Abstract
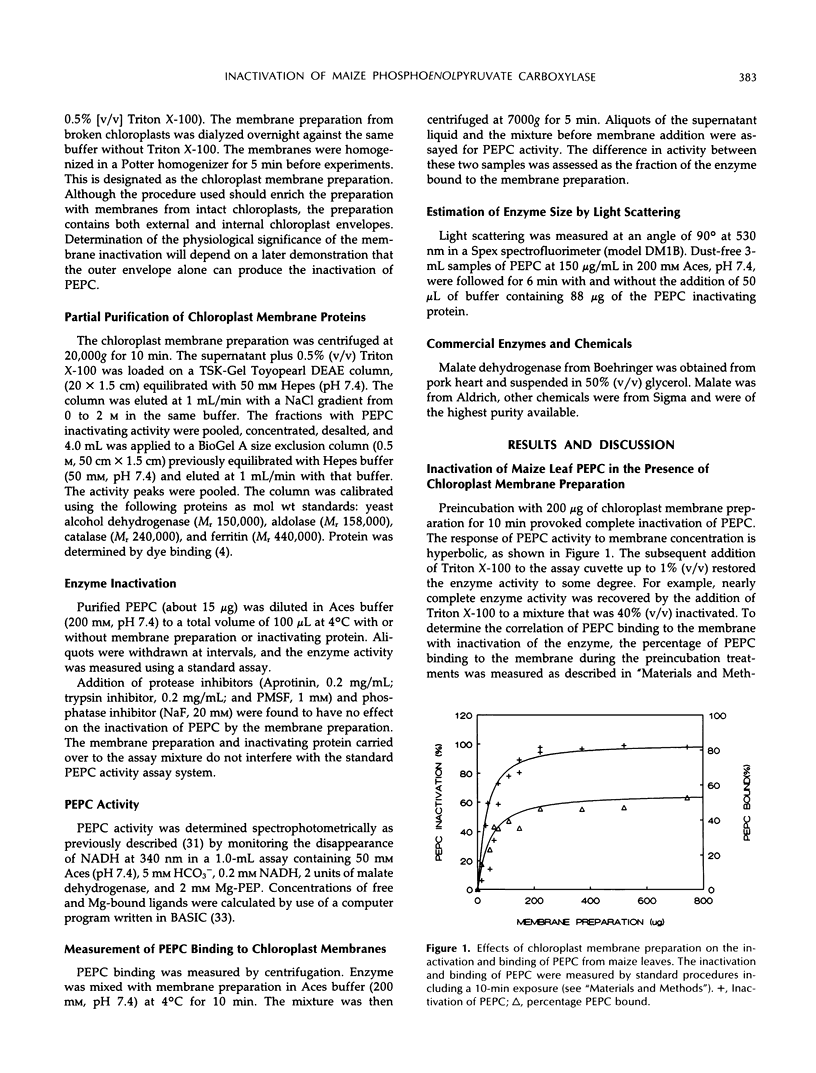
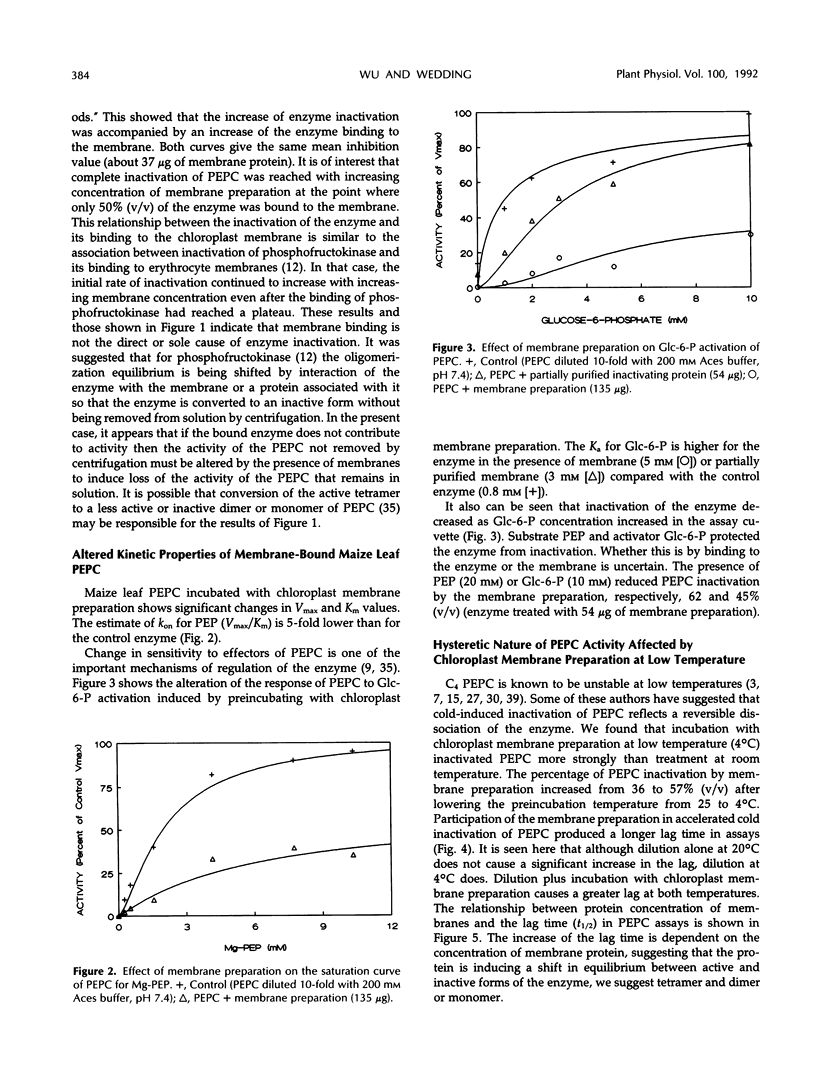
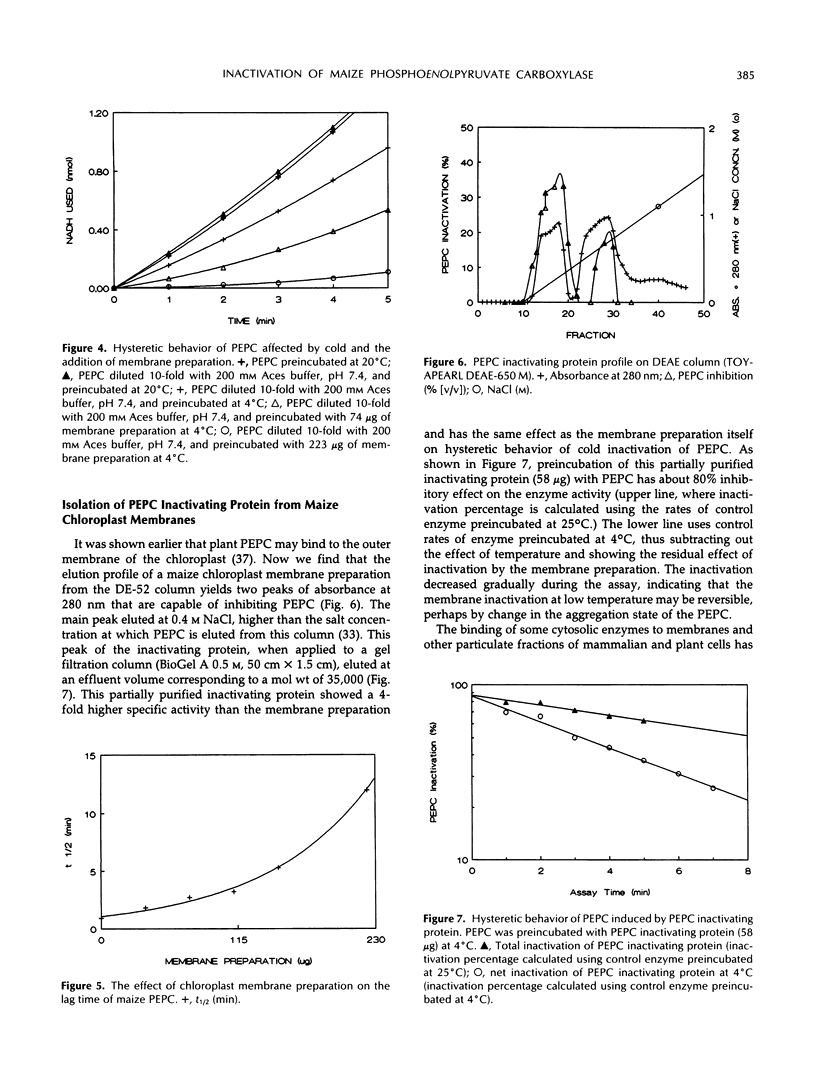
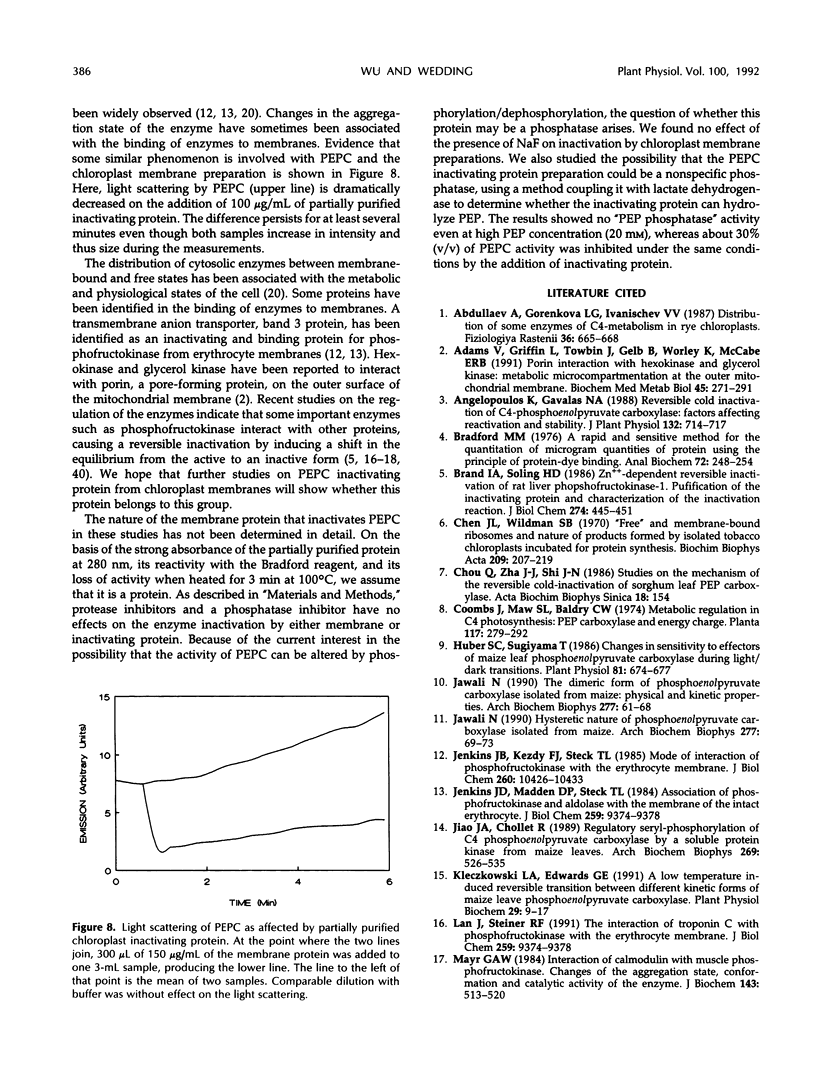
Phosphoenolpyruvate carboxylase (PEPC) purified from maize (Zea mays L.) leaves associates with maize leaf chloroplast membrane in vitro. The binding of PEPC to the membrane results in enzyme inactivation. A protein isolated from a maize leaf chloroplast membrane preparation inactivated PEPC. Treatment with membrane preparation or with partially purified inactivating protein accelerates PEPC inactivation at low temperature (4°C). Interaction of PEPC with chloroplast membrane or inactivating protein may inactivate the enzyme by influencing dissociation of the enzyme active tetramer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams V., Griffin L., Towbin J., Gelb B., Worley K., McCabe E. R. Porin interaction with hexokinase and glycerol kinase: metabolic microcompartmentation at the outer mitochondrial membrane. Biochem Med Metab Biol. 1991 Jun;45(3):271–291. doi: 10.1016/0885-4505(91)90032-g. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen J. L., Wildman S. G. "Free" and membrane-bound ribosomes, and nature of products formed by isolated tobacco chloroplasts incubated for protein synthesis. Biochim Biophys Acta. 1970 May 21;209(1):207–219. doi: 10.1016/0005-2787(70)90677-5. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Sugiyama T. Changes in Sensitivity to Effectors of Maize Leaf Phosphoenolypyruvate Carboxylase during Light/Dark Transitions. Plant Physiol. 1986 Jun;81(2):674–677. doi: 10.1104/pp.81.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawali N. Hysteretic nature of phosphoenolpyruvate carboxylase isolated from maize. Arch Biochem Biophys. 1990 Feb 15;277(1):69–73. doi: 10.1016/0003-9861(90)90551-9. [DOI] [PubMed] [Google Scholar]
