Summary
Background
Half of new HIV acquisitions in Africa occur in adolescent girls and young women (AGYW). Preexposure prophylaxis (PrEP) with oral emtricitabine/tenofovir disoproxil fumarate and the dapivirine ring are efficacious but have lower adherence and effectiveness among AGYW, understanding their adherence and product preference may optimize PrEP use.
Methods
REACH was an open label crossover trial among HIV-negative, non-pregnant AGYW ages 16–21 from South Africa, Uganda, and Zimbabwe with six-month randomized periods of ring or oral PrEP followed by product choice. Study endpoints were ≥Grade 2 adverse events (AEs) and high adherence, defined by >4 mg dapivirine released in returned rings indicating 28 days of use, and tenofovir diphosphate ≥700 fmol/punch correlating with ≥4 tablets/week. The proportion of visits with high adherence for each product was compared between the randomized crossover and choice periods using generalized estimating equations. Adaptive LASSO was used to select variables for a multivariable model of correlates of product choice.
Findings
From February 2019 to April 2021, 247 participants with a median age of 18 years were enrolled; 54 ≥grade 2 product-related AEs were reported during oral PrEP and 5 during ring use with no product-related serious AEs. High adherence was observed in 57% of the ring and oral PrEP visits. For the choice period, 155 (65%) chose the ring, 72 (30%) oral PrEP, and 11 (5%) neither product. AGYW who always had high adherence during the randomized oral PrEP period were six-fold more likely to choose oral PrEP and those reporting vaginal sex in the quarter prior to enrollment were three-fold more likely to choose the ring. Four women acquired HIV during follow-up.
Interpretation
Adherence was moderately high and similar between oral PrEP and the ring with favorable safety and tolerability. Most AGYW chose a PrEP product in the choice period; two-thirds chose the ring.
Keywords: oral HIV preexposure prophylaxis, dapivirine ring, adherence, safety, choice, African young women
Introduction
Adolescent girls and young women (AGYW) in sub-Saharan Africa (SSA) remain disproportionately impacted by HIV in the context of declining population HIV incidence.1 HIV incidence rates among African AGYW in recent HIV prevention efficacy trials have remained at 4% per year, despite the provision of frequent counseling and other prevention services.2–5
Multiple placebo-controlled phase 3 trials of oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate (TDF) or co-formulated with emtricitabine (FTC-TDF) have demonstrated very high efficacy among men and women.6–10 However, two PrEP placebo-controlled efficacy trials which enrolled African AGYW showed no protection in large part due to low adherence to products.6, 7, 11–14 Demonstration projects among African AGYW have shown high initiation rates of oral PrEP with low adherence and persistence to oral PrEP, indicating a high motivation for HIV prevention but that stigma and the burden of daily pill-taking is challenging in this population;14–16 possibly reflecting stage of neurocognitive development, dynamic partnerships and uncertainty regarding HIV exposure.
The monthly dapivirine vaginal ring (‘the ring’) may provide a more discrete and less challenging option for some young women, since no active effort is needed to remain adherent for a month after inserting the ring. Two placebo-controlled phase 3 trials of the ring demonstrated reductions in HIV acquisition of 27 and 31%, respectively; post-hoc analyses indicated lower adherence and either no or lower protection, respectively, in women ages 18–21 years compared to women >21 years old.3, 4 Effectiveness of the ring was approximately 50% in the open-label extension studies in which participants were aware of the efficacy data and HIV incidence was compared to a counterfactual estimate.17–19 Based on these findings, the WHO has recommended the ring as an additional choice for HIV prevention for women at substantial risk of HIV infection.20
To guide implementation of oral PrEP and ring among African AGYW, given previously observed lower adherence in this younger population, MTN-034/ Reversing the Epidemic in Africa with Choices in HIV Prevention (REACH), a crossover trial of oral PrEP and the ring was conducted to assess product adherence, safety, and choice among African AGYW.
