Abstract
With the development of organoboron chemistry, boron-centered radicals have become increasingly attractive. However, their synthetic applications remain limited in that they have been used only as substrates for addition reactions or as initiators for catalytic reactions. We have achieved a new reaction pathway in which tetraarylborate salts are used as precursors for aryl radicals via boron radicals, by introducing a simple activation reagent. In addition, we carried out a diverse array of transformations involving these aryl radical precursors, which allowed the construction of new C–B, C–C, and C–X bonds in the presence of visible light.
Short abstract
A reaction pathway is achieved in which tetraarylborate salts are used as precursors for aryl radicals via boron radicals, and transformations are carried out involving these aryl radical precursors.
Introduction
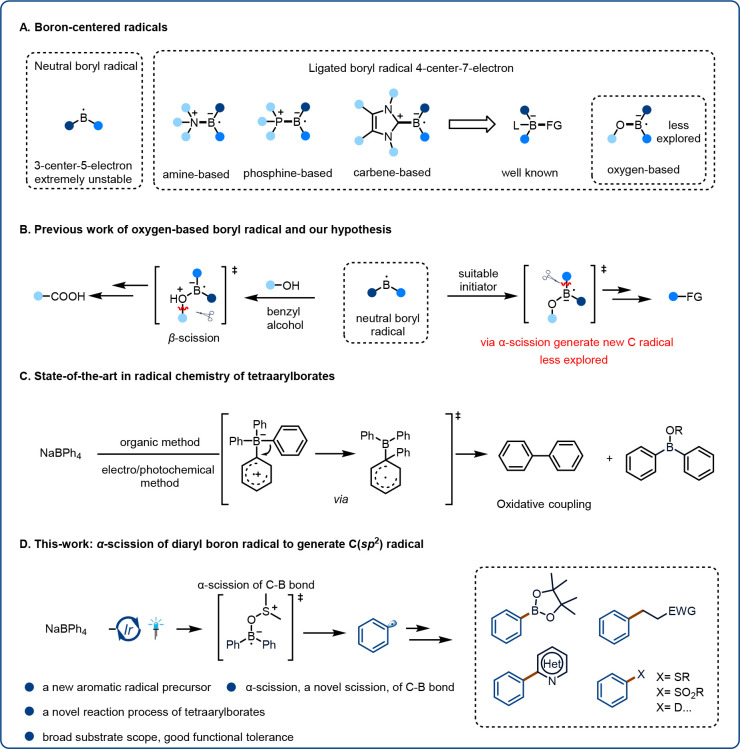
Radicals, which can be generated from many feedstock chemicals, are among the most fundamental intermediates in synthetic chemistry and have become useful tools for developing novel methodologies.1−12 With the development of organoboron chemistry, boron-centered radicals have become more and more attractive, but their synthetic applications remain limited.13−16 For example, the applications of neutral boryl radicals, which are three-center–five-electron radicals, are limited because of their extreme electron deficiency (Figure 1A). In contrast, four-center–seven-electron boryl radicals ligated with a Lewis base (usually a carbene, a phosphine, or an amine) are relatively stable and have been extensively studied.17−25 These Lewis base-based boryl radicals are known to react with alkenes and heteroaromatic rings, and such reactions have been used to modify drug molecules.26−35 In recent years, the groups of Wang4 and Li5 have reported some elegant uses of amine-based boron free radicals as catalysts.34,35 In 2022, Xia’s group7 reported a method for alkyl radical generation by direct splitting of the C–O bonds of alcohol–boron radical intermediates; in these reactions, various alcohols were successfully used as alkyl radical precursors (Figure 1B).
Figure 1.
From inspiration to reaction design. (A) Typical boron-centered radicals. (B) Boryl radical activation by a C(sp3)–OH bond, β-scission, and our hypothesis. (C) State of the art in the radical chemistry of tetraarylborates. (D) α-Scission of a diaryl boryl radical to generate a C(sp2) radical.
Despite the beautiful work that has been accomplished with boron radicals, they have been used only as substrates for addition reactions or as initiators for catalytic reactions, which is still limited. We envisioned that boron free radicals with unique electronic properties could have more special reaction types. For example, we hypothesized that when coordinated by a simple reagent, neutral boron-centered radicals would undergo α-scission to generate carbon radicals (Figure 1B). To test our hypothesis, we needed to consider several criteria. First, the precursor of the neutral boron radical should be inexpensive, stable, and readily available. Second, the conditions for producing the radical should be mild and operationally simple. Third, both the neutral boron radical and the reagent-coordinated boron radical should be weakly nucleophilic and should not readily participate in addition reactions. If we could satisfy these criteria, we would be able expand the application scope of boron radicals and provide new free-radical precursors for the development of new synthetic methodologies.
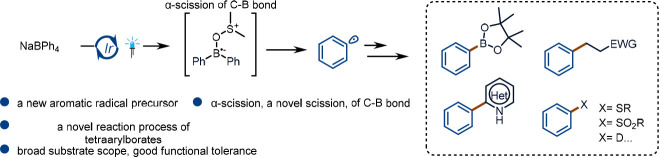
In chemistry pioneered by Xia36 and others37−47 sodium tetraphenylborate, which can be easily synthesized or purchased commercially, can be oxidized under electrochemical, thermal, or photochemical conditions to produce biphenyl compounds (Figure 1C). In these reactions, the most electron-rich aryl moiety undergoes one-electron oxidation; the resulting intermediate undergoes an intramolecular 1,2-aryl shift to afford a cyclohexadienyl radical, and, finally, departure of biphenyl generates a diaryl boron radical, which is captured by other chemical species in the reaction system. Inspired by this work, we thought that tetraarylborate salts would be an ideal source of diaryl boron radicals because boron radicals can be produced under mild conditions and the byproducts do not affect the reaction system. Furthermore, we envisioned that after the introduction of a suitable initiator, the diaryl boron radicals would undergo α-scission of the C–B bond to produce aryl radicals in situ. Indeed, we herein report a novel strategy for the generation of aryl radicals from sodium tetraarylborates, which have previously been used for aryl coupling reactions,37−47 enabled by introduction of a suitable initiator to induce α-scission of the C–B bond (Figure 1D).