- Jawali N. The dimeric form of phosphoenolpyruvate carboxylase isolated from maize: physical and kinetic properties. Arch Biochem Biophys. 1990 Feb 15;277(1):61–68. doi: 10.1016/0003-9861(90)90550-i. [DOI] [PubMed] [Google Scholar]
- Jenkins J. D., Kezdy F. J., Steck T. L. Mode of interaction of phosphofructokinase with the erythrocyte membrane. J Biol Chem. 1985 Sep 5;260(19):10426–10433. [PubMed] [Google Scholar]
- Jenkins J. D., Madden D. P., Steck T. L. Association of phosphofructokinase and aldolase with the membrane of the intact erythrocyte. J Biol Chem. 1984 Aug 10;259(15):9374–9378. [PubMed] [Google Scholar]
- Jiao J. A., Chollet R. Regulatory seryl-phosphorylation of C4 phosphoenolpyruvate carboxylase by a soluble protein kinase from maize leaves. Arch Biochem Biophys. 1989 Mar;269(2):526–535. doi: 10.1016/0003-9861(89)90136-7. [DOI] [PubMed] [Google Scholar]
- Mayr G. W. Interaction of calmodulin with muscle phosphofructokinase. Changes of the aggregation state, conformation and catalytic activity of the enzyme. Eur J Biochem. 1984 Sep 17;143(3):513–520. doi: 10.1111/j.1432-1033.1984.tb08400.x. [DOI] [PubMed] [Google Scholar]
- Mayr G. W. Interaction of calmodulin with muscle phosphofructokinase. Interplay with metabolic effectors of the enzyme under physiological conditions. Eur J Biochem. 1984 Sep 17;143(3):521–529. doi: 10.1111/j.1432-1033.1984.tb08401.x. [DOI] [PubMed] [Google Scholar]