Methods
Study Design and Trial Population
MTN-034/REACH was an open-label phase 2a crossover trial of oral PrEP and the ring (https://www.mtnstopshiv.org/research/studies/mtn-034/mtn-034-protocols). Participants were randomized 1:1 to one of two sequences of either the ring or oral PrEP for six months, then switched to the other product option for six months, followed by a third six-month period in which participants were given a choice of oral PrEP, the ring, or neither product, for a total of 18 months study participation (Supplement, Figure 1). The primary objectives were to evaluate safety and adherence to oral PrEP and the ring during the crossover periods, and the secondary objective was choice of product after the randomized periods with use of each product. The crossover design allowed for intra-individual comparisons of safety, adherence and acceptability. The study enrolled healthy, sexually-active, HIV seronegative non-pregnant participants between the ages of 16–21 years at four MTN-affiliated clinical research sites: Cape Town and Johannesburg, South Africa; Kampala, Uganda; and Harare, Zimbabwe. By design, one third of participants were aged 16–17 years to increase data on safety and adherence in adolescent girls. At screening, participants were assumed to have been assigned female sex at birth and were not asked to report their gender identity. However, required safety pelvic exams that occurred during the screening process confirmed sex at birth. The initial enrollment goal was 300; the sample size was reduced to 247 in June 2020 due to the impact of COVID-19 on active recruitment efforts. Sites utilized youth-focused recruitment strategies, adolescent-friendly clinics, staff training on nonjudgmental counseling, and parent/guardian education about the study.
Randomization and masking
A window of 70 days from screening to enrollment allowed participants to initiate and become familiar with the highly effective contraceptive method of their choice and reduce potential confounding of side effects during initiation of a contraceptive method with oral PrEP or ring. At enrollment, participants were assigned in a 1:1 ratio, with the use of a fixed block randomization stratified by site generated by the MTN Statistical and Data Management Center, to first receive either six months of daily oral FTC/TDF tablets (fixed dose of 200mg of FTC and 300mg of TDF) or six months of the monthly ring. Participants and clinical research staff were not blinded to randomization allocation.
Study Procedures
Participants were counseled on methods for easy swallowing of tablets and ring insertion. Ingestion of the first tablet and first ring insertion were observed in the study clinics. Participants were counseled to wear the ring for the entire month until their next visit and to return with the used ring still inserted for removal in clinic. A new ring or bottle with 30 PrEP tablets was dispensed at each monthly visit. There was no washout between crossover periods based on the ethical obligation to not withhold efficacious HIV prevention products in a population at high risk of acquiring HIV.
Study products were permanently discontinued for confirmed HIV acquisition, an allergic reaction to either product, reported use of oral PrEP for HIV prevention outside of the study, and injection drug use. At the onset of the study, there was less experience with oral PrEP and ring use during pregnancy, so product holds were initiated for pregnancy, breastfeeding, reported use of post-exposure prophylaxis, suspected HIV acquisition or grade 3 or higher product-related AEs. Oral PrEP was held when creatinine clearance levels were below 60mL/min. Pelvic examinations were performed at enrollment, 6, 9, 13, 16 and 20 months, and when clinically indicated. Ring use was temporarily held in instances of deep epithelial disruption, generalized erythema, severe edema, or cervicitis. Study product was resumed eight weeks after end of pregnancy based on a negative pregnancy test, unless the participant was breastfeeding.
Participants received contraceptive counseling and access to effective contraceptive methods, with encouragement to use long-acting reversible methods to minimize time off product during holds for unplanned pregnancy. Participants also received female and/or male condoms, testing and treatment for sexually transmitted infections (STIs), partner referral for STI treatment, and risk reduction counselling. STI testing included nucleic acid amplification for N. gonorrhoeae and C. trachomatis (GeneXpert™, Cepheid, Sunnyvale, California), a rapid test for T. vaginalis (Osom Rapid Trichomonas test, Massachusetts), and syphilis serologies (rapid plasma reagin screening and Treponema pallidum specific confirmatory assay) at screening, if indicated at enrollment, every three months as per protocol and when participants reported symptoms. STIs were treated per national guidelines. HIV testing was conducted with two parallel rapid tests, at least one of which was FDA approved. If the rapid results were positive or discordant, a confirmatory assay (Geenius HIV 1/2 Supplemental Assay, Bio-RAD Hercules, California) was performed.
Participants with confirmed HIV acquisition were counseled about living with HIV, and access to HIV care was facilitated. Participants had the option to continue in the study without product use.