Results and Discussion
Given the importance of alkylboron compounds as synthetic precursors for a wide range of valuable functional groups,48 we first applied the above-described strategy to a boronization reaction with the goal of identifying the most suitable initiator and optimizing the reaction conditions. For our initial experiments, we chose sodium tetraphenylborate (1a) and the boronating reagent B2pin2 (2) as model substrates (Table 1). A solution of the substrates in N,N-dimethylacetamide (DMA, [1a] = 0.2 M) containing methanol (2.0 equiv) as an activation reagent, Co(dmgH)2pyCl (20 mol %) as a transition-metal catalyst, and Ir[dF(CF3)ppy]2(dtbbpy)PF6 (2 mol %) as a photocatalyst was irradiated with a 36 W blue LED at room temperature under an air atmosphere for 24 h. Unfortunately, no borylation products were detected under these conditions (entry 1).
Table 1. Optimization of Conditions for Borylation of Sodium Tetraphenylborate (1a) with Boronating Reagent B2pin2 (2)a.

| entry | activation reagent | catalyst or oxidation reagent | yield (%)b |
|---|---|---|---|
| 1 | methanol | Co(dmgH)2pyCl | NR |
| 2 | phenol | Co(dmgH)2pyCl | NR |
| 3 | N-methyl-2-pyrrolidone | Co(dmgH)2pyCl | NR |
| 4 | DMSO | Co(dmgH)2pyCl | 19 |
| 5 | DMSO | Co(dmgH)2Cl2 | trace |
| 6 | DMSO | Co(dmgH)2(4-CO2Et)PyCl | trace |
| 7c | DMSO | (NH4)2S2O8 | 32 |
| 8d | DMSO | (NH4)2S2O8 | 47 |
| 9e | DMSO | (NH4)2S2O8 | 75 |
| 10f | DMSO | (NH4)2S2O8 | NR |
| 11c | none | (NH4)2S2O8 | NR |
Reaction conditions, unless otherwise noted: 1a (0.4 mmol), 2 (0.8 mmol), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (0.008 mmol), activation reagent (0.8 mmol, 2.0 equiv), catalyst (0.08 mmol, 0.2 equiv), N,N-dimethylacetamide (DMA, 2 mL), air, 36 W blue LED, rt, 24 h.
Isolated yields are provided.
(NH4)2S2O8 (0.8 mmol, 2.0 equiv).
(NH4)2S2O8 (0.8 mmol, 2.0 equiv), DMSO (1.6 mmol, 4.0 equiv).
(NH4)2S2O8 (0.8 mmol, 2.0 equiv), 5:1 (v/v) DMSO/DMA (2.0 mL).
No light or no photocatalysis.
However, when we tested other activation reagents (entries 2–4 and the Supporting Information (SI)), we were delighted to find that when DMSO was present, borylated product 3a was obtained in 19% yield (entry 4), which confirmed the feasibility of our strategy. In addition, we screened two different Co catalysts, but they afforded only a trace of the desired product (entries 5 and 6). Surprisingly, however, when we replaced the transition-metal catalyst with the inorganic oxidant (NH4)2S2O8 (2.0 equiv), the yield of 3a increased from 19% to 32% (entry 7). Given this promising result, we carried out reactions with this oxidant and different amounts of the activation reagent (DMSO, entries 8 and 9). These experiments revealed that when DMSO was the solvent (2 mL of 5:1 [v/v] DMSO/DMA), the yield of 3a increased from 32% to 75% (entry 9). Control experiments proved that the photocatalyst and light (entry 10) and the activation reagent (entry 11) were essential for the transformation.
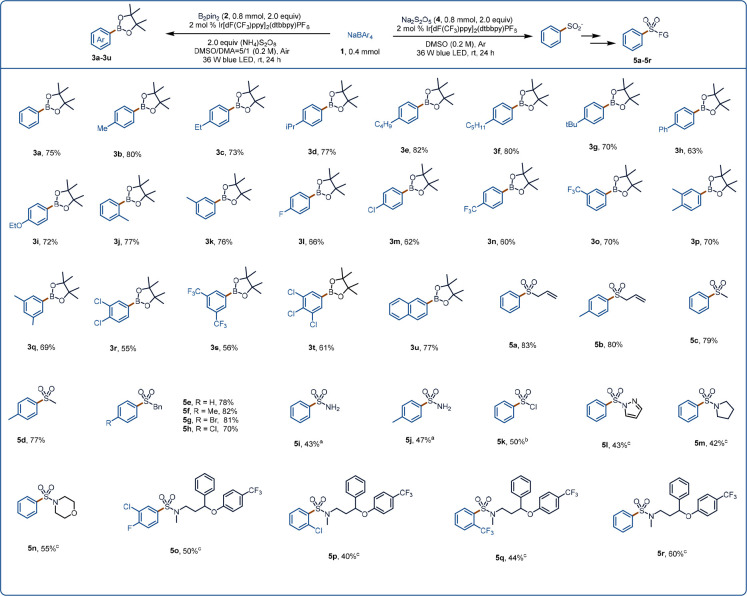
Using the optimized conditions (Table 1, entry 9), we evaluated the substrate scope of the photocatalytic borylation reaction by testing a series of these new aryl radical precursors (Figure 2). A wide range of sodium tetraarylborates were viable, furnishing desired aryl boronic ester products 3. Tetraarylborates with an alkyl substituent were suitable substrates, giving 3b–3g in 70–82% yields. Substrates with other electron-donating groups (i.e., phenyl [3h] and alkoxy [3i]) were also compatible with the reaction conditions. The yields were approximately the same regardless of the position of the substituent (compare 3b, 3j, and 3k). Tetraarylborates with electron-withdrawing substituents were converted to the corresponding boronic esters (3l–3o) in moderate yields, as were disubstituted tetraarylborates (3p–3s). A trisubstituted tetraarylborate was tolerated as well (3t, 61%). Even sodium tetranaphthylborate gave the corresponding product (3u, 77%).
