | 753 | Narrative synthesis | – Included studies were of low quality – Besides improving ADHD symptoms at 3-to – 6-month posttreatment follow-up, mindfulness meditation improved executive function, emotional regulation, and post-intervention cognitive task performance |
Future research should address methodological issues like lack of randomization and control groups, sample size variations, and duration of intervention | Moderate (7/9) |
| Bruce et al. [217] | Assess and identify the most effective behavioral interventions that improves driving outcomes in novice drivers with ADHD | Behavioral interventions, Driving, Hazard perception | Database inception – 2013 | 13 | Not given | Narrative synthesis | Training led to significant enhancement in driving performance in two studies, but methodological issues compromised its validity. Situation awareness training, like commentary driving, was found to be useful | Long-term effect of nonpharmacological interventions (situation awareness, and particularly hazard perception training) in young drivers with ADHD are required | Moderate (6/9) |
| Salehinejad et al. [218] | Assess the effectiveness of tDCS on neuropsychological deficits in ADHD | Transcranial direct current stimulation, Inhibitory control, Working memory | Database inception – January 2019 | 10 | 159 | Random-effect meta-analysis | tDCSs appeared as an effective technique for improving inhibitory control and working memory. However, neuropsychological deficits may not correspond directly with clinical symptoms, so the clinical application of this method cannot be yet confirmed | Further research is needed to develop optimal stimulation parameters for improving cognitive function using tDCS and implementing rigorous experimental design across different ADHD subtypes | Moderate (7/9) |
Abbreviations: CBT, cognitive behavioral therapy; DBRC, daily behavior report cards; ED, emotional dysregulation; EQS, equine-assisted services; k, total number of included studies; MBI, mindfulness-based interventions; NF, neurofeedback; PUFA, polyunsaturated fatty acid; SMD, standardized mean difference; tDCS, transcranial direct current stimulation.
Total participants included in the systematic review and meta-analysis unless otherwise indicated.
For findings from meta-analysis, if given effect estimates with 95% CI are presented unless otherwise indicated.
Children and adolescents
Behavioral interventions, including social and academic skills training, cognitive behavioral therapy (CBT), parent coaching on social skills, and stress management, were found to decrease the child’s ADHD symptoms and conduct problems and improve social skills, academic performance, and positive parenting when reported by proximal observers [175]. Effects on parenting and children’s conduct problems persisted when assessment was blinded [175]. Parental training was an efficacious intervention for reducing ADHD symptoms in preschool children (Hedges’ g = 0.51, 95% CI: 0.33–0.65) and negative parenting style, as based on parents’ rating [176].
In a school-based setting, combined interventions, including social skills training, behavior modification technique, study and organizations skills training, were found to improve ADHD symptoms, as rated by both teachers and parents [177]. Daily behavior report cards were associated with reductions in teacher-rated ADHD symptoms [178] and improvement in academic outcomes [177]. Peer-inclusion interventions led to moderate improvements in pre-post measure of social functioning (Hedges’ g = 0.58, 0.45–0.70) among those receiving treatment [179].
Results were mixed for the efficacy of cognitive interventions in reducing ADHD symptoms and for improving working memory performance [180–182]. Virtual reality-based interventions were more effective than other nonpharmacological interventions or no intervention in improving sustained attention in children and adolescents with ADHD [183]. Interventions based on mind–body therapies [184] and few-foods diet, that is diet elimination of many foods and additives, have shown positive effects on ADHD core symptoms [185]. However, homeopathy did not show positive effects in reducing ADHD symptoms [186].
Several reviews suggested that despite their wide applications, significant knowledge gaps exist regarding the effectiveness of various nonpharmacological interventions. These include mindfulness [187–189], neurofeedback [190–193], behavioral interventions [193], cognitive training [193], dietary interventions [194–201], herbal interventions [202, 203], parent and teacher training [204, 205], social skills training [206], school-based interventions [207], equine-assisted therapies [208, 209], video modeling [210], acupuncture [211], and physical exercise [212].
Adults
In adults, long-term, that is at least 12 months follow-up of psychotherapies (CBT, dialectical behavioral therapy, mindfulness-based cognitive therapy) showed greater improvement in self-reported total ADHD symptoms (SMD = 0.71, 95% CI: 0.22–1.21), inattention, and hyperactivity/impulsivity in intervention than control groups [213]. One systematic review suggested that CBT might improve the core symptoms of ADHD, but the evidence was of low quality [214]. CBT was also efficacious in treating comorbid-internalizing symptoms in adults with ADHD [215]. Mind–body therapies including meditation were efficacious in reducing ADHD core symptoms compared to, for example, treatment as usual, although the evidence was of low quality [216].
Evidence for behavioral intervention to improve driving skills in young train novice drivers with ADHD was inconclusive [217]. Transcranial direct current stimulation might have some effect on neuropsychological and cognitive deficits, but there was no evidence to suggest that it decreases ADHD symptoms [218].
Pharmacological versus nonpharmacological treatment (n = 7, Table 10)
Table 10.
Pharmacological versus nonpharmacological interventions for ADHD
| References | Objective | Keywords | Timeframe of database search | k | Sample size a | Analytical design | Major findings Effect estimates; 95% CI) b | Conclusion and comments from authors | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Yang [219] | Meta-regress the effect sizes of stimulant (MPH and LDX), nonstimulant (ATX and alpha-2 agonists), psychosocial therapy (PBT), combination therapy (psychostimulant plus PBT), and alternative/complementary interventions to determine the right treatment for ADHD | Behavioral therapy, Pharmacotherapy, Treatment efficacy | January 1980–July 2018 | 107 | 9,883 | Random-effect meta-analysis and meta-regression | – Included studies were of high quality – Compared with the stimulant, nonstimulant and alternative or complement intervention were less effective (effect size = −0.38, −0.64 to −0.12) and (effect size = −0.41, −0.79 to −0.04) respectively – However, compared with stimulant, PBT and the combination of stimulant and PBT trials did not differ significantly |
These findings will help clinicians, healthcare providers, parents, and caregivers in choosing treatment for ADHD in children and adolescents | Moderate (7/9) |
| Catala-Lopez et al. [220] | Compare the effectiveness of pharmacological and nonpharmacological treatment for ADHD | Pharmacological treatment, Nonpharmacological treatment, Children and adolescents | Not given | 190 | 26,114 | Network meta-analysis | – Included studies were of low quality – Stimulants appeared to be superior to behavioral treatment, cognitive training and nonstimulants, while a combination of stimulants and behavioral therapy had the best combined effect and acceptance rate |
RCTs with robust methodology are urgently needed | High (9/9) |
| Chan et al. [221] | Assess the effectiveness of pharmacological and nonpharmcological treatment for ADHD | Pharmacological treatment, Psychosocial treatment, Adolescents | January 1999–January 2016 | 17 | 2,668 | Narrative synthesis | Medications were associated with improvements in total ADHD symptoms, while psychosocial treatments led to improvements in functional outcomes like academic and organizational skills | Both pharmacological and nonpharmacological interventional studies should examine how dosage, frequency, intensity and duration affect clinical outcomes, including their effectiveness over longer-term | Moderate (6/9) |
| Yan et al. [222] | Compare the effectiveness of MPH and NF on ADHD | MPH, Neurofeedback, Children and adolescents | Database inception – August 2018 | 18 | NF: 778 MPH:757 |
Random-effect meta-analysis | High risk of bias, inconsistency in findings between main analysis and subgroup analysis and nonfunded studies, and mixed findings at the follow-up endpoint restricted from making any firm conclusion about the efficacy of MPH versus NF in reducing ADHD symptoms in children and adolescents | High quality and larger studies are needed to compare the effectiveness | Moderate (7/9) |
| Lan et al. [223] | Compare the effectiveness of traditional Chinese medicine with MPH in treating ADHD symptoms | Traditional Chinese Medicine, MPH, ADHD | Database inception – June 2008 | 34 | 3,167 | Random and fixed-effect meta-analysis | – Majority of included studies were of poor quality – No conclusion can be made about the efficacy of Chinese medicine compared to MPH due to lack of high-quality clinical trials |
High-quality, multicenter studies are needed to make a firm conclusion | High (8/9) |
| Dijk et al. [224] | Present full economic evaluations of ADHD treatments | Economic evaluations, Cost-effectiveness | Not given | 29 | NA | Narrative synthesis | – Almost all studies that compared medication or psychosocial treatment to no treatment, placebo, or care as usual indicated that medication and psychosocial treatment were cost-effective compared to the control group. Stimulant treatment appeared to be cost-effective for the treatment of ADHD in children and adolescents | More cost-effectiveness research of higher quality is warranted to aid in the optimal use of available treatments and resources for individuals with ADHD | Moderate (7/9) |
| Wu et al. [225] | Assess cost-effectiveness of different pharmacological treatment to better inform payers in the allocation of limited resources | Economic evaluation, ADHD medications, | January 1990–September 2011 | 13 | NA | Narrative synthesis | – Included studies were of moderate quality. – Pharmacological treatment was found to be cost-effective compared to placebo, no medication, or behavioral treatment among children and adolescents. – However, economic evidence comparing different medications was limited and inconclusive |
Future research should study the cost effectiveness of medications on ADHD adult population and should assess the long-term cost effectiveness of pharmacological treatment | Moderate (6/9) |
Abbreviations: k, total number of included studies; LDX, lisdexamphetamine; MPH, methylphenidate; NA, not applicable; NF, neurofeedback; PBT, parental behavioral therapy; RCTs, randomized controlled trials.
Total participants included in the systematic review and meta-analysis unless otherwise indicated.
For findings from meta-analysis, if given effect estimates with 95% CI are presented or otherwise specified.
Two meta-analyses have reported the efficacy of stimulant treatment compared to nonstimulant or other interventions [219, 220]. A network meta-analysis of 190 RCTs found stimulants to be superior compared to nonpharmacological treatment in children and adolescents with ADHD [220]. While medications like extended release-MPH, amphetamine formulations, ATX, and extended release-guanfacine improved ADHD symptoms, psychosocial treatments were beneficial for academic and organizational skills in adolescents [221]. Results from head-to-head trials comparing MPH and neurofeedback were too inconsistent to conclude [222]. Similarly, findings for the efficacy of MPH versus traditional Chinese medicine were suggested by earlier review [223].
A recent systematic review revealed that stimulant treatment appeared to be cost-effective compared to other treatments or no treatment for ADHD in children and adolescents [224]. Similar findings for pharmacotherapy as a whole were suggested by earlier review [225].
Patients’ and caregivers’ experience of ADHD beyond symptoms (n = 13, Table 11)
Impact on quality of life
Of the two included reviews on health-related quality of life of children and adolescents with ADHD, one found that the parents reported significantly worse health-related quality of life of their children than that reported by the children with ADHD themselves [226], while the other review suggested no such differences [227].