Assessment of adherence and adherence counseling
Adherence assessments included participant self-report by computer-assisted self interview (CASI), interviewer-administered questionnaires, and objective drug level evaluation, using intracellular concentrations of tenofovir diphosphate (TFV-DP) in dried blood spots (DBS) and residual dapivirine levels in returned rings. TFV-DP in red blood cells provides a cumulative measure of dosing and average adherence to oral PrEP in the prior four to six weeks.21 TFV-DP levels were analyzed by a liquid chromatography-tandem mass spectrometry assay (University of Cape Town Pharmacology Lab, South Africa). Assessment of ring adherence was based on residual drug levels in returned rings, determined by acetone extraction and high pressure liquid chromatography (Farmovs, South Africa). Adherence measurements were obtained monthly during the randomized and product choice periods. The dapivirine release rate was calculated by subtracting the amount of residual dapivirine in returned rings from the amount of dapivirine in control rings from the same lot divided by the duration of time during which the participant had the ring.17 Drug level feedback counseling was required at the second and fifth month of each product use period, reflecting the first and third months of use, respectively. Study counselors were permitted to share drug level feedback at other visits, if available, per their discretion or if results were requested by the participant.
For counseling and adherence outcome measures, categorical adherence thresholds were defined. Counseling was provided when the drug level result was available in order to initiate conversations with participants about what adherence support might be needed to achieve optimal adherence. Client-facing adherence counseling used images to show the semi-quantitative thresholds (Supplement, Figure 2); a green zone for oral PrEP use depicted the threshold for high adherence of intracellular TFV-DP levels ≥700 fmol/DBS punch, which correlates with ≥4 doses/per week on average in directly-observed dosing studies and was associated with 100% efficacy in an open-label extension study among men who have sex with men.8, 22 The yellow zone depicted any to moderate oral PrEP adherence, defined as 16.6–700 fmol/punch, and the red zone depicted no use (<16.6 fmol/punch, the lower limit of detection). Because steady-state levels for FTC/TDF are not reached until approximately six weeks, lower thresholds were used for counseling about FTC/TDF based on the month one DBS result: >500 fmol/punch for high and 16.6–499 fmol/punch for medium adherence.
The semi-quantitative images and counseling messages for ring adherence were categorized as a green zone for high use (>4.0 mg dapivirine released per month, associated with 28 days of use), yellow zone as some to moderate use (0.9 mg-4.0 mg dapivirine released per month, associated with 1–27 days of use), and red zone as no use (<0.9 mg dapivirine released), based on the rate of dapivirine release over 28 days and the correlation of >4 mg released per month with reduced risk of HIV acquisition in the MTN 025/HOPE trial. 17, 19
Participants were offered a menu of adherence support options, including digital support (text messages daily or weekly), group support (in-person or WhatsApp group meetings), and individual support (extra counseling sessions, peer buddies).23
Assessment of choice of PrEP product
The secondary outcome of study product choice was assessed 12 months after the two six-month crossover periods (Supplement, Figure 1). Participants were asked to select the ring, oral PrEP or neither product for the final six month period. Participants could switch or restart a product during the final six month product choice period.
Assessment of product safety
Safety assessments were conducted at each visit based on laboratory parameters, symptoms, and physical and pelvic examinations by trained study clinicians. AEs were reported per the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events.24 Adverse events were classified into Period 1, if the reported onset date was between randomization and 30 days after the month 6 visit, and Period 2, if the onset date was between months 6 and 7. The number and percentage with at least one grade 2 or higher AE were ascertained by product and period. Each participant-period contributed once with the highest severity AE for that period. The five most common product-related grade 2 and higher AEs were tabulated for each product across the two randomized periods.
Primary and secondary endpoints
The primary adherence endpoint was use of each product during the randomized periods, with high use defined as TFV-DP levels ≥700 fmol/punch (associated with taking an average of ≥4 tablets per week in the prior month) and ≥4 mg dapivirine released from the returned ring (continuous use for 28 days) in the prior month based on residual drug levels. A secondary adherence measurement for oral PrEP was ≥1200 TFV-DP fmol/punch which is associated with 6–7 doses and aligns with the threshold of >4 mg DPV release indicating continuous use of the ring. The primary safety endpoint was Grade 2 or higher AEs during each randomized period of 24 weeks of ring and oral PrEP. Given the open label design, the analysis focused on AEs considered related to the product.
