Abstract
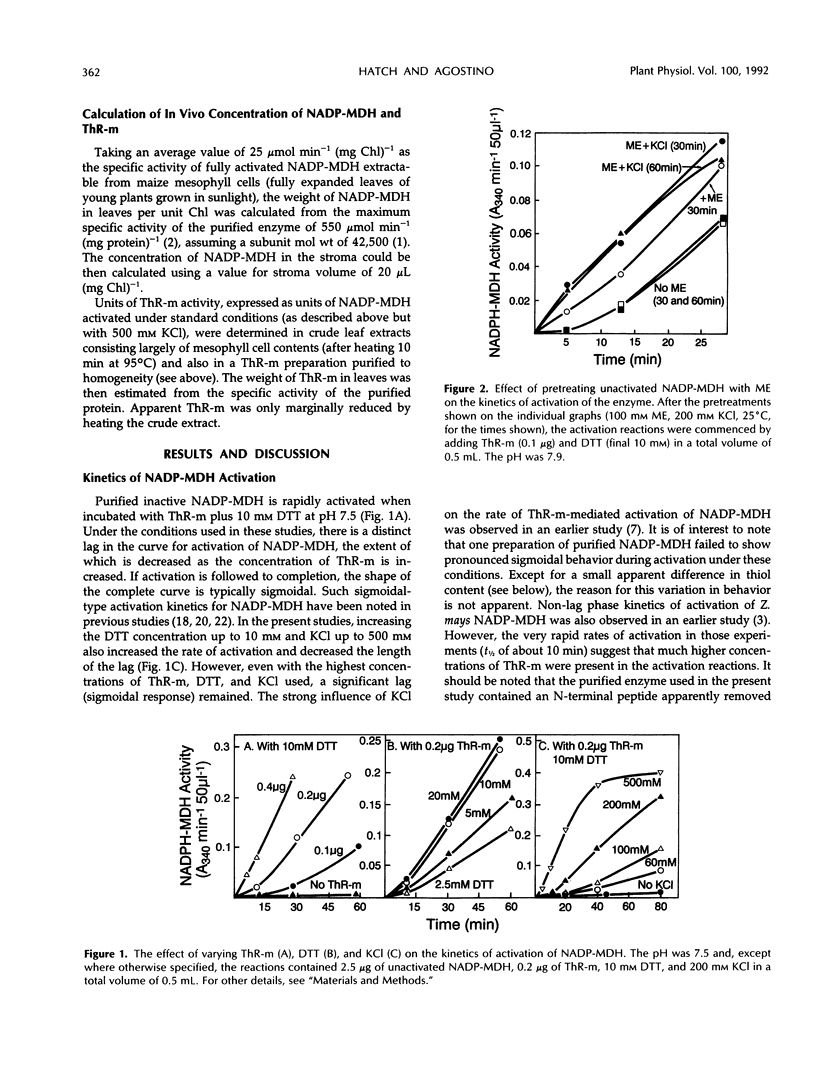
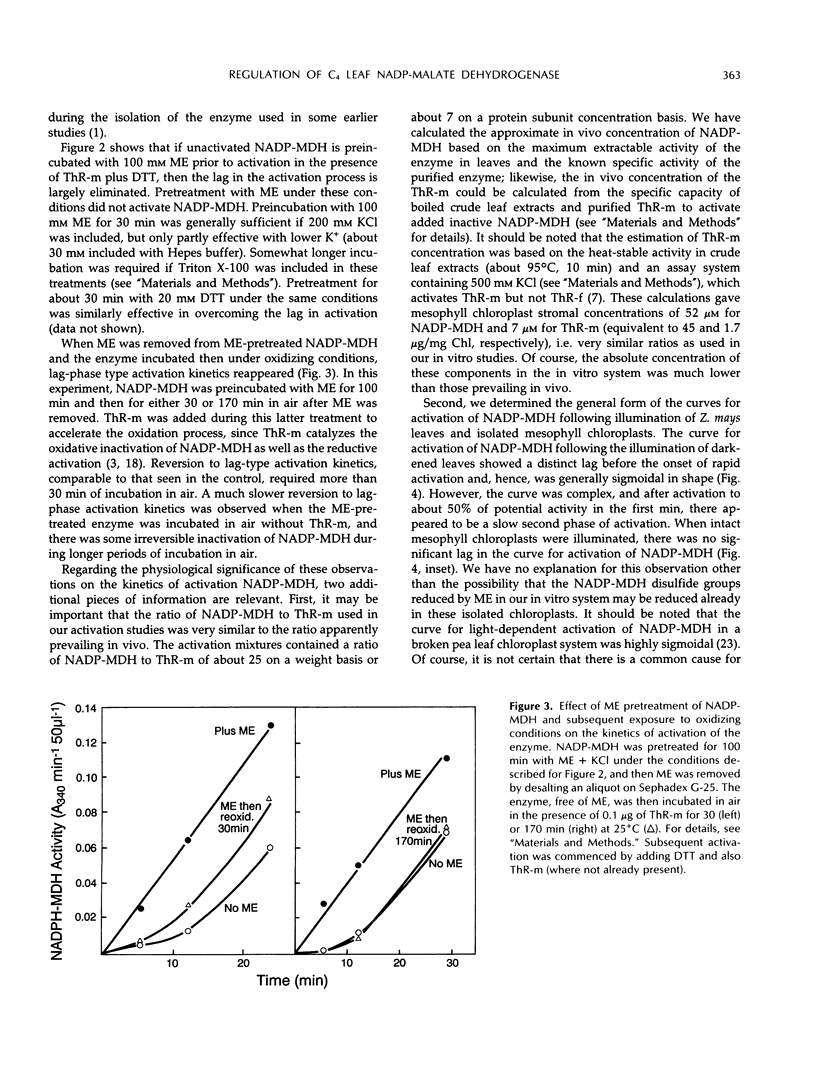
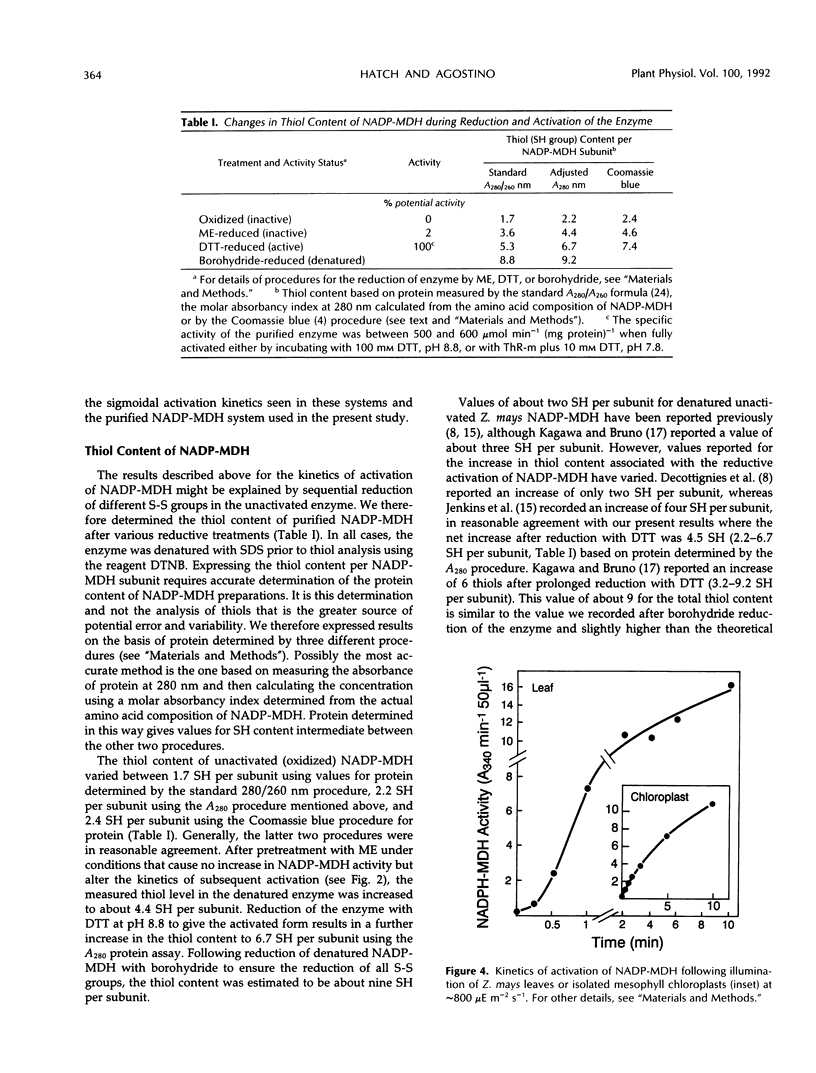
The time course of thioredoxin-mediated reductive activation of isolated Zea mays nicotinamide adenine dinucleotide phosphatemalate dehydrogenase is highly sigmoidal in nature. We examined the factors affecting these kinetics, including the thiol-disulfide status of unactivated and activated forms of the enzyme. The maximum steady rate of activation was increased, and the length of the lag in activation decreased, as the concentrations of thioredoxin-m, dithiothreitol, and KCl were increased. The lag in activation (sigmoidicity) was eliminated by preincubating the unactivated enzyme with 100 mm 2-mercaptoethanol; this pretreatment did not activate the enzyme. Unactivated nicotinamide adenine dinucleotide phosphate-malate dehydrogenase was found to contain approximately two SH groups per subunit, increasing to about four SH per subunit after pretreatment with 2-mercaptoethanol and six SH per subunit after activation by incubating the enzyme with dithiothreitol. We suggest that reduction of one particular higher redox potential disulfide group in unactivated nicotinamide adenine dinucleotide phosphate-malate dehydrogenase facilitates the subsequent reduction of the critical S-S group (regulatory S-S) necessary to generate the active form of the enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostino A., Jeffrey P., Hatch M. D. Amino Acid Sequence and Molecular Weight of Native NADP Malate Dehydrogenase from the C(4) Plant Zea mays. Plant Physiol. 1992 Apr;98(4):1506–1510. doi: 10.1104/pp.98.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Hatch M. D. Regulation of C4 photosynthesis: physical and kinetic properties of active (dithiol) and inactive (disulfide) NADP-malate dehydrogenase from Zea mays. Arch Biochem Biophys. 1983 Dec;227(2):406–415. doi: 10.1016/0003-9861(83)90470-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B. Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch Biochem Biophys. 1991 Jul;288(1):1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- Cavallini D., Graziani M. T., Dupré S. Determination of disulphide groups in proteins. Nature. 1966 Oct 15;212(5059):294–295. doi: 10.1038/212294a0. [DOI] [PubMed] [Google Scholar]
- Crawford N. A., Yee B. C., Hutcheson S. W., Wolosiuk R. A., Buchanan B. B. Enzyme regulation in C4 photosynthesis: purification, properties, and activities of thioredoxins from C4 and C3 plants. Arch Biochem Biophys. 1986 Jan;244(1):1–15. doi: 10.1016/0003-9861(86)90088-3. [DOI] [PubMed] [Google Scholar]
- Decottignies P., Schmitter J. M., Miginiac-Maslow M., Le Maréchal P., Jacquot J. P., Gadal P. Primary structure of the light-dependent regulatory site of corn NADP-malate dehydrogenase. J Biol Chem. 1988 Aug 25;263(24):11780–11785. [PubMed] [Google Scholar]