Experience with ADHD, pharmacological and nonpharmacological interventions
Individuals with ADHD experience poor academic functioning, pressure to fit in with societal rules and expectations, fear of stigma, and difficulties in being part of the workplace and performing work tasks [228–230]. However, they may also recognize the positive and rewarding aspects of having ADHD [228–230].
Patients with ADHD and their caregivers experience medication as a last resort [231] or as a coping strategy [230]. Some experience that starting medication trigger off an identity crisis [228, 231]. There were reports of concerns about the long-term side effects of medication and financial costs, and the decision of taking medication was based on a process of “pro and con” considerations [228, 231]. Educators, children, and adolescents with ADHD, and their parents felt that school-based nonpharmacological interventions can lead to stigma, but also improve relationships and attitudes [232].
Parenting a child with ADHD was felt as exhausting and emotional journey filled with feelings of guilt, frustration, although “not all bad” [229, 233]. The most commonly used coping strategy of parents seemed to be avoidant-focused coping and was linked to distress and depression [234, 235].
Societal and familial barriers to ADHD treatment
Reviews of qualitative studies found perceived stigma as a barrier for acknowledging ADHD by primary care practitioners [236] and for implementing nonpharmacological intervention in schools [237]. There was no sufficient evidence to conclude if poverty moderates psychosocial treatment efficacy for ADHD [238].
Discussion
To the best of our knowledge, this is the first systematic meta-review that summarizes the main findings of 231 existing systematic reviews and meta-analyses on ADHD of moderate to high quality.
Different from earlier narrative reviews of evidence-based conclusions about ADHD [239–241], we have pre-registered the protocol, adhered to the PRISMA and JBI guidelines to perform a systematic quality assessment of the included reviews. However, our review has some limitations that should be considered. Importantly, this meta-review is not an overview of all published literature on ADHD but limited to academic publications on ADHD covered by systematic reviews and meta-analyses of moderate to high quality, indexed in the five most relevant databases or found in reference lists. Grey literature publications were not covered in our search. Secondly, our inclusion criteria and threshold for quality assessment were quite strict, thus excluding some of the pertinent systematic reviews on topics including genetics [242–249], neurobiology [250, 251], prevalence including young adults [252–254], comorbidities like dyslexia [255, 256], speech disorder [257], borderline personality disorder [258] and nonpharmacological interventions in adults [259].
Nevertheless, this meta-review will aid both researchers and clinicians to get an update on the main conclusions from ADHD research. Importantly our review may also be used as a bird’s eye view by identifying areas where there is sufficient evidence, insufficient evidence, and systematic weaknesses in reviews in various fields of ADHD. In the following, we will summarize some of our key findings with emphasis on weaknesses in the literature of systematic reviews on ADHD.
Prevalence of ADHD
According to included meta-analyses, the prevalence of ADHD is 7.2% in children [12] and 2.5% in adults [18]. However, there is considerable variation in the reported prevalence between the original studies included in these reviews. This strong variation is not fully understood, although probably due to other factors than true phenotype differences within or between populations studied. More plausible explanations for the variation in prevalence might include: (i) There are differences in research design, applied diagnostic instruments, and source of information which may cause bias [252]. (ii) There may be variation in provider-preference, that is clinician-dependent variation in assessment and diagnostic decisions [260]. This variation may be caused by variation in clinicians’ attitudes toward ADHD diagnosis and medications, for example, from a liberal to a restrictive position, even within uniform health systems [261]. (iii) There may be variation in supply [262–264] and demand [265] of health services, which in turn ultimately will affect variation in rates of diagnosed ADHD. (iv) Finally, the rate of diagnosed ADHD has increased over the last decades, both in children [12, 17, 266] and adults [18]. This may reflect a previous under-recognition of ADHD or increasing over-diagnosis [17]. Hence, this has left the field of ADHD research with the question of how certain are we of the prevalence of ADHD? The uncertainty is not merely a question of narrowing the confidence interval in meta-analyses by including more studies. More sophisticated reviews are needed, addressing the issues of various forms of bias in original studies.
Risk factors for ADHD: Correlations versus causality
There is evidence of a whole range of ADHD “risk factors,” including for example biological [20], maternal [21–28, 30, 31, 35], environmental [39, 41, 46], social [47], and nutritional factors [49, 50]. However, this literature is mostly based on research designs precluding conclusions on causality due to problems with confounding, and reverse causality. Adjustment for confounders usually makes a difference in such correlational studies, indicating residual confounding due to a lack of information on potential confounders or the reliability of measured confounders. As an example, maternal smoking has, and still is, consistently associated with offspring ADHD in classical epidemiological studies. However, later years’ research, combining different approaches, has shown that this association is not causal, but mainly due to genetic confounding [267–269]. Hence, we need more original studies that integrate results from different methodological approaches [270], allowing for causal inference.
Long-term prognosis of ADHD
The included reviews indicate a rather bleak prognosis over months and years of follow-up for young patients with ADHD in terms of criminal behavior [75, 76], school dropout [61, 62], vocational challenges [61, 62], injuries [68–70], comorbidities [80, 83, 84], and welfare dependency [61]. However, ADHD is correlated with a whole range of “risk factors” which themselves may be causally linked to challenging life trajectories. Studies of prognosis in ADHD may thus be confounded, and overestimate the negative prognosis of ADHD.
Since ADHD is a very heterogeneous condition, studies focusing more on predictors for different prognostic trajectories would be more informative and fruitful both for increasing our understanding of underlying mechanisms, targeting treatment, and preventing negative outcomes. Future systematic reviews should specifically address issues of publication bias, confounding, and residual confounding, and aim to highlight studies allowing for causal inference.
Pharmacological treatment of ADHD
There is strong evidence for ADHD symptom reduction during weeks or even months of stimulant use [113, 115, 121, 131] and also some evidence for nonstimulant medication [121, 137] in both children, adolescents, and adults. There is, however, an interesting controversy on how solid the trial evidence on ADHD medication efficacy is. Several reviews on both children and adults, and for both stimulant and nonstimulant pharmacotherapy, conclude with caution as to conclusions on efficacy [123, 124, 128, 130, 135, 139, 157, 158].
The follow-up time in trials is generally in terms of weeks, and the focus has mainly been on symptom scales rather than real-life outcomes [113, 115]. We need systematic review evidence on the efficacy of ADHD pharmacotherapy to mitigate the negative prognosis in ADHD. Can we prevent criminal behavior, school dropout, poor academic performance, vocational challenges, accidents, suicide, comorbidities, and welfare dependency, during years of follow-up, with pharmacological therapies? This is perhaps the most pressing question in the ADHD community, and it is yet to be answered. It should be noted that RCTs due to ethical and practical reasons, and conventional epidemiological studies due to the issue of residual confounding, have not been able to address this evidence gap [260]. We need evidence for efficacy based on large-scale, population-based studies with research designs allowing for causal inference as to the efficacy of ADHD medication in mitigating the rather bleak life trajectory in ADHD. These studies need to reach beyond symptom relief over weeks follow-up, but rather address outcomes regarding life-trajectories with over several years follow-up as to criminal behavior [75, 76], school dropout [61, 62], vocational challenges [61, 62], injuries [68–70], comorbidities [80, 83, 84], and welfare dependency [61, 271].
Nonpharmacological treatment of ADHD
The evidence on the efficacy of nonpharmacological treatment for ADHD is more mixed than that of pharmacotherapies. Mixed evidence exists for almost all type of nonpharmacological interventions: behavioral intervention for children and adolescents [175, 177, 193] and for adults [213, 214], parental training [175, 176, 204, 205], dietary interventions [194–201], mindfulness [184, 187–189], and other interventions. One reason may be that these interventions are more complex both to deliver and study, for example, therapies being less standardized than drugs, and also that it is more difficult to design blinded and “placebo”-controlled conditions for these more complex intervention. Despite this mixed evidence, ADHD treatments beyond pharmacotherapies are commonly administered and recommended in clinical guidelines [7].
Conclusion
In this meta-review, we found a large number of reviews that have reasonably well elucidated the evidence for different topics on ADHD. However, when summarizing the findings from the included reviews we still see some important knowledge gaps, for example on prevalence and risk factors. The most pressing knowledge gap is probably that of the efficacy of current ADHD treatments in mitigating the rather bleak life trajectory in ADHD. Hence, future systematic reviews and meta-analyses should address the identified knowledge gaps related to ADHD. To some extent, the lack of systematic review and meta-analysis evidence reflects lack of relevant original studies on ADHD.
Supporting information
Chaulagain et al. supplementary material
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1192/j.eurpsy.2023.2451.
Author contribution
Conceptualization: A.C., A.M., I.L., A.H., I.B., and T.W.-H.; Protocol: A.C. and A.M.; Search strategy: A.C. in consultation with A.M., I.L., T.W.-H., A.H., and I.B.; Screening: A.C., I.L., O.N. (for screening of abstract and full text published on 2021), and A.M.; Quality assessment: A.C. and I.L.; Data extraction: A.C., I.L., and O.N. (for articles published in 2021); Writing – original draft preparation: A.C. and I.L.; Writing – review and editing: A.H., A.M., I.B., T.W.-H., and O.N.; Supervision: A.M., A.H., and I.B.
Financial support
This work was supported by the Research Council of Norway (RCN) under the program FRIMEDBIO (project number 288585). The funders have not been involved in the creation or carrying out of the study.
Competing interest
The authors declare no conflicts of interest.
References
- [1].Closs SJ, Dowding D, Allcock N, et al. Towards improved decision support in the assessment and management of pain for people with dementia in hospital: a systematic meta-review and observational study. Southampton (UK): NIHR Journals Library; 2016 Oct. (Health Services and Delivery Research, No. 4.30.) Chapter 3, Meta-review: methods. Available from: https://www.ncbi.nlm.nih.gov/books/NBK390801/. [PubMed]
- [2].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40. [DOI] [PubMed] [Google Scholar]
- [4].Endnote X7.2. https://endnote.com/.
- [5].Covidence. https://www.covidence.org/.