Sample size and statistical analyses
The sample size and power calculations were based on the primary comparisons of safety and adherence between the first and second periods of the study (i.e., the randomized periods). For the primary safety outcome, the study had 80% power to detect a minimum difference of 5.8% to 9.2% depending on the rate of AEs in the treatment regimen with the lower AE rate, if there was no intra-participant correlation for safety outcomes. If the intra-participant correlation was moderately high (i.e., 0.5), the minimum detectable difference range was 4.1% to 6.5%. For the primary adherence outcome, the study had >80% power to detect a difference of 8.4% (with an intra-participant correlation of 0.5) to 11.9% (with an intra-participant correlation of 0.0), assuming that 60% of participant visits with lower adherence would have low levels of TFV-DP or high residual dapivirine levels in returned rings.
Data from all randomized participants were included in the intention to treat analysis of the first two randomized periods. For the primary safety endpoint, paired analyses were conducted using generalized estimating equation models with a Poisson (log) link, an offset of the number of visits per study product use period, an exchangeable correlation structure and robust errors (controlling for study product use periods) were used to compare the two treatment regimens for the safety endpoints during the first two randomized study product use periods. Because there was no washout period between the two randomized study product periods, it was not possible to assign AEs that occured in the first month of the second and third period to one product or the other, so AEs reported in the first month in period 2 (month 6) and 3 (month 7), reflecting potential ongoing AEs in the prior period, are reported separately (Supplement Table 2).
For the secondary outcome of product choice, the initial product choice at the beginning of the third period was calculated. To select and model correlates of product choice, we used an adaptive LASSO with 10-fold cross validation to select the tuning parameter. Candidates for inclusion in the final multivariable model included age and perceived risk of acquiring HIV at enrollment, randomization sequence, any product-related Grade 2 or higher AE (disaggregated for the ring and oral PrEP), drug level results during ring and oral PrEP randomized periods (always green versus at least one non-green report), STIs at baseline and self-reported behavioral and demographic variables at baseline and month 12 at the beginning of the choice period (i.e., having a primary partner, vaginal and anal sex in the past 3 months, and currently in school).