- Gill S. C., von Hippel P. H. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989 Nov 1;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Jacquot J. P., Buchanan B. B. Enzyme Regulation in C(4) Photosynthesis : PURIFICATION AND PROPERTIES OF THIOREDOXIN-LINKED NADP-MALATE DEHYDROGENASE FROM CORN LEAVES. Plant Physiol. 1981 Aug;68(2):300–304. doi: 10.1104/pp.68.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot J. P., Gadal P., Nishizawa A. N., Yee B. C., Crawford N. A., Buchanan B. B. Enzyme regulation in C4 photosynthesis: mechanism of activation of NADP-malate dehydrogenase by reduced thioredoxin. Arch Biochem Biophys. 1984 Jan;228(1):170–178. doi: 10.1016/0003-9861(84)90058-4. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Bruno P. L. NADP-malate dehydrogenase from leaves of Zea mays: purification and physical, chemical, and kinetic properties. Arch Biochem Biophys. 1988 Feb 1;260(2):674–695. doi: 10.1016/0003-9861(88)90497-3. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Hatch M. D. Regulation of C4 photosynthesis: characterization of a protein factor mediating the activation and inactivation of NADP-malate dehydrogenase. Arch Biochem Biophys. 1977 Nov;184(1):290–297. doi: 10.1016/0003-9861(77)90353-8. [DOI] [PubMed] [Google Scholar]