- [6].Aromataris EFR, Godfrey C, Holly C, Khalil H, Tungpunkom P. Chapter 10: Umbrella reviews. In: Aromataris EMZ, editor. JBI manual for evidence synthesis JBI. Adelaide: Joanna Briggs Institute; 2020. [Google Scholar]
- [7].Razzak HA, Ghader N, Qureshi AA, Zafar M, Shaijan JF, Al Kuwari M. Clinical practice guidelines for the evaluation and diagnosis of attention-deficit/hyperactivity disorder in children and adolescents: a systematic review of the literature. Sultan Qaboos Univ Med J. 2021;21(1):e12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Loyer Carbonneau M, Demers M, Bigras M, Guay MC. Meta-analysis of sex differences in ADHD symptoms and associated cognitive deficits. J Atten Disord. 2021;25(12):1640–56. [DOI] [PubMed] [Google Scholar]
- [9].Chang LY, Wang MY, Tsai PS. Diagnostic accuracy of rating scales for attention-deficit/hyperactivity disorder: a meta-analysis. Pediatrics. 2016;137(3):e20152749. [DOI] [PubMed] [Google Scholar]
- [10].Staff AI, Oosterlaan J, van der Oord S, Hoekstra PJ, Vertessen K, de Vries R, et al. The validity of teacher rating scales for the assessment of ADHD symptoms in the classroom: a systematic review and meta-analysis. J Atten Disord. 2020;25(11):1578–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Taylor A, Deb S, Unwin G. Scales for the identification of adults with attention deficit hyperactivity disorder (ADHD): a systematic review. Res Dev Disabil. 2011;32(3):924–38. [DOI] [PubMed] [Google Scholar]
- [12].Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135 (4):e994–1001. [DOI] [PubMed] [Google Scholar]
- [13].Ayano G, Yohannes K, Abraha M. Epidemiology of attention-deficit/hyperactivity disorder (ADHD) in children and adolescents in Africa: a systematic review and meta-analysis. Ann General Psychiatry. 2020;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang T, Liu K, Li Z, Xu Y, Liu Y, Shi W, et al. Prevalence of attention deficit/hyperactivity disorder among children and adolescents in China: a systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cenat JM, Blais-Rochette C, Morse C, Vandette MP, Noorishad PG, Kogan C, et al. Prevalence and risk factors associated with attention-deficit/hyperactivity disorder among US black individuals: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hakim Shooshtari M, Shariati B, Kamalzadeh L, Naserbakht M, Tayefi B, Taban M. The prevalence of attention deficit hyperactivity disorder in Iran: an updated systematic review. Med J Islam Repub Iran. 2021;35:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kazda L, Bell K, Thomas R, McGeechan K, Sims R, Barratt A. Overdiagnosis of attention-deficit/hyperactivity disorder in children and adolescents: a systematic scoping review. JAMA Netw Open. 2021;4(4):e215335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Song P, Zha M, Yang Q, Zhang Y, Li X, Rudan I. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dobrosavljevic M, Solares C, Cortese S, Andershed H, Larsson H. Prevalence of attention-deficit/hyperactivity disorder in older adults: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2020;118:282–9. [DOI] [PubMed] [Google Scholar]
- [20].Ronald A, de Bode N, Polderman TJC. Systematic review: how the attention-deficit/hyperactivity disorder polygenic risk score adds to our understanding of ADHD and associated traits. J Am Acad Child Adolesc Psychiatry. 2021;60(10):1234–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li L, Lagerberg T, Chang Z, Cortese S, Rosenqvist MA, Almqvist C, et al. Maternal pre-pregnancy overweight/obesity and the risk of attention-deficit/hyperactivity disorder in offspring: a systematic review, meta-analysis and quasi-experimental family-based study. Int J Epidemiol. 2020;49(3):857–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ai Y, Zhao J, Shi J, Zhu TT. Antibiotic exposure and childhood attention-deficit/hyperactivity disorder: systematic review and meta-analysis. Psychopharmacology. 2021;238(11):3055–62. [DOI] [PubMed] [Google Scholar]
- [23].Gou XY, Wang Y, Tang Y, Qu Y, Tang J, Shi J, et al. Association of maternal prenatal acetaminophen use with the risk of attention deficit/hyperactivity disorder in offspring: a meta-analysis. Aust N Z J Psychiatry. 2019;53(3):195–206. [DOI] [PubMed] [Google Scholar]
- [24].Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, et al. Prenatal antidepressant exposure and the risk of attention-deficit hyperactivity disorder in children: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;86:1–11. [DOI] [PubMed] [Google Scholar]
- [25].Guo D, Ju R, Zhou Q, Mao J, Tao H, Jing H, et al. Association of maternal diabetes with attention deficit/hyperactivity disorder (ADHD) in offspring: a meta-analysis and review. Diabetes Res Clin Pract. 2020;165:108269. [DOI] [PubMed] [Google Scholar]
- [26].Rowland J, Wilson CA. The association between gestational diabetes and ASD and ADHD: a systematic review and meta-analysis. Sci Rep. 2021;11(1):5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dong T, Hu W, Zhou X, Lin H, Lan L, Hang B, et al. Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: a meta-analysis. Reprod Toxicol. 2018;76:63–70. [DOI] [PubMed] [Google Scholar]
- [28].Schwartz AN, Reyes LM, Meschke LL, Kintziger KW. Prenatal opioid exposure and ADHD childhood symptoms: a meta-analysis. Children (Basel, Switzerland). 2021;8(2):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Qu A, Cao T, Li Z, Wang W, Liu R, Wang X, et al. The association between maternal perfluoroalkyl substances exposure and early attention deficit hyperactivity disorder in children: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2021;28(47):67066–81. [DOI] [PubMed] [Google Scholar]
- [30].Zhu T, Gan J, Huang J, Li Y, Qu Y, Mu D. Association between perinatal hypoxic-ischemic conditions and attention-deficit/hyperactivity disorder: a meta-analysis. J Child Neurol. 2016;31(10):1235–44. [DOI] [PubMed] [Google Scholar]
- [31].Franz AP, Bolat GU, Bolat H, Matijasevich A, Santos IS, Silveira RC, et al. Attention-deficit/hyperactivity disorder and very preterm/very low birth weight: a meta-analysis. Pediatrics. 2018;141(1):e20171645. [DOI] [PubMed] [Google Scholar]
- [32].Serati M, Barkin JL, Orsenigo G, Altamura AC, Buoli M. Research review: the role of obstetric and neonatal complications in childhood attention deficit and hyperactivity disorder – a systematic review. J Child Psychol Psychiatry. 2017;58(12):1290–300. [DOI] [PubMed] [Google Scholar]
- [33].Curran EA, O’Neill SM, Cryan JF, Kenny LC, Dinan TG, Khashan AS, et al. Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Child Psychol Psychiatry. 2015;56(5):500–8. [DOI] [PubMed] [Google Scholar]
- [34].Xu L-L, Zhang X, Zhou G-L, Jiang C-M, Jiang H-Y, Zhou Y-Y. Meta-analysis found that studies may have overestimated caesarean section risks for attention-deficit hyperactivity disorder by ignoring confounding factors. Acta Paediatr. 2020;109(2):258–65. [DOI] [PubMed] [Google Scholar]
- [35].Min X, Li C, Yan Y. Parental age and the risk of ADHD in offspring: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(9):4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tseng PT, Yen CF, Chen YW, Stubbs B, Carvalho AF, Whiteley P, et al. Maternal breastfeeding and attention-deficit/hyperactivity disorder in children: a meta-analysis. Eur Child Adolesc Psychiatry. 2019;28(1):19–30. [DOI] [PubMed] [Google Scholar]
- [37].Zeng Y, Tang Y, Tang J, Shi J, Zhang L, Zhu T, et al. Association between the different duration of breastfeeding and attention deficit/hyperactivity disorder in children: a systematic review and meta-analysis. Nutr Neurosci. 2020;23:811–23. [DOI] [PubMed] [Google Scholar]
- [38].Huang A, Wu K, Cai Z, Lin Y, Zhang X, Huang Y. Association between postnatal second-hand smoke exposure and ADHD in children: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2021;28(2):1370–80. [DOI] [PubMed] [Google Scholar]
- [39].Asarnow RF, Newman N, Weiss RE, Su E. Association of attention-deficit/hyperactivity disorder diagnoses with Pediatric traumatic brain injury: a meta-analysis. JAMA Pediatr. 2021;175(10):1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sun JJ, Zhu CY, Jiang HY. Exposure to general anaesthesia in childhood and the subsequent risk of attention-deficit hyperactivity disorder: a meta-analysis of cohort studies. Asian J Psychiatr. 2021;62:102708. [DOI] [PubMed] [Google Scholar]
- [41].Daneshparvar M, Mostafavi SA, Zare Jeddi M, Yunesian M, Mesdaghinia A, Mahvi AH, et al. The role of lead exposure on attention-deficit/ hyperactivity disorder in children: a systematic review. Iran J Psychiatry. 2016;11(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- [42].Kalantary RR, Jaffarzadeh N, Rezapour M, Arani MH. Association between exposure to polycyclic aromatic hydrocarbons and attention deficit hyperactivity disorder in children: a systematic review and meta-analysis. Environ Sci Pollut Res. 2020;27(11):11531–40. [DOI] [PubMed] [Google Scholar]
- [43].Zhang M, Wang C, Zhang X, Song H, Li Y. Association between exposure to air pollutants and attention-deficit hyperactivity disorder (ADHD) in children: a systematic review and meta-analysis. Int J Environ Health Res. 2022;32(1):207–19. [DOI] [PubMed] [Google Scholar]
- [44].Aghaei M, Janjani H, Yousefian F, Jamal A, Yunesian M. Association between ambient gaseous and particulate air pollutants and attention deficit hyperactivity disorder (ADHD) in children; a systematic review. Environ Res. 2019;173:135–56. [DOI] [PubMed] [Google Scholar]
- [45].Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, et al. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ Health Perspect. 2017;125(8):086001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Caye A, Petresco S, de Barros AJD, Bressan RA, Gadelha A, Goncalves H, et al. Relative age and attention-deficit/hyperactivity disorder: data from three epidemiological cohorts and a meta-analysis. J Am Acad Child Adolesc Psychiatry. 2020;59:990–7. [DOI] [PubMed] [Google Scholar]
- [47].Russell AE, Ford T, Williams R, Russell G. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440–58. [DOI] [PubMed] [Google Scholar]
- [48].Langevin R, Marshall C, Wallace A, Gagné ME, Kingsland E, Temcheff C. Disentangling the associations between attention deficit hyperactivity disorder and child sexual abuse: a systematic review. Trauma Violence Abuse. 2023;24:369–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Del-Ponte B, Quinte GG, Cruz S, Grellert M, Santos IS. Dietary patterns and attention deficit/hyperactivity disorder (ADHD): a systematic review and meta-analysis. J Affect Disord. 2019;252:160–73. [DOI] [PubMed] [Google Scholar]
- [50].Farsad-Naeimi A, Asjodi F, Omidian M, Askari M, Nouri M, Pizarro AB, et al. Sugar consumption, sugar sweetened beverages and attention deficit hyperactivity disorder: a systematic review and meta-analysis. Complement Ther Med. 2020;53:102512. [DOI] [PubMed] [Google Scholar]