Figure 2.
Substrate scope. Borylation conditions: 1 (0.4 mmol), B2pin2 (2, 0.8 mmol, 2.0 equiv), (NH4)2S2O8 (0.8 mmol, 2.0 equiv), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (2 mol %), 5:1 (v/v) DMSO/DMA (2.0 mL), air, 36 W blue LED, rt, 24 h. Sulfinylation conditions: 1 (0.4 mmol), Na2S2O5 (4, 0.8 mmol, 2.0 equiv), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (2 mol %), DMSO (2.0 mL), Ar, 36 W blue LED, 24 h; then addition of NaHCO3 (2.0 equiv), EtOH (1 mL), and R–X (1.5 equiv), stirring at rt for 16 h. See the SI for complete experimental details. DMA, N,N-dimethylacetamide. aOne-pot access to sulfonamides; see the SI for complete experimental details. bOne-pot access to a sulfonylhalide; see the SI for complete experimental details. cTwo-step sulfonamide synthesis via a sulfonyl chloride; see the SI for complete experimental details.
We envisioned that these radical precursors could be used for the synthesis of sulfones, sulfonamides, which are widely used functional groups and are present in a variety of functional materials, agricultural chemicals, and pharmaceuticals.49−51 The abundance of these functional groups in biologically active molecules underscores their importance: in approved drugs, sulfur-containing functional groups are even more common than fluorine- or phosphorus-containing groups.52 Therefore, we used our radical precursors to develop a new method for the synthesis of sulfur-containing compounds, starting with sulfones (Figure 2). We were pleased to find that when we changed the reaction conditions, we were able to convert radical precursors 1 to sulfur-containing products by Ir-catalyzed reactions with Na2S2O5 (4) in DMSO under irradiation with a blue LED and subsequent reactions with alkyl halides. Although the isolation of crude aryl sulfonates was possible, to facilitate the characterization and isolation of the final products, we instead used a one-pot procedure to convert the intermediate sulfites to benzyl sulfones 5a–5h by reactions with various alkyl halides (R–X). To further illustrate the wide range of synthetic uses for our method, we carried out one-pot reactions of 1 with 4 to afford aryl sulfonamides 5i and 5j and sulfonyl chloride 5k. In addition, a two-step protocol was developed for the conversion of aryl sulfonates into sulfonamides 5l–5r via the corresponding sulfonyl chloride intermediates.
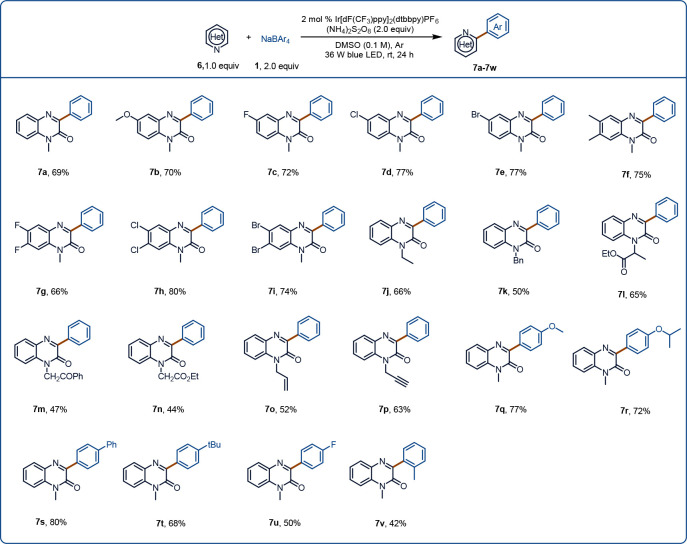
To demonstrate the universality of our approach, we planned to use it for other types of reactions, such as reactions of compounds with heteroaryl groups, which are widely found in natural products, organic materials, small-molecule drugs, and ligands for metal catalysts.53,54 Substituted heteroaryl groups can be obtained by Minisci reactions, which involve attack of a radical on a protonated heteroaromatic compound to generate a dearomatized intermediate, which then undergoes rearomatization. To realize our plan, we chose quinoxalin-2(1H)-ones as heteroaryl substrates and screened various reaction conditions, finally achieving efficient rearomatization of the intermediates under photocatalytic conditions. We then investigated the substrate scope by carrying out reactions of various quinoxalin-2(1H)-ones 6 with sodium tetraphenylborate (1a, Figure 3). N-Methyl quinoxalin-2(1H)-ones with methoxy, (di)fluoro, (di)chloro, (di)bromo, ester, or dimethyl substituents on the heteroaromatic ring reacted smoothly with 1a, producing the corresponding products (7a–7i) in 66–80% yields. Moreover, quinoxalin-2(1H)-ones bearing substituents other than a methyl group on the nitrogen atom were also suitable substrates, providing 7j–7p. Notably, the allyl group of 6o and the alkyne of 6p were retained under the reaction conditions. In addition, we were pleased to find that tetraarylborates with various substituents on the aryl ring showed good tolerance for the reaction conditions, affording desired products 7q–7v in 42–80% yields.
Figure 3.
Substrate scope of the Minisci reaction. Reaction conditions: 1 (0.4 mmol, 2.0 equiv), 6 (0.2 mmol, 1.0 equiv), (NH4)2S2O8 (0.4 mmol, 2.0 equiv), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (2 mol %), DMSO (2.0 mL), Ar, 36 W blue LED, rt, 24 h.