- Moorhead G. B., Plaxton W. C. Binding of glycolytic enzymes to a particulate fraction in carrot and sugar beet storage roots : dependence on metabolic state. Plant Physiol. 1988 Feb;86(2):348–351. doi: 10.1104/pp.86.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngam-Ek A., Seery T. A., Amis E. J., Grover S. D. Malate-Induced Hysteresis of Phosphoenolpyruvate Carboxylase from Crassula argentea. Plant Physiol. 1989 Nov;91(3):954–960. doi: 10.1104/pp.91.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R., Gonzalez D. H., Podesta F. E., Andreo C. S. Changes in the quaternary structure of phosphoenolpyruvate carboxylase induced by ionic strength affect its catalytic activity. Eur J Biochem. 1987 May 4;164(3):661–666. doi: 10.1111/j.1432-1033.1987.tb11177.x. [DOI] [PubMed] [Google Scholar]
- Walker G. H., Ku M. S., Edwards G. E. Catalytic activity of maize leaf phosphoenolpyruvate carboxylase in relation to oligomerization. Plant Physiol. 1986 Apr;80(4):848–855. doi: 10.1104/pp.80.4.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K., Meyer C. R. Activation of higher plant phosphoenolpyruvate carboxylases by glucose-6-phosphate. Plant Physiol. 1989 Jun;90(2):648–652. doi: 10.1104/pp.90.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K., Meyer C. R. Inhibition of phosphoenolpyruvate carboxylase by malate. Plant Physiol. 1990 Feb;92(2):456–461. doi: 10.1104/pp.92.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeford K. O., Wu M. X., Meyer C. R., Wedding R. T. The role of oligomerization in regulation of maize phosphoenolpyruvate carboxylase activity. Influence of Mg-PEP and malate on the oligomeric equilibrium of PEP carboxylase. Biochem Biophys Res Commun. 1990 Apr 30;168(2):778–785. doi: 10.1016/0006-291x(90)92389-h. [DOI] [PubMed] [Google Scholar]