- [51].Khoshbakht Y, Bidaki R, Salehi-Abargouei A. Vitamin D status and attention deficit hyperactivity disorder: a systematic review and meta-analysis of observational studies. Adv Nutr. 2018;9(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].LaChance L, McKenzie K, Taylor VH, Vigod SN. Omega-6 to omega-3 fatty acid ratio in patients with ADHD: a meta-analysis. J Can Acad Child Adolesc Psychiatry. 2016;25(2):87–96. [PMC free article] [PubMed] [Google Scholar]
- [53].Huang YH, Zeng BY, Li DJ, Cheng YS, Chen TY, Liang HY, et al. Significantly lower serum and hair magnesium levels in children with attention deficit hyperactivity disorder than controls: a systematic review and meta-analysis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;90:134–41. [DOI] [PubMed] [Google Scholar]
- [54].Effatpanah M, Rezaei M, Effatpanah H, Effatpanah Z, Varkaneh HK, Mousavi SM, et al. Magnesium status and attention deficit hyperactivity disorder (ADHD): a meta-analysis. Psychiatry Res. 2019;274:228–34. [DOI] [PubMed] [Google Scholar]
- [55].Shih J-H, Zeng B-Y, Lin P-Y, Chen T-Y, Chen Y-W, Wu C-K, et al. Association between peripheral manganese levels and attention-deficit/hyperactivity disorder: a preliminary meta-analysis. Neuropsychiatr Dis Treat. 2018;14:1831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Degremont A, Jain R, Philippou E, Latunde-Dada GO. Brain iron concentrations in the pathophysiology of children with attention deficit/hyperactivity disorder: a systematic review. Nutr Rev. 2021;79(5):615–26. [DOI] [PubMed] [Google Scholar]
- [57].Cortese S, Angriman M, Lecendreux M, Konofal E. Iron and attention deficit/hyperactivity disorder: what is the empirical evidence so far? a systematic review of the literature. Expert Rev Neurother. 2012;12(10):1227–40. [DOI] [PubMed] [Google Scholar]
- [58].Tseng PT, Cheng YS, Yen CF, Chen YW, Stubbs B, Whiteley P, et al. Peripheral iron levels in children with attention-deficit hyperactivity disorder: a systematic review and meta-analysis. Sci Rep. 2018;8(1):788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ghoreishy SM, Ebrahimi Mousavi S, Asoudeh F, Mohammadi H. Zinc status in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis of observational studies. Sci Rep. 2021;11(1):14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Luo J, Mo Y, Liu M. Blood and hair zinc levels in children with attention deficit hyperactivity disorder: a meta-analysis. Asian J Psychiatr. 2020;47:101805. [DOI] [PubMed] [Google Scholar]
- [61].Christiansen MS, Labriola M, Kirkeskov L, Lund T. The impact of childhood diagnosed ADHD versus controls without ADHD diagnoses on later labour market attachment - a systematic review of longitudinal studies. Child Adolesc Psychiatry Ment Health. 2021;15(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Erskine HE, Norman RE, Ferrari AJ, Chan GCK, Copeland WE, Whiteford HA, et al. Long-term outcomes of attention-deficit/hyperactivity disorder and conduct disorder: a systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2016;55(10):841–50. [DOI] [PubMed] [Google Scholar]
- [63].Tosto MG, Momi SK, Asherson P, Malki K. A systematic review of attention deficit hyperactivity disorder (ADHD) and mathematical ability: current findings and future implications. BMC Med. 2015;13:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Di Lorenzo R, Balducci J, Poppi C, Arcolin E, Cutino A, Ferri P, et al. Children and adolescents with ADHD followed up to adulthood: a systematic review of long-term outcomes. Acta Neuropsychiatr. 2021;33(6):283–98. [DOI] [PubMed] [Google Scholar]
- [65].Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50(1):9–21. [DOI] [PubMed] [Google Scholar]
- [66].Fond G, Loundou A, Guillaume S, Quantin X, Macgregor A, Lopez R, et al. Smoking behavior characteristics of non-selected smokers with childhood attention-deficit/hyperactivity disorder (AD/HD) history: a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264(5):379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Oliva F, Mangiapane C, Nibbio G, Berchialla P, Colombi N, Vigna-Taglianti FD. Prevalence of cocaine use and cocaine use disorder among adult patients with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatr Res. 2020;143:587–98. [DOI] [PubMed] [Google Scholar]
- [68].Amiri S, Sadeghi-Bazargani H, Nazari S, Ranjbar F, Abdi S. Attention deficit/hyperactivity disorder and risk of injuries: a systematic review and meta-analysis. J Inj Violence Res. 2017;9(2):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Seens H, Modarresi S, MacDermid JC, Walton DM, Grewal R. Prevalence of bone fractures among children and adolescents with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. BMC Pediatr. 2021;21(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ruiz-Goikoetxea M, Cortese S, Aznarez-Sanado M, Magallon S, Zallo NA, Luis EO, et al. Risk of unintentional injuries in children and adolescents with ADHD and the impact of ADHD medications: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;84:63–71. [DOI] [PubMed] [Google Scholar]
- [71].Cook NE, Iaccarino MA, Karr JE, Iverson GL. Attention-deficit/hyperactivity disorder and outcome after concussion: a systematic review. J Dev Behav Pediatr. 2020;41(7):571–82. [DOI] [PubMed] [Google Scholar]
- [72].Ruiz-Goikoetxea M, Cortese S, Magallon S, Aznarez-Sanado M, Zallo NA, Luis EO, et al. Risk of poisoning in children and adolescents with ADHD: a systematic review and meta-analysis. Sci Rep. 2018;8:7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Septier M, Stordeur C, Zhang J, Delorme R, Cortese S. Association between suicidal spectrum behaviors and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;103:109–18. [DOI] [PubMed] [Google Scholar]
- [74].Nourredine M, Gering A, Fourneret P, Rolland B, Falissard B, Cucherat M, et al. Association of attention-deficit/hyperactivity disorder in childhood and adolescence with the risk of subsequent psychotic disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(5):519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Baggio S, Fructuoso A, Guimaraes M, Fois E, Golay D, Heller P, et al. Prevalence of attention deficit hyperactivity disorder in detention settings: a systematic review and meta-analysis. Front Psych. 2018;9:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mohr-Jensen C, Steinhausen HC. A meta-analysis and systematic review of the risks associated with childhood attention-deficit hyperactivity disorder on long-term outcome of arrests, convictions, and incarcerations. Clin Psychol Rev. 2016;48:32–42. [DOI] [PubMed] [Google Scholar]
- [77].Buitelaar NJ, Posthumus JA, Buitelaar JK. ADHD in childhood and/or adulthood as a risk factor for domestic violence or intimate partner violence: a systematic review. J Atten Disord. 2020;24:1203–14. [DOI] [PubMed] [Google Scholar]
- [78].Kittel-Schneider S, Quednow BB, Leutritz AL, McNeill RV, Reif A. Parental ADHD in pregnancy and the postpartum period – a systematic review. Neurosci Biobehav Rev. 2021;124:63–77. [DOI] [PubMed] [Google Scholar]
- [79].Chhibber A, Watanabe AH, Chaisai C, Veettil SK, Chaiyakunapruk N. Global economic burden of attention-deficit/hyperactivity disorder: a systematic review. PharmacoEconomics. 2021;39(4):399–420. [DOI] [PubMed] [Google Scholar]
- [80].Hollingdale J, Woodhouse E, Young S, Fridman A, Mandy W. Autistic spectrum disorder symptoms in children and adolescents with attention-deficit/hyperactivity disorder: a meta-analytical review. Psychol Med. 2020;50:2240–53. [DOI] [PubMed] [Google Scholar]
- [81].Carruthers S, Taylor L, Sadiq H, Tripp G. The profile of pragmatic language impairments in children with ADHD: a systematic review. Dev Psychopathol. 2021;11:1–23. [DOI] [PubMed] [Google Scholar]
- [82].Jacobsson P, Hopwood CJ, Söderpalm B, Nilsson T. Adult ADHD and emerging models of maladaptive personality: a meta-analytic review. BMC Psychiatry. 2021;21(1):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Schiweck C, Arteaga-Henriquez G, Aichholzer M, Thanarajah SE, Vargas-Caceres S, Matura S, et al. Comorbidity of ADHD and adult bipolar disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;124:100–23. [DOI] [PubMed] [Google Scholar]
- [84].Nazar BP, Bernardes C, Peachey G, Sergeant J, Mattos P, Treasure J. The risk of eating disorders comorbid with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Int J Eat Disord. 2016;49(12):1045–57. [DOI] [PubMed] [Google Scholar]
- [85].Kaisari P, Dourish CT, Higgs S. Attention deficit hyperactivity disorder (ADHD) and disordered eating behaviour: a systematic review and a framework for future research. Clin Psychol Rev. 2017;53:109–21. [DOI] [PubMed] [Google Scholar]
- [86].Curtin C, Pagoto SL, Mick E. The association between ADHD and eating disorders/pathology in adolescents: a systematic review. Open J Epidemiol. 2013;03(04):10. [Google Scholar]
- [87].Dullur P, Krishnan V, Diaz AM. A systematic review on the intersection of attention-deficit hyperactivity disorder and gaming disorder. J Psychiatr Res. 2021;133:212–22. [DOI] [PubMed] [Google Scholar]
- [88].Wang BQ, Yao NQ, Zhou X, Liu J, Lv ZT. The association between attention deficit/hyperactivity disorder and internet addiction: a systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Keenan L, Sherlock C, Bramham J, Downes M. Overlapping sleep disturbances in persistent tic disorders and attention-deficit hyperactivity disorder: a systematic review and meta-analysis of polysomnographic findings. Neurosci Biobehav Rev. 2021;126:194–212. [DOI] [PubMed] [Google Scholar]
- [90].Lee SH, Kim HB, Lee KW. Association between sleep duration and attention-deficit hyperactivity disorder: a systematic review and meta-analysis of observational studies. J Affect Disord. 2019;256:62–9. [DOI] [PubMed] [Google Scholar]
- [91].Díaz-Román A, Hita-Yáñez E, Buela-Casal G. Sleep characteristics in children with attention deficit hyperactivity disorder: systematic review and meta-analyses. J Clin Sleep Med. 2016;12(5):747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].De Crescenzo F, Licchelli S, Ciabattini M, Menghini D, Armando M, Alfieri P, et al. The use of actigraphy in the monitoring of sleep and activity in ADHD: a meta-analysis. Sleep Med Rev. 2016;26:9–20. [DOI] [PubMed] [Google Scholar]
- [93].Diaz-Roman A, Mitchell R, Cortese S. Sleep in adults with ADHD: systematic review and meta-analysis of subjective and objective studies. Neurosci Biobehav Rev. 2018;89:61–71. [DOI] [PubMed] [Google Scholar]
- [94].Instanes JT, Klungsoyr K, Halmoy A, Fasmer OB, Haavik J. Adult ADHD and comorbid somatic disease: a systematic literature review. J Atten Disord. 2018;22(3):203–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lugo J, Fadeuilhe C, Gisbert L, Setien I, Delgado M, Corrales M, et al. Sleep in adults with autism spectrum disorder and attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2020;38:1–24. [DOI] [PubMed] [Google Scholar]