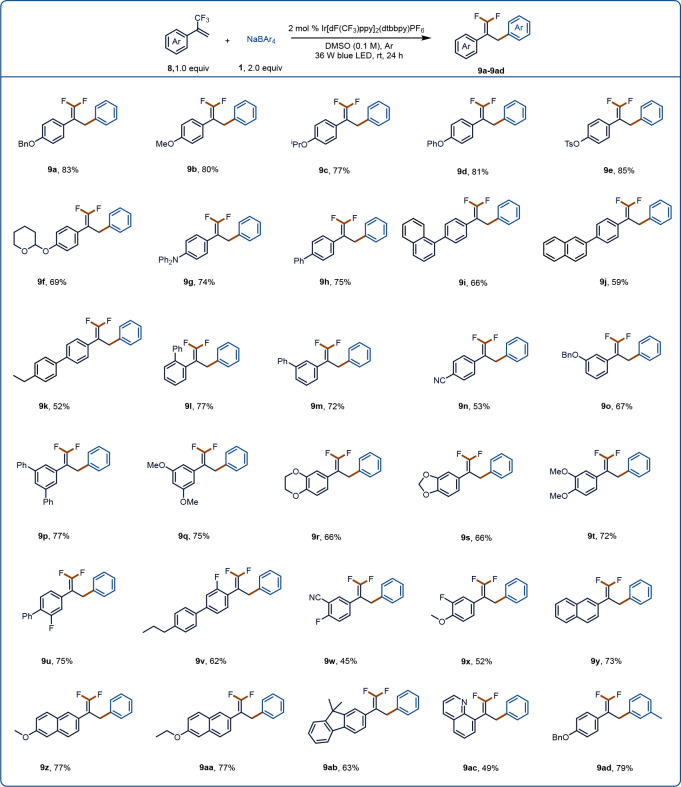
Because fluorinated groups are frequently incorporated into organic molecules to impart desirable pharmacological properties such as increased metabolic stability, enhanced lipophilicity, and improved bioavailability, the development of methods for the synthesis of new and unusual fluorinated groups is of increasing interest to chemists. Therefore, in a further demonstration of the generality of our method, we used it to accomplish intramolecular radical polarity cross-elimination reactions, namely, defluorinative alkylation and allylation.55−59 We were pleased to find that when α-trifluoromethyl aryl alkenes 8 and sodium tetraphenylborate 1a in DMSO containing Ir[dF(CF3)ppy]2(dtbbpy)PF6 were irradiated with a blue LED at rt, E1cb-type fluoride elimination prevailed over protonation and yielded desired gem-difluoroalkene products 9 (Figure 4). Specifically, α-trifluoromethyl aryl alkenes with an electron-donating group at the para position (alkoxy, arylamino, or phenyl) gave 9a–9h in moderate to good yields. In addition, para-naphthyl-substituted compounds were suitable substrates (9i and 9j), and a 4-(4-ethylphenyl)-substituted compound gave 9k, albeit in a relatively low yield. The position of a phenyl group on the aromatic ring of the aryl alkene had little effect on the yield (compare 9h, 9l, and 9m). The yield was relatively low for an aryl alkene with an electron-withdrawing cyano group (9n). Products with disubstituted aromatic rings (9p–9x) were obtained in 45–77% yields. Substrates containing naphthalene (9y–9aa), fluorene (9ab), and quinoline (9ac) rings, which are useful for further synthetic manipulations, were well tolerated. Sodium tetra(p-tolyl)borate was also tested and found to give desired product 9ad.
Figure 4.
Substrate scope of the defluorinative arylation reaction. Reaction conditions: 1 (0.4 mmol, 2.0 equiv), 8 (0.2 mmol, 1.0 equiv), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (2 mol %), DMSO (2.0 mL), Ar, 36 W blue LED, rt, 24 h.
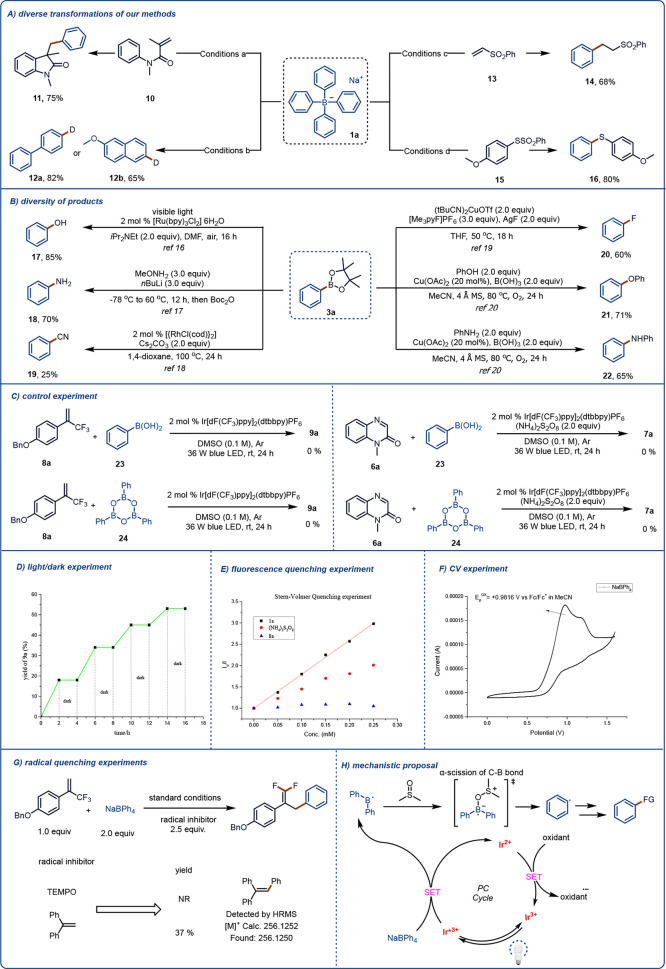
In addition to the reactions described above, photocatalytic reactions of sodium tetraarylborates with several other coupling partners could also be carried out at ambient temperature. For example, by using a catalytic amount of Ir[(dF(CF3)2ppy)2dtbpy]PF6 and irradiation with visible light, we could obtain tandem cyclization product 11 from the reaction of sodium tetraphenylborate (1a) and 10 in the presence of (NH4)2S2O8 as an oxidizing agent. By using the same photocatalyst, along with tert-butylthiol as a hydrogen-atom-transfer reagent, we achieved deuteration of this method. Alternatively, 1a could be used for a Giese radical addition reaction with 13 to afford 14, as well as for a direct deboronization sulfide reaction with 15 to afford 16 (Figure 5A). Moreover, phenylboronic ester 3a could be efficiently synthesized on a gram scale from B2pin2 and 1a, and the C–B bond of 3a could be transformed into various C–O, C–N, C–F, and C–CN bonds by means of previously described methods (17–22, Figure 5B).60−64
Figure 5.