- Winter K. Day/Night Changes in the Sensitivity of Phosphoenolpyruvate Carboxylase to Malate during Crassulacean Acid Metabolism. Plant Physiol. 1980 May;65(5):792–796. doi: 10.1104/pp.65.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Meyer C. R., Willeford K. O., Wedding R. T. Regulation of the aggregation state of maize phosphoenolpyruvate carboxylase: evidence from dynamic light-scattering measurements. Arch Biochem Biophys. 1990 Sep;281(2):324–329. doi: 10.1016/0003-9861(90)90451-4. [DOI] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Diurnal regulation of phosphoenolpyruvate carboxylase from crassula. Plant Physiol. 1985 Mar;77(3):667–675. doi: 10.1104/pp.77.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Regulation of Phosphoenolpyruvate Carboxylase from Crassula argentea: Further Evidence on the Dimer-Tetramer Interconversion. Plant Physiol. 1987 Aug;84(4):1080–1083. doi: 10.1104/pp.84.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Regulation of phosphoenolpyruvate carboxylase from Crassula by interconversion of oligomeric forms. Arch Biochem Biophys. 1985 Aug 1;240(2):655–662. doi: 10.1016/0003-9861(85)90073-6. [DOI] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Temperature Effects on Phosphoenolpyruvate Carboxylase from a CAM and a C(4) Plant : A Comparative Study. Plant Physiol. 1987 Oct;85(2):497–501. doi: 10.1104/pp.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. Z., Malencik D. A., Anderson S. R. Protein-induced inactivation and phosphorylation of rabbit muscle phosphofructokinase. Biochemistry. 1991 Feb 26;30(8):2204–2216. doi: 10.1021/bi00222a026. [DOI] [PubMed] [Google Scholar]