- [96].Souto-Souza D, Mourao PS, Barroso HH, Douglas-de-Oliveira DW, Ramos-Jorge ML, Falci SGM, et al. Is there an association between attention deficit hyperactivity disorder in children and adolescents and the occurrence of bruxism? a systematic review and meta-analysis. Sleep Med Rev. 2020;53:101330. [DOI] [PubMed] [Google Scholar]
- [97].Li YJ, Xie XN, Lei X, Li YM, Lei XY. Global prevalence of obesity, overweight and underweight in children, adolescents and adults with autism spectrum disorder, attention-deficit hyperactivity disorder: a systematic review and meta-analysis. Obes Rev. 2020;21(12):e13123. [DOI] [PubMed] [Google Scholar]
- [98].Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Penalver C, Rohde LA, Faraone SV. Association between ADHD and obesity: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(1):34–43. [DOI] [PubMed] [Google Scholar]
- [99].Mocanu V, Tavakoli I, MacDonald A, Dang JT, Switzer N, Birch DW, et al. The impact of ADHD on outcomes following bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2019;29(4):1403–9. [DOI] [PubMed] [Google Scholar]
- [100].Pan PY, Jonsson U, Şahpazoğlu Çakmak SS, Häge A, Hohmann S, Nobel Norrman H, et al. Headache in ADHD as comorbidity and a side effect of medications: a systematic review and meta-analysis. Psychol Med. 2021;52(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kaas TH, Vinding RK, Stokholm J, Bønnelykke K, Bisgaard H, Chawes BL. Association between childhood asthma and attention deficit hyperactivity or autism spectrum disorders: a systematic review with meta-analysis. Clin Exp Allergy. 2021;51(2):228–52. [DOI] [PubMed] [Google Scholar]
- [102].Cortese S, Sun SH, Zhang JH, Sharma E, Chang Z, Kuja-Halkola R, et al. Association between attention deficit hyperactivity disorder and asthma: a systematic review and meta-analysis and a Swedish population-based study. Lancet Psychiatry. 2018;5(9):717–26. [DOI] [PubMed] [Google Scholar]
- [103].Schans JV, Cicek R, de Vries TW, Hak E, Hoekstra PJ. Association of atopic diseases and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. Neurosci Biobehav Rev. 2017;74(Pt A):139–48. [DOI] [PubMed] [Google Scholar]
- [104].Miyazaki C, Koyama M, Ota E, Swa T, Mlunde LB, Amiya RM, et al. Allergic diseases in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Robe A, Dobrean A, Cristea IA, Păsărelu CR, Predescu E. Attention-deficit/hyperactivity disorder and task-related heart rate variability: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;99:11–22. [DOI] [PubMed] [Google Scholar]
- [106].de Sena Oliveira AC, BdS Athanasio, FCdC Mrad, MMdA Vasconcelos, Albuquerque MR, Miranda DM, et al. Attention deficit and hyperactivity disorder and nocturnal enuresis co-occurrence in the pediatric population: a systematic review and meta-analysis. Pediatr Nephrol. 2021;36(11):3547–59. [DOI] [PubMed] [Google Scholar]
- [107].Athanasiadou A, Buitelaar JK, Brovedani P, Chorna O, Fulceri F, Guzzetta A, et al. Early motor signs of attention-deficit hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2020;29(7):903–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Havmoeller SR, Thomsen PH, Lemcke S. The early motor development in children diagnosed with ADHD: a systematic review. Atten Defic Hyperact Disord. 2019;11(3):233–40. [DOI] [PubMed] [Google Scholar]
- [109].Bishop C, Mulraney M, Rinehart N, Sciberras E. An examination of the association between anxiety and social functioning in youth with ADHD: a systematic review. Psychiatry Res. 2019;273:402–21. [DOI] [PubMed] [Google Scholar]
- [110].Li SL, Kam KW, Chee ASH, Zhang XJ, Chen LJ, Yip WWK, et al. The association between attention-deficit/hyperactivity disorder and retinal nerve fiber/ganglion cell layer thickness measured by optical coherence tomography: a systematic review and meta-analysis. Int Ophthalmol. 2021;41(9):3211–21. [DOI] [PubMed] [Google Scholar]
- [111].Chamorro Y, Betz LT, Philipsen A, Kambeitz J, Ettinger U. The eyes have it: a meta-analysis of oculomotor inhibition in attention-deficit/hyperactivity disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7(11):1090–102. [DOI] [PubMed] [Google Scholar]
- [112].Bellato A, Norman L, Idrees I, Ogawa CY, Waitt A, Zuccolo PF, et al. A systematic review and meta-analysis of altered electrophysiological markers of performance monitoring in obsessive-compulsive disorder (OCD), gilles de la tourette syndrome (GTS), attention-deficit/hyperactivity disorder (ADHD) and autism. Neurosci Biobehav Rev. 2021;131:964–87. [DOI] [PubMed] [Google Scholar]
- [113].Cerrillo-Urbina AJ, Garcia-Hermoso A, Pardo-Guijarro MJ, Sanchez-Lopez M, Santos-Gomez JL, Martinez-Vizcaino V. The effects of long-acting stimulant and nonstimulant medications in children and adolescents with attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials. J Child Adolesc Psychopharmacol. 2018;28(8):494–507. [DOI] [PubMed] [Google Scholar]
- [114].Riera M, Castells X, Tobias A, Cunill R, Blanco L, Capella D. Discontinuation of pharmacological treatment of children and adolescents with attention deficit hyperactivity disorder: meta-analysis of 63 studies enrolling 11,788 patients. Psychopharmacology. 2017;234(17):2657–71. [DOI] [PubMed] [Google Scholar]
- [115].Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Maia CRM, Cortese S, Caye A, Deakin TK, Polanczyk GV, Polanczyk CA, et al. Long-term efficacy of methylphenidate immediate-release for the treatment of childhood ADHD: a systematic review and meta-analysis. J Atten Disord. 2017;21(1):3–13. [DOI] [PubMed] [Google Scholar]
- [117].Rezaei G, Hosseini SA, Akbari Sari A, Olyaeemanesh A, Lotfi MH, Yassini M, et al. Comparative efficacy of methylphenidate and atomoxetine in the treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review and meta-analysis. Med J Islam Repub Iran. 2016;30:325. [PMC free article] [PubMed] [Google Scholar]
- [118].Liu Q, Zhang H, Fang Q, Qin L. Comparative efficacy and safety of methylphenidate and atomoxetine for attention-deficit hyperactivity disorder in children and adolescents: meta-analysis based on head-to-head trials. J Clin Exp Neuropsychol. 2017;39(9):854–65. [DOI] [PubMed] [Google Scholar]
- [119].Maneeton N, Maneeton B, Woottiluk P, Suttajit S, Likhitsathian S, Charnsil C, et al. Comparative efficacy, acceptability, and tolerability of dexmethylphenidate versus placebo in child and adolescent ADHD: a meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat. 2015;11:2943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Punja S, Zorzela L, Hartling L, Urichuk L, Vohra S. Long-acting versus short-acting methylphenidate for paediatric ADHD: a systematic review and meta-analysis of comparative efficacy. BMJ Open. 2013;3(3):e002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Stuhec M, Munda B, Svab V, Locatelli I. Comparative efficacy and acceptability of atomoxetine, lisdexamfetamine, bupropion and methylphenidate in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis with focus on bupropion. J Affect Disord. 2015;178:149–59. [DOI] [PubMed] [Google Scholar]
- [122].Maneeton B, Maneeton N, Likhitsathian S, Suttajit S, Narkpongphun A, Srisurapanont M, et al. Comparative efficacy, acceptability, and tolerability of lisdexamfetamine in child and adolescent ADHD: a meta-analysis of randomized, controlled trials. Drug Des Devel Ther. 2015;9:1927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev. 2015;11:CD009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2:CD009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ruggiero S, Clavenna A, Reale L, Capuano A, Rossi F, Bonati M. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(10):1578–90. [DOI] [PubMed] [Google Scholar]
- [126].Cheng JY, Chen RY, Ko JS, Ng EM. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents-meta-analysis and meta-regression analysis. Psychopharmacology. 2007;194(2):197–209. [DOI] [PubMed] [Google Scholar]
- [127].Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(12):1551–9. [DOI] [PubMed] [Google Scholar]
- [128].Maneeton N, Maneeton B, Intaprasert S, Woottiluk P. A systematic review of randomized controlled trials of bupropion versus methylphenidate in the treatment of attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2014;10:1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Otasowie J, Castells X, Ehimare UP, Smith CH. Tricyclic antidepressants for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2014;9:CD006997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Matsui Y, Matsunaga S, Matsuda Y, Kishi T, Iwata N. Azapirones for attention deficit hyperactivity disorder: a systematic review. Pharmacopsychiatry. 2016;49(3):97–106. [DOI] [PubMed] [Google Scholar]
- [131].Stuhec M, Lukic P, Locatelli I. Efficacy, acceptability, and tolerability of lisdexamfetamine, mixed amphetamine salts, methylphenidate, and modafinil in the treatment of attention-deficit hyperactivity disorder in adults: a systematic review and meta-analysis. Ann Pharmacother. 2019;53(2):121–33. [DOI] [PubMed] [Google Scholar]
- [132].Maneeton N, Maneeton B, Suttajit S, Reungyos J, Srisurapanont M, Martin SD. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Castells X, Cunill R, Capella D. Treatment discontinuation with methylphenidate in adults with attention deficit hyperactivity disorder: a meta-analysis of randomized clinical trials. Eur J Clin Pharmacol. 2013;69(3):347–56. [DOI] [PubMed] [Google Scholar]
- [134].Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology. 2008;197(1):1–11. [DOI] [PubMed] [Google Scholar]
- [135].Cândido RCF, Menezes de Padua CA, Golder S, Junqueira DR. Immediate-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2021;1:CD013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Castells X, Blanco-Silvente L, Cunill R. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2018;8:CD007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Cunill R, Castells X, Tobias A, Capellà D. Atomoxetine for attention deficit hyperactivity disorder in the adulthood: a meta-analysis and meta-regression. Pharmacoepidemiol Drug Saf. 2013;22(9):961–9. [DOI] [PubMed] [Google Scholar]
- [138].Verbeeck W, Bekkering GE, Van den Noortgate W, Kramers C. Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2017;10(10):CD009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Elliott J, Johnston A, Husereau D, Kelly SE, Eagles C, Charach A, et al. Pharmacologic treatment of attention deficit hyperactivity disorder in adults: a systematic review and network meta-analysis. PLoS One. 2020;15(10):e0240584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Liang EF, Lim SZ, Tam WW, Ho CS, Zhang MW, McIntyre RS, et al. The effect of methylphenidate and atomoxetine on heart rate and systolic blood pressure in young people and adults with attention-deficit hyperactivity disorder (ADHD): systematic review, meta-analysis, and meta-regression. Int J Environ Res Public Health. 2018;15(8):1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Liu H, Feng WJ, Zhang DF. Association of ADHD medications with the risk of cardiovascular diseases: a meta-analysis. Eur Child Adolesc Psychiatry. 2019;28(10):1283–93. [DOI] [PubMed] [Google Scholar]