(A) Utility of sodium tetraarylborates for a diverse array of transformations. Conditions a: 1a (0.4 mmol, 2.0 equiv), 10 (0.2 mmol, 1.0 equiv), (NH4)2S2O8 (0.4 mmol, 2.0 equiv), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (2 mol %), DMSO (2.0 mL), Ar, 36 W blue LED, rt, 24 h. Conditions b: 1a (0.4 mmol, 2.0 equiv), tert-butylthiol (20 mol %), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (2 mol %), 5:1 (v/v) d6-DMSO/D2O (2.0 mL), Ar, 36 W blue LED, rt, 24 h. Conditions c: 1a (0.4 mmol, 2.0 equiv), 13 (0.2 mmol, 1.0 equiv), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (2 mol %), DMSO (2.0 mL), Ar, 36 W blue LED, rt, 24 h. Conditions d: 1a (0.2 mmol, 2.0 equiv), 15 (0.4 mmol, 2.0 equiv), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (2 mol %), DMSO (2.0 mL), Ar, 36 W blue LED, rt, 24 h. (B) Transformations of product 3a (0.4 mmol). (C) Control experiments. (D) Light/dark experiment. (E) Fluorescence quenching experiment. (F) Cyclic voltammetry experiment. (G) Radical quenching experiments. (H) Proposed mechanism.
We then performed several experiments to gain insight into the mechanism of reaction. According to Xia’s report,723 and 24 can be produced by oxidation of sodium tetraphenylborate (1a) under photocatalytic conditions (Figure 5C). Therefore, we used these two compounds instead of 1a to react with radical receptor 8a or 6a under otherwise standard conditions. However, none of the desired product (9a or 7a, respectively) was detected, so we concluded that neither 23 nor 24 was the source of the aryl radicals. Next, we carried out a light/dark experiment, which showed that the reaction of 8a and 1a stopped when there was no light (Figure 5D). This result suggests that any chain propagation process was transient and that light was essential for product formation. We then performed UV–vis spectroscopy and fluorescence quenching experiments and prepared Stern–Volmer diagrams (Figure 5E). The UV–vis spectra confirmed that the photocatalyst was quenched by 1a. Electrochemical analysis of 1a showed that 1a had a low oxidation peak that completely quenched the photocatalyst (Figure 5F). Finally, we found that the reaction was stopped by free-radical scavengers, and we detected a free-radical-trapping product by means of high-resolution mass spectrometry (Figure 5G). This experiment clearly shows that the reaction proceeded via a free-radical pathway.
On the basis of literature reports and the results of our mechanistic experiments, we propose the reaction mechanism shown in Figure 5H. The excited-state photocatalyst is quenched by 1a to produce a diarylboron radical, which then forms a complex with DMSO. The complex undergoes α-scission to produce an aryl radical, which is subsequently captured by a radical acceptor, resulting in aryl functionalization of the acceptor.
Conclusion
In conclusion, we have described the use of tetraarylborate salts as new precursors for aryl radicals, which are generated upon irradiation of the salts with visible light in the presence of DMSO as an activation reagent. Our findings also offer new reaction pathways and applications for tetraarylborate salts. We used the radical precursors to accomplish various transformations. By addition of DMSO, the initially generated diarylboron radicals could be made to produce aryl radicals, which in turn enabled the formation of C–B, C–C, and C–X bonds. The extension of tetraarylborate salts to other challenging and useful transformations is currently being explored in our laboratory.
Acknowledgments
We are grateful to the National Natural Science Foundation of China (22271166, 22077071) and the Frontiers Science Center for New Organic Matter, Nankai University (63181206), for generous financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.3c00993.