Statistical analyses were conducted in SAS 9.4 and R version 4.0.4.
Ethical review
The study protocol was approved by the ethics review committees at each study site: University of Cape Town Faculty of Health Sciences Human Research Ethics Committee, University of Witwatersrand Human Research Ethics Committee, University of Zimbabwe Joint Research Ethics Committee, and Makerere University. All participants provided written informed consent in English or their local language (i.e., Xhosa, i-Zulu, Setswana, and Luganda). Following local regulations, participants below the legal age for consent provided assent and parent or guardian permission was obtained.
Role of the funder and study oversight
The National Institutes of Health funded the trial and was the regulatory sponsor. The authors designed the trial, gathered and analyzed the data, prepared the manuscript, and were responsible for the decision to submit the manuscript for publication. The International Partnership for Microbicides (Silver Spring, MD, USA) supplied the rings, and participated in the design of the trial, the interpretation of the results, and manuscript preparation. Emtricitabine-tenofovir tablets were supplied by Gilead Sciences (Foster City, CA, USA) which participated in the design of the trial, interpretation of the results, and manuscript preparation. The authors vouch for the accuracy and completeness of the data and analyses.
Results
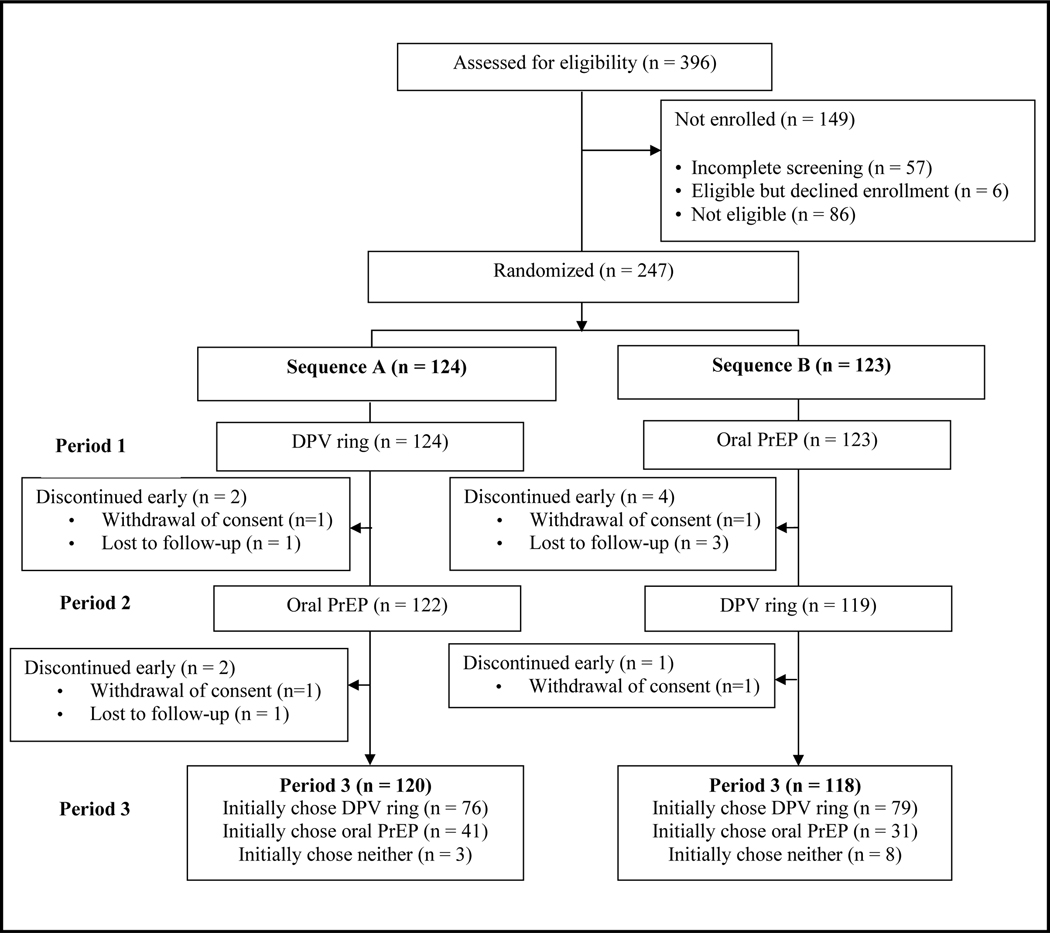
Between February 2019 and September 2021, the REACH study screened 396 potential participants, 247 enrolled, of whom 124 were randomized to Sequence A (six months of the ring followed by six months of oral PrEP) and 123 to Sequence B (six months of oral PrEP followed by six months of the ring (Figure 1). Participants’ median age was 18 years. The majority (87%) were single, 89% reported a primary sex partner, 74% of whom were reported to be HIV-negative and 25% were unsure of their partner’s HIV status (Table 1). At enrollment, almost half reported they were very or somewhat worried about their HIV risk in the next year. One-third were diagnosed with gonorrhea or chlamydia. Perceived risk of HIV in the next year, STI prevalence at baseline, and specific type of contraception used at baseline differed by site (Supplement, Table 1).
Figure 1: Consort flow diagram for MTN-034, the REACH Study.
DPV ring is the monthly dapivirine ring. Oral PrEP is daily oral emtricitabine/tenofovir. Participants were first randomized 1:1 to one of two sequences of either the ring or oral PrEP for six months, then switched to the other product option for six months (Periods 1 and 2 and Sequences A and B). At month 12, participants were given a choice of oral PrEP, the ring, or neither product for 6 months (Period 3).