- [142].Kidwell KM, Van Dyk TR, Lundahl A, Nelson TD. Stimulant medications and sleep for youth with ADHD: a meta-analysis. Pediatrics. 2015;136(6):1144–53. [DOI] [PubMed] [Google Scholar]
- [143].De Crescenzo F, Armando M, Mazzone L, Ciliberto M, Sciannamea M, Figueroa C, et al. The use of actigraphy in the monitoring of methylphenidate versus placebo in ADHD: a meta-analysis. Atten Defic Hyperact Disord.. 2014;6(1):49–58. [DOI] [PubMed] [Google Scholar]
- [144].Holmskov M, Storebo OJ, Moreira-Maia CR, Ramstad E, Magnusson FL, Krogh HB, et al. Gastrointestinal adverse events during methylphenidate treatment of children and adolescents with attention deficit hyperactivity disorder: a systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. PLoS One. 2017;12(6):e0178187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chierrito de Oliveira D, Guerrero de Sousa P, Borges Dos Reis C, Tonin FS, Maria Steimbach L, Virtuoso S, et al. Safety of treatments for ADHD in adults: pairwise and network meta-analyses. J Atten Disord. 2019;23(2):111–20. [DOI] [PubMed] [Google Scholar]
- [146].Li L, Sujan AC, Butwicka A, Chang Z, Cortese S, Quinn P, et al. Associations of prescribed ADHD medication in pregnancy with pregnancy-related and offspring outcomes: a systematic review. CNS Drugs. 2020;34(7):731–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Jiang HY, Zhang X, Jiang CM, Fu HB. Maternal and neonatal outcomes after exposure to ADHD medication during pregnancy: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2019;28(3):288–95. [DOI] [PubMed] [Google Scholar]
- [148].Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non-randomised studies. Cochrane Database Syst Rev. 2018;5:CD012069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Rodrigues R, Lai MC, Beswick A, Gorman DA, Anagnostou E, Szatmari P, et al. Practitioner review: pharmacological treatment of attention-deficit/hyperactivity disorder symptoms in children and youth with autism spectrum disorder: a systematic review and meta-analysis. J Child Psychol Psychiatry. 2021;62(6):680–700. [DOI] [PubMed] [Google Scholar]
- [150].Sun CK, Tseng PT, Wu CK, Li DJ, Chen TY, Stubbs B, et al. Therapeutic effects of methylphenidate for attention-deficit/hyperactivity disorder in children with borderline intellectual functioning or intellectual disability: a systematic review and meta-analysis. Sci Rep. 2019;9(1):15908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Tarrant N, Roy M, Deb S, Odedra S, Retzer A, Roy A. The effectiveness of methylphenidate in the management of attention deficit hyperactivity disorder (ADHD) in people with intellectual disabilities: a systematic review. Res Dev Disabil. 2018;83:217–32. [DOI] [PubMed] [Google Scholar]
- [152].Froehlich TE, Fogler J, Barbaresi WJ, Elsayed NA, Evans SW, Chan E. Using ADHD medications to treat coexisting ADHD and reading disorders: a systematic review. Clin Pharmacol Ther. 2018;104(4):619–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Osland ST, Steeves TDL, Pringsheim T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2018;6:CD007990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Woon LS, Hazli Z, Gan LLY. Pharmacotherapy for comorbid adult attention-deficit hyperactivity disorder and stimulant dependence: a systematic review. Int Med J Malays. 2018;17(2):149–61. [Google Scholar]
- [155].Anand S, Tong H, Besag FMC, Chan EW, Cortese S, Wong ICK. Safety, tolerability and efficacy of drugs for treating behavioural insomnia in children with attention-deficit/hyperactivity disorder: a systematic review with methodological quality assessment. Paediatr Drugs. 2017;19(3):235–50. [DOI] [PubMed] [Google Scholar]
- [156].Tsujii N, Usami M, Naya N, Tsuji T, Mishima H, Horie J, et al. Efficacy and safety of medication for attention-deficit hyperactivity disorder in children and adolescents with common comorbidities: a systematic review. Neurol Ther. 2021;10(2):499–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Thomson A, Maltezos S, Paliokosta E, Xenitidis K. Amfetamine for attention deficit hyperactivity disorder in people with intellectual disabilities. Cochrane Database Syst Rev. 2009;1:CD007009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Thomson A, Maltezos S, Paliokosta E, Xenitidis K. Risperidone for attention-deficit hyperactivity disorder in people with intellectual disabilities. Cochrane Database Syst Rev. 2009;2009(2):CD007011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Fluyau D, Revadigar N, Pierre CG. Systematic review and meta-analysis: treatment of substance use disorder in attention deficit hyperactivity disorder. Am J Addict. 2021;30:110–21. [DOI] [PubMed] [Google Scholar]
- [160].Man KKC, Ip P, Chan EW, Law SL, Leung MTY, Ma EXY, et al. Effectiveness of pharmacological treatment for attention-deficit/hyperactivity disorder on physical injuries: a systematic review and meta-analysis of observational studies. CNS Drugs. 2017;31(12):1043–55. [DOI] [PubMed] [Google Scholar]
- [161].Zhang SW, Shen D, Yan YT. ADHD, stimulant medication use, and the risk of fracture: a systematic review and meta-analysis. Arch Osteoporos. 2021;16(1):81. [DOI] [PubMed] [Google Scholar]
- [162].Schoenfelder EN, Faraone SV, Kollins SH. Stimulant treatment of ADHD and cigarette smoking: a meta-analysis. Pediatrics. 2014;133(6):1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Liu W-J, Mao H-J, Hu L-L, Song M-F, Jiang H-Y, Zhang L. Attention-deficit/hyperactivity disorder medication and risk of suicide attempt: a meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2020;29(11):1364–72. [DOI] [PubMed] [Google Scholar]
- [164].Prasad V, Brogan E, Mulvaney C, Grainge M, Stanton W, Sayal K. How effective are drug treatments for children with ADHD at improving on-task behaviour and academic achievement in the school classroom? A systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2013;22(4):203–16. [DOI] [PubMed] [Google Scholar]
- [165].Hulsbosch AK, De Meyer H, Beckers T, Danckaerts M, Van Liefferinge D, Tripp G, et al. Systematic review: attention-deficit/hyperactivity disorder and instrumental learning. J Am Acad Child Adolesc Psychiatry. 2021;60(11):1367–81. [DOI] [PubMed] [Google Scholar]
- [166].Pievsky MA, McGrath RE. Neurocognitive effects of methylphenidate in adults with attention-deficit/hyperactivity disorder: a meta-analysis. Neurosci Biobehav Rev. 2018;90:447–55. [DOI] [PubMed] [Google Scholar]
- [167].Villas-Boas CB, Chierrito D, Fernandez-Llimos F, Tonin FS, Sanches ACC. Pharmacological treatment of attention-deficit hyperactivity disorder comorbid with an anxiety disorder: a systematic review. Int Clin Psychopharmacol. 2019;34(2):57–64. [DOI] [PubMed] [Google Scholar]
- [168].Lenzi F, Cortese S, Harris J, Masi G. Pharmacotherapy of emotional dysregulation in adults with ADHD: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;84:359–67. [DOI] [PubMed] [Google Scholar]
- [169].Connor DF, Glatt SJ, Lopez ID, Jackson D, Melloni RH Jr. Psychopharmacology and aggression. I: a meta-analysis of stimulant effects on overt/covert aggression-related behaviors in ADHD. J Am Acad Child Adolesc Psychiatry. 2002;41(3):253–61. [DOI] [PubMed] [Google Scholar]
- [170].Pringsheim T, Hirsch L, Gardner D, Gorman DA. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatr. 2015;60(2):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Yuan D, Zhang M, Huang Y, Wang X, Jiao J, Huang Y. Noradrenergic genes polymorphisms and response to methylphenidate in children with ADHD: a systematic review and meta-analysis. Medicine (Baltimore). 2021;100(46):e27858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Soleimani R, Salehi Z, Soltanipour S, Hasandokht T, Jalali MM. SLC6A3 polymorphism and response to methylphenidate in children with ADHD: a systematic review and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2018;177(3):287–300. [DOI] [PubMed] [Google Scholar]
- [173].Leffa DT, Panzenhagen AC, Salvi AA, Bau CHD, Pires GN, Torres ILS, et al. Systematic review and meta-analysis of the behavioral effects of methylphenidate in the spontaneously hypertensive rat model of attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2019;100:166–79. [DOI] [PubMed] [Google Scholar]
- [174].Massuti R, Moreira-Maia CR, Campani F, Sônego M, Amaro J, Akutagava-Martins GC, et al. Assessing undertreatment and overtreatment/misuse of ADHD medications in children and adolescents across continents: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;128:64–73. [DOI] [PubMed] [Google Scholar]
- [175].Daley D, van der Oord S, Ferrin M, Danckaerts M, Doepfner M, Cortese S, et al. Behavioral interventions in attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry. 2014;53(8):835–47. [DOI] [PubMed] [Google Scholar]
- [176].Rimestad ML, Lambek R, Zacher Christiansen H, Hougaard E. Short- and long-term effects of parent training for preschool children with or at risk of ADHD: a systematic review and meta-analysis. J Atten Disord. 2019;23(5):423–34. [DOI] [PubMed] [Google Scholar]
- [177].Moore DA, Russell AE, Matthews J, Ford TJ, Rogers M, Ukoumunne OC, et al. School-based interventions for attention-deficit/hyperactivity disorder: a systematic review with multiple synthesis methods. Rev Educ. 2018;6(3):209–63. [Google Scholar]
- [178].Iznardo M, Rogers MA, Volpe RJ, Labelle PR, Robaey P. The effectiveness of daily behavior report cards for children with ADHD: a meta-analysis. J Atten Disord. 2020;24:1623–36. [DOI] [PubMed] [Google Scholar]
- [179].Cordier R, Vilaysack B, Doma K, Wilkes-Gillan S, Speyer R. Peer inclusion in interventions for children with ADHD: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:7693479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Chen S, Yu J, Zhang Q, Zhang J, Zhang Y, Wang J. Which factor is more relevant to the effectiveness of the cognitive intervention? a meta-analysis of randomized controlled trials of cognitive training on symptoms and executive function behaviors of children with attention deficit hyperactivity disorder. Front Psychol. 2021;12:810298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Pauli-Pott U, Mann C, Becker K. Do cognitive interventions for preschoolers improve executive functions and reduce ADHD and externalizing symptoms? a meta-analysis of randomized controlled trials. Eur Child Adolesc Psychiatry. 2021;30(10):1503–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Cortese S, Ferrin M, Brandeis D, Buitelaar J, Daley D, Dittmann RW, et al. Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54(3):164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Romero-Ayuso D, Toledano-González A, Rodríguez-Martínez MDC, Arroyo-Castillo P, Triviño-Juárez JM, González P, et al. Effectiveness of virtual reality-based interventions for children and adolescents with ADHD: a systematic review and meta-analysis. Children (Basel). 2021;8(2):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Barranco-Ruiz Y, Etxabe BE, Ramirez-Velez R, Villa-Gonzalez E. Interventions based on mind-body therapies for the improvement of attention-deficit/hyperactivity disorder symptoms in youth: a systematic review. Medicina (Kaunas). 2019;55(7):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Pelsser LM, Frankena K, Toorman J, Rodrigues Pereira R. Diet and ADHD, reviewing the evidence: a systematic review of meta-analyses of double-blind placebo-controlled trials evaluating the efficacy of diet interventions on the behavior of children with ADHD. PLoS One. 2017;12(1):e0169277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Heirs M, Dean ME. Homeopathy for attention deficit/hyperactivity disorder or hyperkinetic disorder. Cochrane Database Syst Rev. 2007;4:CD005648. [DOI] [PubMed] [Google Scholar]