Experimental procedures and spectroscopic data for all new compounds (PDF)
Author Contributions
F.Y. conceived the chemistry and designed the experiments under the guidance of Professor Q.W. The experiments and data analysis were conducted by all authors. F.Y. wrote the manuscript. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Ravelli D.; Protti S.; Fagnoni M. Carbon–Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850–9913. 10.1021/acs.chemrev.5b00662. [DOI] [PubMed] [Google Scholar]
- Skubi K. L.; Blum T. R.; Yoon T. P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074. 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.-Y.; Chen J.-R.; Xiao W.-J. Visible Light-Driven Radical-Mediated C–C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561. 10.1021/acs.chemrev.0c00030. [DOI] [PubMed] [Google Scholar]
- Wu X.; Zhu C. Radical-Mediated Remote Functional Group Migration. Acc. Chem. Res. 2020, 53, 1620–1636. 10.1021/acs.accounts.0c00306. [DOI] [PubMed] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Sanchez R. A.; Tlahuext-Aca A.; Tavakoli G.; Glorius F. Visible Light-Mediated Direct Decarboxylative C–H Functionalization of Heteroarenes. ACS Catal. 2017, 7, 4057–4061. 10.1021/acscatal.7b01133. [DOI] [Google Scholar]
- Liu P.; Liu W.; Li C.-J. Catalyst-Free and Redox-Neutral Innate Trifluoromethylation and Alkylation of Aromatics Enabled by Light. J. Am. Chem. Soc. 2017, 139, 14315–14321. 10.1021/jacs.7b08685. [DOI] [PubMed] [Google Scholar]
- Liu W.; Liu P.; Lv L.; Li C.-J. Metal-Free and Redox-Neutral Conversion of Organotrifluoroborates into Radicals Enabled by Visible Light. Angew. Chem., Int. Ed. 2018, 57, 13499–13503. 10.1002/anie.201807181. [DOI] [PubMed] [Google Scholar]
- Zheng D.; Studer A. Angew. Chem., Int. Ed. 2019, 131, 15950–15954. 10.1002/ange.201908987. [DOI] [Google Scholar]
- Huang C.; Li J.; Li C.-J. Photocatalytic C(sp3) radical generation via C–H, C–C, and C–X bond cleavage. Chem. Sci. 2022, 13, 5465–5504. 10.1039/D2SC00202G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z.; MacMillan D. W. C. Decarboxylative Arylation of α-Amino Acids via Photoredox Catalysis: A One-Step Conversion of Biomass to Drug Pharmacophore. J. Am. Chem. Soc. 2014, 136, 5257–5260. 10.1021/ja501621q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.; Schwenzer M.; Studer A. Radical NHC Catalysis. ACS Catal. 2022, 12, 11984–11999. 10.1021/acscatal.2c03996. [DOI] [Google Scholar]
- Ollivier C.; Renaud P. Organoboranes as a Source of Radicals. Chem. Rev. 2001, 101, 3415–3434. 10.1021/cr010001p. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Boryl Radical Addition to Multiple Bonds in Organic Synthesis. Eur. J. Org. Chem. 2019, 2019, 6308–6421. 10.1002/ejoc.201901010. [DOI] [Google Scholar]
- Jin J.; Xia H.; Zhang F.; Wang Y. Lewis-Base Boryl Radicals Enabled Borylation, Radical Catalysis and Reduction Reactions. Chin J. Org. Chem. 2020, 40, 2185–2194. 10.6023/cjoc202005017. [DOI] [Google Scholar]
- Taniguchi T. Advances in chemistry of N-heterocyclic carbene boryl radicals. Chem. Soc. Rev. 2021, 50, 8995–9021. 10.1039/D1CS00385B. [DOI] [PubMed] [Google Scholar]
- Aramaki Y.; Omiya H.; Yamashita M.; Nakabayashi K.; Ohkoshi S.-i.; Nozaki Synthesis and Characterization of B-Heterocyclic π-Radical and Its Reactivity as a Boryl Radical. K. J. Am. Chem. Soc. 2012, 134, 19989–19992. 10.1021/ja3094372. [DOI] [PubMed] [Google Scholar]
- Wu C.; Hou X.; Zheng Y.; Li P.; Lu D. Electrophilicity and Nucleophilicity of Boryl Radicals. J. Org. Chem. 2017, 82, 2898–2905. 10.1021/acs.joc.6b02849. [DOI] [PubMed] [Google Scholar]
- Kim J.; Constantin T.; Simonetti M.; Llaveria J.; Sheikh N. S.; Leonori D. A radical approach for the selective C–H borylation of azines. Nature 2021, 595, 677–683. 10.1038/s41586-021-03637-6. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Chen J.; Lin Z.; Quan Y. Photoinduced Dehydrogenative Borylation via Dihydrogen Bond Bridged Electron Donor and Acceptor Complexes. Chem.—Eur. J. 2023, 29, e20220305. 10.1002/chem.202203053. [DOI] [PubMed] [Google Scholar]
- Choi W.; Kim M.; Lee K.; Park S.; Hong S. C4-Selective C–H Borylation of Pyridinium Derivatives Driven by Electron Donor–Acceptor Complexes. Org. Lett. 2022, 24, 9452–9457. 10.1021/acs.orglett.2c03882. [DOI] [PubMed] [Google Scholar]
- Ren S.; Zhang F.; Qi J.; Huang Y.; Xu A.; Yan H.; Wang Y. Radical Borylation/Cyclization Cascade of 1,6-Enynes for the Synthesis of Boron-Handled Hetero- and Carbocycles. J. Am. Chem. Soc. 2017, 139, 6050–6053. 10.1021/jacs.7b01889. [DOI] [PubMed] [Google Scholar]
- Jin J.; Zheng W.; Xia H.; Zhang F.; Wang Y. Regioselective Radical Hydroboration of gem-Difluoroalkenes: Synthesis of α-Borylated Organofluorines. Org. Lett. 2019, 21, 8414–8418. 10.1021/acs.orglett.9b03173. [DOI] [PubMed] [Google Scholar]