- [187].Oliva F, Malandrone F, di Girolamo G, Mirabella S, Colombi N, Carletto S, et al. The efficacy of mindfulness-based interventions in attention-deficit/hyperactivity disorder beyond core symptoms: a systematic review, meta-analysis, and meta-regression. J Affect Disord. 2021;292:475–86. [DOI] [PubMed] [Google Scholar]
- [188].Evans S, Ling M, Hill B, Rinehart N, Austin D, Sciberras E. Systematic review of meditation-based interventions for children with ADHD. Eur Child Adolesc Psychiatry. 2018;27(1):9–27. [DOI] [PubMed] [Google Scholar]
- [189].Zhang J, Diaz-Roman A, Cortese S. Meditation-based therapies for attention-deficit/hyperactivity disorder in children, adolescents and adults: a systematic review and meta-analysis. Evid Based Ment Health. 2018;21(3):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Sampedro Baena L, Fuente GAC, Martos-Cabrera MB, Gómez-Urquiza JL, Albendín-García L, Romero-Bejar JL, et al. Effects of neurofeedback in children with attention-deficit/hyperactivity disorder: a systematic review. J Clin Med. 2021;10(17):3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Goode AP, Coeytaux RR, Maslow GR, Davis N, Hill S, Namdari B, et al. Nonpharmacologic treatments for attention-deficit/hyperactivity disorder: a systematic review. Pediatrics. 2018;141(6):e20180094. [DOI] [PubMed] [Google Scholar]
- [192].Cortese S, Ferrin M, Brandeis D, Holtmann M, Aggensteiner P, Daley D, et al. Neurofeedback for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2016;55(6):444–55. [DOI] [PubMed] [Google Scholar]
- [193].Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170(3):275–89. [DOI] [PubMed] [Google Scholar]
- [194].Händel MN, Rohde JF, Rimestad ML, Bandak E, Birkefoss K, Tendal B, et al. Efficacy and safety of polyunsaturated fatty acids supplementation in the treatment of attention deficit hyperactivity disorder (ADHD) in children and adolescents: a systematic review and meta-analysis of clinical trials. Nutrients [Internet]. 2021;13(4):1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Gillies D, Sinn JKH, Lad SS, Leach MJ, Ross MJ. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50(10):991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Abdullah M, Jowett B, Whittaker PJ, Patterson L. The effectiveness of omega-3 supplementation in reducing ADHD associated symptoms in children as measured by the Conners’ rating scales: a systematic review of randomized controlled trials. J Psychiatr Res. 2019;110:64–73. [DOI] [PubMed] [Google Scholar]
- [198].Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P. The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: a systematic review and meta-analysis. J Affect Disord. 2016;190:474–82. [DOI] [PubMed] [Google Scholar]
- [199].Talebi S, Miraghajani M, Ghavami A, Mohammadi H. The effect of zinc supplementation in children with attention deficit hyperactivity disorder: a systematic review and dose-response meta-analysis of randomized clinical trials. Crit Rev Food Sci Nutr. 2022;62(32):9093–102. [DOI] [PubMed] [Google Scholar]
- [200].Granero R, Pardo-Garrido A, Carpio-Toro IL, Ramírez-Coronel AA, Martínez-Suárez PC, Reivan-Ortiz GG. The role of iron and zinc in the treatment of ADHD among children and adolescents: a systematic review of randomized clinical trials. Nutrients. 2021;13(11):4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Gan J, Galer P, Ma D, Chen C, Xiong T. The effect of vitamin D supplementation on attention-deficit/hyperactivity disorder: a systematic review and meta-analysis of randomized controlled trials. J Child Adolesc Psychopharmacol. 2019;29(9):670–87. [DOI] [PubMed] [Google Scholar]
- [202].Bruton A, Nauman J, Hanes D, Gard M, Senders A. Phosphatidylserine for the treatment of pediatric attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Altern Complement Med. 2021;27(4):312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Anheyer D, Lauche R, Schumann D, Dobos G, Cramer H. Herbal medicines in children with attention deficit hyperactivity disorder (ADHD): a systematic review. Complement Ther Med. 2017;30:14–23. [DOI] [PubMed] [Google Scholar]
- [204].Zwi M, Jones H, Thorgaard C, York A, Dennis JA. Parent training interventions for attention deficit hyperactivity disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst Rev. 2011;12:CD003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Montoya A, Colom F, Ferrin M. Is psychoeducation for parents and teachers of children and adolescents with ADHD efficacious? a systematic literature review. Eur Psychiatry. 2011;26(3):166–75. [DOI] [PubMed] [Google Scholar]
- [206].Storebø OJ, Elmose Andersen M, Skoog M, Joost Hansen S, Simonsen E, Pedersen N, et al. Social skills training for attention deficit hyperactivity disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst Rev. 2019;6:CD008223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Richardson M, Moore DA, Gwernan-Jones R, Thompson-Coon J, Ukoumunne O, Rogers M, et al. Non-pharmacological interventions for attention-deficit/hyperactivity disorder (ADHD) delivered in school settings: systematic reviews of quantitative and qualitative research. Health Technol Assess 2015;19(45):1–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Helmer A, Wechsler T, Gilboa Y. Equine-assisted Services for children with attention-deficit/hyperactivity disorder: a systematic review. J Altern Complement Med. 2021;27(6):477–88. [DOI] [PubMed] [Google Scholar]
- [209].Perez-Gomez J, Amigo-Gamero H, Collado-Mateo D, Barrios-Fernandez S, Munoz-Bermejo L, Garcia-Gordillo MA, et al. Equine-assisted activities and therapies in children with attention-deficit/hyperactivity disorder: a systematic review. J Psychiatr Mental Health Nurs. 2021;28:1079–91. [DOI] [PubMed] [Google Scholar]
- [210].Wilkes-Gillan S, Cordier R, Chen YW, Swanton R, Mahoney N, Trimboli C, et al. A systematic review of video-modelling interventions for children and adolescents with attention-deficit hyperactivity disorder. Aust Occup Ther J. 2021;68(5):454–71. [DOI] [PubMed] [Google Scholar]
- [211].Lee MS, Choi TY, Kim JI, Kim L, Ernst E. Acupuncture for treating attention deficit hyperactivity disorder: a systematic review and meta-analysis. Chin J Integr Med. 2011;17(4):257–60. [DOI] [PubMed] [Google Scholar]
- [212].Cerrillo-Urbina AJ, García-Hermoso A, Sánchez-López M, Pardo-Guijarro MJ, Santos Gómez JL, Martínez-Vizcaíno V. The effects of physical exercise in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis of randomized control trials. Child Care Health Dev. 2015;41(6):779–88. [DOI] [PubMed] [Google Scholar]
- [213].López-Pinar C, Martínez-Sanchís S, Carbonell-Vayá E, Fenollar-Cortés J, Sánchez-Meca J. Long-term Efficacy of psychosocial treatments for adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Front Psychol. 2018;9:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Lopez PL, Torrente FM, Ciapponi A, Lischinsky AG, Cetkovich-Bakmas M, Rojas JI, et al. Cognitive-behavioural interventions for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2018;3:CD010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Lopez-Pinar C, Martinez-Sanchis S, Carbonell-Vaya E, Sanchez-Meca J, Fenollar-Cortes J. Efficacy of nonpharmacological treatments on comorbid internalizing symptoms of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. J Atten Disord. 2020;24(3):456–78. [DOI] [PubMed] [Google Scholar]
- [216].Poissant H, Mendrek A, Talbot N, Khoury B, Nolan J. Behavioral and cognitive impacts of mindfulness-based interventions on adults with attention-deficit hyperactivity disorder: a systematic review. Behav Neurol. 2019;2019:5682050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Bruce C, Unsworth C, Tay R. A systematic review of the effectiveness of behavioural interventions for improving driving outcomes in novice drivers with attention deficit hyperactivity disorder (ADHD). Br J Occup Ther. 2014;77(7):348–57. [Google Scholar]
- [218].Salehinejad MA, Wischnewski M, Nejati V, Vicario CM, Nitsche MA. Transcranial direct current stimulation in attention-deficit hyperactivity disorder: a meta-analysis of neuropsychological deficits. PLoS One. 2019;14(4):e0215095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Yang KH, Lane HY, Chang YC, Tzang RF. Exploring the effects of pharmacological, psychosocial, and alternative/complementary interventions in children and adolescents with attention-deficit/hyperactivity disorder: meta-regression approach. Int J Neuropsychopharmacol. 2021;24(10):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Catala-Lopez F, Hutton B, Nunez-Beltran A, Page MJ, Ridao M, St-Gerons DM, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta-analyses of randomised trials. PLoS One. 2017;12(7):e0180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Chan E, Fogler JM, Hammerness PG. Treatment of attention-deficit/hyperactivity disorder in adolescents: a systematic review. JAMA. 2016;315(18):1997–2008. [DOI] [PubMed] [Google Scholar]
- [222].Yan LX, Wang SY, Yuan Y, Zhang JH. Effects of neurofeedback versus methylphenidate for the treatment of ADHD: systematic review and meta-analysis of head-to-head trials. Evid Based Ment Health. 2019;22(3):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Lan Y, Zhang LL, Luo R. Attention deficit hyperactivity disorder in children: comparative efficacy of traditional Chinese medicine and methylphenidate. J Int Med Res. 2009;37(3):939–48. [DOI] [PubMed] [Google Scholar]
- [224].Dijk HH, Wessels LM, Constanti M, van den Hoofdakker BJ, Hoekstra PJ, Groenman AP. Cost-effectiveness and cost utility of treatment of attention-deficit/hyperactivity disorder: a systematic review. J Child Adolesc Psychopharmacol. 2021;31(9):578–96. [DOI] [PubMed] [Google Scholar]
- [225].Wu EQ, Hodgkins P, Ben-Hamadi R, Setyawan J, Xie JP, Sikirica V, et al. Cost effectiveness of pharmacotherapies for attention-deficit hyperactivity disorder a systematic literature review. CNS Drugs. 2012;26(7):581–600. [DOI] [PubMed] [Google Scholar]
- [226].Lee YC, Yang HJ, Lee WT, Teng MJ. Do parents and children agree on rating a child’s HRQOL? a systematic review and meta-analysis of comparisons between children with attention deficit hyperactivity disorder and children with typical development using the PedsQL(TM). Disabil Rehabil. 2019;41(3):265–75. [DOI] [PubMed] [Google Scholar]
- [227].Lee YC, Yang HJ, Chen VC, Lee WT, Teng MJ, Lin CH, et al. Meta-analysis of quality of life in children and adolescents with ADHD: by both parent proxy-report and child self-report using PedsQL. Res Dev Disabil. 2016;51–52:160–72. [DOI] [PubMed]
- [228].Eccleston L, Williams J, Knowles S, Soulsby L. Adolescent experiences of living with a diagnosis of ADHD: a systematic review and thematic synthesis. Emot Behav Diffic. 2019;24(2):119–35. [Google Scholar]
- [229].Wong IYT, Hawes DJ, Clarke S, Kohn MR, Dar-Nimrod I. Perceptions of ADHD among diagnosed children and their parents: a systematic review using the common-sense model of illness representations. Clin Child Fam Psychol Rev. 2018;21(1):57–93. [DOI] [PubMed] [Google Scholar]
- [230].Bjerrum MB, Pedersen PU, Larsen P. Living with symptoms of attention deficit hyperactivity disorder in adulthood: a systematic review of qualitative evidence. JBI Database System Rev Implement Rep. 2017;15(4):1080–153. [DOI] [PubMed] [Google Scholar]
- [231].Rashid MA, Lovick S, Llanwarne NR. Medication-taking experiences in attention deficit hyperactivity disorder: a systematic review. Fam Pract. 2018;35(2):142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Moore DA, Gwernan-Jones R, Richardson M, Racey D, Rogers M, Stein K, et al. The experiences of and attitudes toward non-pharmacological interventions for attention-deficit/hyperactivity disorder used in school settings: a systematic review and synthesis of qualitative research. Emot Behav Diffic 2016;21(1):61–82. [Google Scholar]
- [233].Laugesen B, Groenkjaer M. Parenting experiences of living with a child with attention deficit hyperactivity disorder: a systematic review of qualitative evidence. JBI Database System Rev Implement Rep. 2015;13(11):169–234. [DOI] [PubMed] [Google Scholar]
- [234].Martin CA, Papadopoulos N, Chellew T, Rinehart NJ, Sciberras E. Associations between parenting stress, parent mental health and child sleep problems for children with ADHD and ASD: Systematic review. Res Dev Disabil. 2019;93:103463. [DOI] [PubMed] [Google Scholar]
- [235].Craig F, Savino R, Fanizza I, Lucarelli E, Russo L, Trabacca A. A systematic review of coping strategies in parents of children with attention deficit hyperactivity disorder (ADHD). Res Dev Disabil. 2020;98:103571. [DOI] [PubMed] [Google Scholar]
- [236].French B, Sayal K, Daley D. Barriers and facilitators to understanding of ADHD in primary care: a mixed-method systematic review. Eur Child Adolesc Psychiatry. 2019;28(8):1037–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Gwernan-Jones R, Moore DA, Cooper P, Russell AE, Richardson M, Rogers M, et al. A systematic review and synthesis of qualitative research: the influence of school context on symptoms of attention deficit hyperactivity disorder. Emot Behav Diffic. 2016;21(1):83–100. [Google Scholar]
- [238].Ogle RR, Frazier SL, Helseth SA, Cromer K, Lesperance N. Does poverty moderate psychosocial treatment Efficacy for ADHD? a systematic review. J Atten Disord. 2020;24(10):1377–91. [DOI] [PubMed] [Google Scholar]
- [239].Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. The world federation of ADHD international consensus statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387(10024):1240–50. [DOI] [PubMed] [Google Scholar]
- [241].ADHD. Nat Rev Dis Primers. 2015;1(1):15027. [DOI] [PubMed] [Google Scholar]
- [242].Liu YS, Dai X, Wu W, Yuan FF, Gu X, Chen JG, et al. The association of SNAP25 gene polymorphisms in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Mol Neurobiol. 2017;54(3):2189–200. [DOI] [PubMed] [Google Scholar]
- [243].Grünblatt E, Werling AM, Roth A, Romanos M, Walitza S. Association study and a systematic meta-analysis of the VNTR polymorphism in the 3’-UTR of dopamine transporter gene and attention-deficit hyperactivity disorder. J Neural Transm. 2019;126(4):517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Hou YW, Xiong P, Gu X, Huang X, Wang M, Wu J. Association of Serotonin Receptors with attention deficit hyperactivity disorder: a systematic review and meta-analysis. Curr Med Sci. 2018;38(3):538–51. [DOI] [PubMed] [Google Scholar]
- [245].Lee YH, Song GG. Meta-analysis of case-control and family-based associations between the 5-HTTLPR L/S polymorphism and susceptibility to ADHD. J Atten Disord. 2018;22(9):901–8. [DOI] [PubMed] [Google Scholar]
- [246].Lee YH, Song GG. BDNF 196 G/A and COMT Val158Met polymorphisms and susceptibility to ADHD: a meta-analysis. J Atten Disord. 2018;22(9):872–7. [DOI] [PubMed] [Google Scholar]
- [247].Srivastav S, Walitza S, Grünblatt E. Emerging role of miRNA in attention deficit hyperactivity disorder: a systematic review. Atten Defic Hyperact Disord. 2018;10(1):49–63. [DOI] [PubMed] [Google Scholar]
- [248].Forero DA, Arboleda GH, Vasquez R, Arboleda H. Candidate genes involved in neural plasticity and the risk for attention-deficit hyperactivity disorder: a meta-analysis of 8 common variants. J Psychiatry Neurosci. 2009;34(5):361–6. [PMC free article] [PubMed] [Google Scholar]
- [249].Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti-Hümmer A, et al. Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology. 2010;35(3):656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Kaiser ML, Schoemaker MM, Albaret JM, Geuze RH. What is the evidence of impaired motor skills and motor control among children with attention deficit hyperactivity disorder (ADHD)? systematic review of the literature. Res Dev Disabil. 2015;36c:338–57. [DOI] [PubMed] [Google Scholar]
- [251].Lei D, Du M, Wu M, Chen T, Huang X, Du X, et al. Functional MRI reveals different response inhibition between adults and children with ADHD. Neuropsychology. 2015;29:874–81. [DOI] [PubMed] [Google Scholar]
- [252].Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43(2):434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Bonvicini C, Faraone SV, Scassellati C. Attention-deficit hyperactivity disorder in adults: a systematic review and meta-analysis of genetic, pharmacogenetic and biochemical studies. Mol Psychiatry. 2016;21(7):872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Lonergan A, Doyle C, Cassidy C, MacSweeney Mahon S, Roche RAP, Boran L, et al. A meta-analysis of executive functioning in dyslexia with consideration of the impact of comorbid ADHD. J Cogn Psychol. 2019;31(7):725–49. [Google Scholar]
- [256].McGrath LM, Stoodley CJ. Are there shared neural correlates between dyslexia and ADHD? a meta-analysis of voxel-based morphometry studies. J Neurodev Disord. 2019;11(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Machado-Nascimento N, Melo EKA, Lemos SM. Speech-language pathology findings in attention deficit hyperactivity disorder: a systematic literature review. CoDAS. 2016;28(6):833–42. [DOI] [PubMed] [Google Scholar]
- [258].Ditrich I, Philipsen A, Matthies S. Borderline personality disorder (BPD) and attention deficit hyperactivity disorder (ADHD) revisited – a review-update on common grounds and subtle distinctions. Borderline Personal Disord Emot Dysregulation. 2021;8(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Nimmo-Smith V, Merwood A, Hank D, Brandling J, Greenwood R, Skinner L, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529–41. [DOI] [PubMed] [Google Scholar]
- [260].Mykletun A, Widding-Havneraas T, Chaulagain A, Lyhmann I, Bjelland I, Halmøy A, et al. Causal modelling of variation in clinical practice and long-term outcomes of ADHD using Norwegian registry data: the ADHD controversy project. BMJ Open. 2021;11(1):e041698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Lyhmann I, Widding-Havneraas T, Zachrisson HD, Bjelland I, Chaulagain A, Mykletun A, et al. Variation in attitudes toward diagnosis and medication of ADHD: a survey among clinicians in the Norwegian child and adolescent mental health services. Eur Child Adolesc Psychiatry. 2022. 10.1007/s00787-022-02110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Patel V, Maj M, Flisher AJ, De Silva MJ, Koschorke M, Prince M. Reducing the treatment gap for mental disorders: a WPA survey. World Psychiatry. 2010;9(3):169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Sadeniemi M, Almeda N, Salinas-Pérez JA, Gutiérrez-Colosía MR, García-Alonso C, Ala-Nikkola T, et al. A comparison of mental health care systems in Northern and Southern Europe: a service mapping study. Int J Environ Res Public Health. 2018;15(6):1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Madsen KB, Ersbøll AK, Olsen J, Parner E, Obel C. Geographic analysis of the variation in the incidence of ADHD in a country with free access to healthcare: a Danish cohort study. Int J Health Geogr. 2015;14(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Johnson A, Stukel TA. Medical practice variations. Cham: Springer; 2016. [Google Scholar]
- [266].Xu G, Strathearn L, Liu B, Yang B, Bao W. Twenty-year trends in diagnosed attention-deficit/hyperactivity disorder among US children and adolescents, 1997–2016. JAMA Netw Open. 2018;1(4):e181471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Rice F, Langley K, Woodford C, Davey Smith G, Thapar A. Identifying the contribution of prenatal risk factors to offspring development and psychopathology: what designs to use and a critique of literature on maternal smoking and stress in pregnancy. Dev Psychopathol. 2018;30(3):1107–28. [DOI] [PubMed] [Google Scholar]
- [268].Haan E, Sallis HM, Zuccolo L, Labrecque J, Ystrom E, Reichborn-Kjennerud T, et al. Prenatal smoking, alcohol and caffeine exposure and maternal-reported attention deficit hyperactivity disorder symptoms in childhood: triangulation of evidence using negative control and polygenic risk score analyses. Addiction. 2022;117(5):1458–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Havdahl A, Wootton RE, Leppert B, Riglin L, Ask H, Tesli M, et al. Associations between pregnancy-related predisposing factors for offspring neurodevelopmental conditions and parental genetic liability to attention-deficit/hyperactivity disorder, autism, and schizophrenia: The Norwegian mother, father and child cohort study (MoBa). JAMA Psychiatry. 2022;79(8):799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45(6):1866–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Li L, Chang Z, Sun J, Jangmo A, Zhang L, Andersson LM, et al. Association between pharmacological treatment of attention-deficit/hyperactivity disorder and long-term unemployment among working-age individuals in Sweden. JAMA Netw Open. 2022;5(4):e226815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chaulagain et al. supplementary material