- Qi J.; Zhang F.; Jin J.; Zhao Q.; Li B.; Liu L.; Wang Y. New Radical Borylation Pathways for Organoboron Synthesis Enabled by Photoredox Catalysis. Angew. Chem., Int. Ed. 2020, 59, 12876–12884. 10.1002/anie.201915619. [DOI] [PubMed] [Google Scholar]
- Xia P.; Song D.; Ye Z.; Hu Y.; Xiao J.; Xiang H.; Chen X.; Yang H. Photoinduced Single-Electron Transfer as an Enabling Principle in the Radical Borylation of Alkenes with NHC–Borane. Angew. Chem., Int. Ed. 2020, 59, 6706–6710. 10.1002/anie.201913398. [DOI] [PubMed] [Google Scholar]
- Xia H.; Zhang F.; Ye T.; Wang Y. Selective α-Monomethylation by an Amine-Borane/N,N-Dimethylformamide System as the Methyl Source. Angew. Chem., Int. Ed. 2018, 57, 11770–11775. 10.1002/anie.201804794. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Zhang F.; Peng T.; Wang C.; Cheng J.; Chen C.; Houk K. N.; Wang Y. Sequential C–F bond functionalizations of trifluoroacetamides and acetates via spin-center shifts. Science 2021, 371, 1232–1240. 10.1126/science.abg0781. [DOI] [PubMed] [Google Scholar]
- Peng T.; Xu Z.; Zhang F.; Li B.; Xu W.; Fu Y.; Wang Y. Dehydroxylative Alkylation of α-Hydroxy Carboxylic Acid Derivatives via a Spin-Center Shift. Angew. Chem., Int. Ed. 2022, 61, e2022013. 10.1002/anie.202201329. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Li B.; Zhou X.; Wang Z.; Zhang F.; Li Y.; Zhou X.; Fu Y.; Wang Y. Boryl Radicals Enabled a Three-Step Sequence to Assemble All-Carbon Quaternary Centers from Activated Trichloromethyl Groups. J. Am. Chem. Soc. 2022, 144, 15275–15285. 10.1021/jacs.2c05798. [DOI] [PubMed] [Google Scholar]
- Peng T.; Zhang F.; Wang Y. Lewis Base–Boryl Radicals Enabled Borylation Reactions and Selective Activation of Carbon–Heteroatom Bonds. Acc. Chem. Res. 2023, 56, 169–186. 10.1021/acs.accounts.2c00752. [DOI] [PubMed] [Google Scholar]
- Yu T.; Yang J.; Wang Z.; Ding Z.; Xu M.; Wen J.; Xu L.; Li P. Selective [2σ + 2σ] Cycloaddition Enabled by Boronyl Radical Catalysis: Synthesis of Highly Substituted Bicyclo[3.1.1]heptanes. J. Am. Chem. Soc. 2023, 145, 4304–4310. 10.1021/jacs.2c13740. [DOI] [PubMed] [Google Scholar]
- Xu M.; Wang Z.; Sun Z.; Ouyang Y.; Ding Z.; Yu T.; Xu L.; Li P. Diboron(4)-Catalyzed Remote [3 + 2] Cycloaddition of Cyclopropanes via Dearomative/Rearomative Radical Transmission through Pyridine. Angew. Chem., Int. Ed. 2022, 61, e2022145. 10.1002/anie.202214507. [DOI] [PubMed] [Google Scholar]
- Ding Z.; Liu Z.; Wang Z.; Yu T.; Xu M.; Wen J.; Yang K.; Zhang H.; Xu L.; Li P. Catalysis with Diboron(4)/Pyridine: Application to the Broad-Scope [3 + 2] Cycloaddition of Cyclopropanes and Alkenes. J. Am. Chem. Soc. 2022, 144, 8870–8882. 10.1021/jacs.2c03673. [DOI] [PubMed] [Google Scholar]
- Suga T.; Ukaji Y. Nickel-Catalyzed Cross-Electrophile Coupling between Benzyl Alcohols and Aryl Halides Assisted by Titanium Co-reductant. Org. Lett. 2018, 20, 7846–7850. 10.1021/acs.orglett.8b03367. [DOI] [PubMed] [Google Scholar]
- Dong Z.; MacMillan D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 2021, 598, 451–456. 10.1038/s41586-021-03920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Wu Y.; Li S.; Jiang Y.; Li Y.; Lan Y.; Xia J. Boryl Radical Activation of Benzylic C–OH Bond: Cross-Electrophile Coupling of Free Alcohols and CO2 via Photoredox Catalysis. J. Am. Chem. Soc. 2022, 144, 8551–8559. 10.1021/jacs.1c12463. [DOI] [PubMed] [Google Scholar]
- Geske D. H. The Electroöxidation of the Tetraphenylborate Ion; An Example of a Secondary Chemical Reaction Following the Primary Electrode Process. J. Phys. Chem. 1959, 63, 1062–1070. 10.1021/j150577a008. [DOI] [Google Scholar]
- Geske D. H. EVIDENCE FOR THE FORMATION OF BIPHENYL BY INTRAMOLECULAR DIMERIZATION IN THE ELECTROÖXIDATION OF TETRAPHENYLBORATE ION. J. Phys. Chem. 1962, 66, 1743–1744. 10.1021/j100815a507. [DOI] [Google Scholar]
- Gerleve C.; Studer A. Transition-Metal-Free Oxidative Cross-Coupling of Tetraarylborates to Biaryls Using Organic Oxidants. Angew. Chem., Int. Ed. 2020, 59, 15468–15473. 10.1002/anie.202002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abley P.; Halpern J. Oxidation of tetraphenylborate by hexachloroiridate(IV). J. Chem. Soc. D 1971, 20, 1238–1239. 10.1039/c29710001238. [DOI] [Google Scholar]
- Mizuno H.; Sakurai H.; Amaya T.; Hirao T. Oxovanadium(v)-catalyzed oxidative biaryl synthesis from organoborate under O2. Chem. Commun. 2006, 48, 5042–5044. 10.1039/b610731a. [DOI] [PubMed] [Google Scholar]
- Dhital R. N.; Sakurai H. Oxidative Coupling of Organoboron Compounds. Asian J. Org. Chem. 2014, 3, 668–684. 10.1002/ajoc.201300283. [DOI] [Google Scholar]
- Beil S. B.; Möhle S.; Enders P.; Waldvogel S. R. Electrochemical instability of highly fluorinated tetraphenyl borates and syntheses of their respective biphenyls. Chem. Commun. 2018, 54, 6128–6131. 10.1039/C8CC02996B. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Lavendomme R.; Burghaus O.; Nitschke J. R. A. A Zn4L6 Capsule with Enhanced Catalytic C–C Bond Formation Activity upon C60 Binding. Angew. Chem., Int. Ed. 2019, 58, 9073–9077. 10.1002/anie.201903286. [DOI] [PubMed] [Google Scholar]
- Music A.; Baumann A. N.; Spieß P.; Plantefol A.; Jagau T. C.; Didier D. Electrochemical Synthesis of Biaryls via Oxidative Intramolecular Coupling of Tetra(hetero)arylborates. J. Am. Chem. Soc. 2020, 142, 4341–4348. 10.1021/jacs.9b12300. [DOI] [PubMed] [Google Scholar]
- Baumann A. N.; Music A.; Dechent J.; Müller N.; Jagau T. C.; Didier D. Electro-Olefination—A Catalyst Free Stereoconvergent Strategy for the Functionalization of Alkenes. Chem.—Eur. J. 2020, 26, 8382–8387. 10.1002/chem.202001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Music A.; Nuber C. M.; Lemke Y.; Spieß P.; Didier D. Electro-alkynylation: Intramolecular Rearrangement of Trialkynylorganoborates for Chemoselective C(sp2)–C(sp) Bond Formation. Org. Lett. 2021, 23, 4179–4184. 10.1021/acs.orglett.1c01126. [DOI] [PubMed] [Google Scholar]
- Wang B.; Peng P.; Ma W.; Liu Z.; Huang C.; Cao Y.; Hu P.; Qi X.; Lu Q. Electrochemical Borylation of Alkyl Halides: Fast, Scalable Access to Alkyl Boronic Esters. J. Am. Chem. Soc. 2021, 143, 12985–12991. 10.1021/jacs.1c06473. [DOI] [PubMed] [Google Scholar]
- Feng M.; Tang B.; Liang S. H.; Jiang X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. 10.2174/1568026615666150915111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. A.; Njardarson J. T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5. 10.1007/s41061-018-0184-5. [DOI] [PubMed] [Google Scholar]
- Kheirieh S.; Asghari M.; Afsari M. Application and modification of polysulfone membranes. Rev. Chem. Eng. 2018, 34, 657–693. 10.1515/revce-2017-0011. [DOI] [Google Scholar]
- Ertl P.; Altmann E.; McKenna J. M. The Most Common Functional Groups in Bioactive Molecules and How Their Popularity Has Evolved over Time. J. Med. Chem. 2020, 63, 8408–8418. 10.1021/acs.jmedchem.0c00754. [DOI] [PubMed] [Google Scholar]
- Zheng D.; Studer A. Asymmetric Synthesis of Heterocyclic γ-Amino-Acid and Diamine Derivatives by Three-Component Radical Cascade Reactions. Angew. Chem., Int. Ed. 2019, 58, 15803–15807. 10.1002/anie.201908987. [DOI] [PubMed] [Google Scholar]
- Klauck F. J. R.; Yoon H.; James M. J.; Lautens M.; Glorius F. Visible-Light-Mediated Deaminative Three-Component Dicarbofunctionalization of Styrenes with Benzylic Radicals. ACS Catal. 2019, 9, 236–241. 10.1021/acscatal.8b04191. [DOI] [Google Scholar]
- Zhang C.; Lin Z.; Zhu Y.; Wang C. Chromium-Catalyzed Allylic Defluorinative Ketyl Olefin Coupling. J. Am. Chem. Soc. 2021, 143, 11602–11610. 10.1021/jacs.1c04531. [DOI] [PubMed] [Google Scholar]
- Meanwell N. A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- Bobek M.; Kavai I.; De Clercq E. Synthesis and biological activity of 5-(2,2-difluorovinyl)-2’-deoxyuridine. J. Med. Chem. 1987, 30, 1494–1497. 10.1021/jm00391a036. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Qiu J.; Silverman R. B. Design, Synthesis, and Biological Activity of a Difluoro-Substituted, Conformationally Rigid Vigabatrin Analogue as a Potent γ-Aminobutyric Acid Aminotransferase Inhibitor. J. Med. Chem. 2003, 46, 5292–5293. 10.1021/jm034162s. [DOI] [PubMed] [Google Scholar]
- Yue F.; Ma H.; Song H.; Liu Y.; Dong J.; Wang Q. Chem. Sci. 2022, 13, 13466–13474. 10.1039/D2SC05521J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y.; Chen J.; Liu X.; Lu L.; Davis R. L.; Jo̷rgensen K.; Xiao W. Highly Efficient Aerobic Oxidative Hydroxylation of Arylboronic Acids: Photoredox Catalysis Using Visible Light. Angew. Chem., Int. Ed. 2012, 51, 784–788. 10.1002/anie.201107028. [DOI] [PubMed] [Google Scholar]
- Mlynarski S. N.; Karns A. S.; Morken J. P. Direct Stereospecific Amination of Alkyl and Aryl Pinacol Boronates. J. Am. Chem. Soc. 2012, 134, 16449–16451. 10.1021/ja305448w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malapit C. A.; Reeves J. T.; Busacca C. A.; Howell A. R.; Senanayake C. H. Rhodium-Catalyzed Transnitrilation of Aryl Boronic Acids with Dimethylmalononitrile. Angew. Chem., Int. Ed. 2016, 55, 326–330. 10.1002/anie.201508122. [DOI] [PubMed] [Google Scholar]
- Fier P. S.; Luo J.; Hartwig J. F. Copper-Mediated Fluorination of Arylboronate Esters. Identification of a Copper(III) Fluoride Complex. J. Am. Chem. Soc. 2013, 135, 2552–2559. 10.1021/ja310909q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantourout J. C.; Miras H. N.; Isidro-Llobet A.; Sproules S.; Watson A. J. B. Spectroscopic Studies of the Chan–Lam Amination: A Mechanism-Inspired Solution to Boronic Ester Reactivity. J. Am. Chem. Soc. 2017, 139, 4769–4779. 10.1021/jacs.6b12800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.