Table 1:
Participant baseline characteristics by randomized crossover sequence in the REACH study
| Number of Participants n/% | Sequence A, Period 1DPV Ring; Period 2 Oral FTC/TDF N= 124 (%) |
Sequence B Period 1:Oral FTC/TDF Period 2: DPV Ring N =123 (%) |
Total N = 247 (%) |
|---|---|---|---|
| Demographic characteristics | |||
| Age | |||
| Median (IQR range) | 18 years (17,19) | 18 years (17, 19) | 18 years (17, 19) |
| 16–17 | 41 (33%) | 44 (36%) | 85 (34%) |
| 18–19 | 57 (46%) | 57 (46%) | 114 (46%) |
| 20–21 | 26 (21%) | 22 (18%) | 48 (19%) |
| Marital Status | |||
| Single | 106 (85%) | 108 (88%) | 214 (87%) |
| Married | 7 (6%) | 3 (2%) | 10 (4%) |
| Cohabitating | 9 (7%) | 11 (9%) | 20 (8%) |
| Separated or divorced | 2 (2%) | 1 (1%) | 3 (1%) |
| Highest level of education | |||
| Primary | 15 (12%) | 18 (15%) | 33 (13%) |
| Secondary | 92 (75%) | 97 (79%) | 189 (77%) |
| College or university | 15 (12%) | 9 (7%) | 23 (9%) |
| Currently in school | 51 (41%) | 41 (33%) | 92 (37%) |
| Earns income | 22 (18%) | 31 (25%) | 53 (21%) |
| Partner as a source of income | 34 (27%) | 47 (38%) | 81 (33%) |
| Behavioral characteristics | |||
| Ever pregnant | 52 (42%) | 47 (38%) | 99 (40%) |
| Alcohol consumption, prior month | |||
| Never | 52 (42%) | 51 (41%) | 103 (42%) |
| Monthly or less | 38 (31%) | 33 (27%) | 70 (28%) |
| 2–4 times a month | 24 (19%) | 29 (24%) | 54 (22%) |
| 2–3 times a week | 9 (7%) | 5 (4%) | 14 (6%) |
| 4 or more times a week | 1 (1%) | 5 (4%) | 6 (2%) |
| CES Depression Scale | |||
| < 10 | 78 (67%) | 65 (56%) | 143 (61%) |
| ≥ 10 | 39 (33%) | 51 (44%) | 90 (39%) |
| Primary sexual partner | |||
| Has primary sex partner | 111 (90%) | 108 (89%) | 218 (89%) |
| Age of primary sex partner, mean (SD) | 22.8 (3.3) | 23.2 (4.4) | 23.0 (3.9) |
| In partnership >1 year | 70 (63%) | 63 (59%) | 133 (61%) |
| HIV status | |||
| HIV positive | 2 (2%) | 1 (1%) | 3 (1%) |
| HIV negative | 82 (74%) | 79 (73%) | 161 (74%) |
| Don’t know | 27 (24%) | 28 (26%) | 55 (25%) |
| Has other partners | |||
| Yes or probably | 25 (23%) | 26 (24%) | 51 (23%) |
| No | 30 (27%) | 22 (20%) | 52 (24%) |
| Don’t know | 56 (50%) | 60 (56%) | 116 (53%) |
| Sexual Behavior | |||
| Vaginal sex, past 3 months | 100 (81%) | 103 (84%) | 203 (83%) |
| Condom use with last vaginal sex in past months | 55/100 (55%) | 57/103 (55%) | 112/203 (55%) |
| Anal sex, past 3 months | 6 (5%) | 13 (11%) | 20 (8%) |
| Number sex partners, past 3 months median (IQR) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
| Received goods or money for sex, past 6 months | 33 (27%) | 33 (27%) | 66 (27%) |
| Intimate partner violence, past 6 months | 15 (12%) | 21 (17%) | 36 (15%) |
| Perceived HIV risk in next year | |||
| Very worried | 38 (31%) | 33 (27%) | 71 (29%) |
| Somewhat worried | 18 (15%) | 11 (9%) | 29 (12%) |
| A little worried | 30 (24%) | 38 (31%) | 68 (28%) |
| Not at all worried | 38 (31%) | 41 (33%) | 79 (32%) |
| Laboratory STI diagnosis | |||
| Gonorrhea | 13 (10%) | 8 (7%) | 21 (9%) |
| Chlamydia | 30 (24%) | 41 (33%) | 71 (29%) |
| Trichomonas | 8 (6%) | 5 (4%) | 13 (5%) |
| Syphilis seropositive | 3 (2%) | 3 (2%) | 6 (2%) |
| Any STI | 40 (32%) | 47 (38%) | 87 (35%) |
| Current contraceptive use | |||
| IUD | 13 (10%) | 14 (11%) | 27 (11%) |
| Implant | 60 (48%) | 51 (41%) | 111 (45%) |
| Oral | 0 (0%) | 0 (0%) | 0 (0%) |
| Injectable | 51 (41%) | 58 (47%) | 109 (44%) |
| Baseline creatinine clearance | |||
| ml/min, median (IQR) | 102.0 (94.2 – 118.1) | 100.6 (89.9 – 111.0) | 101.6 (91.2 – 114.0) |
Retention at the end of the product choice period (week 72) was 94%, with similar retention by arm (95% in sequence A and 93% in sequence B). The proportion of visits which were not completed ranged from 1–8% with sites completing remote and off-site visits during COVID lock-down periods.
Adherence during randomized cross-over periods
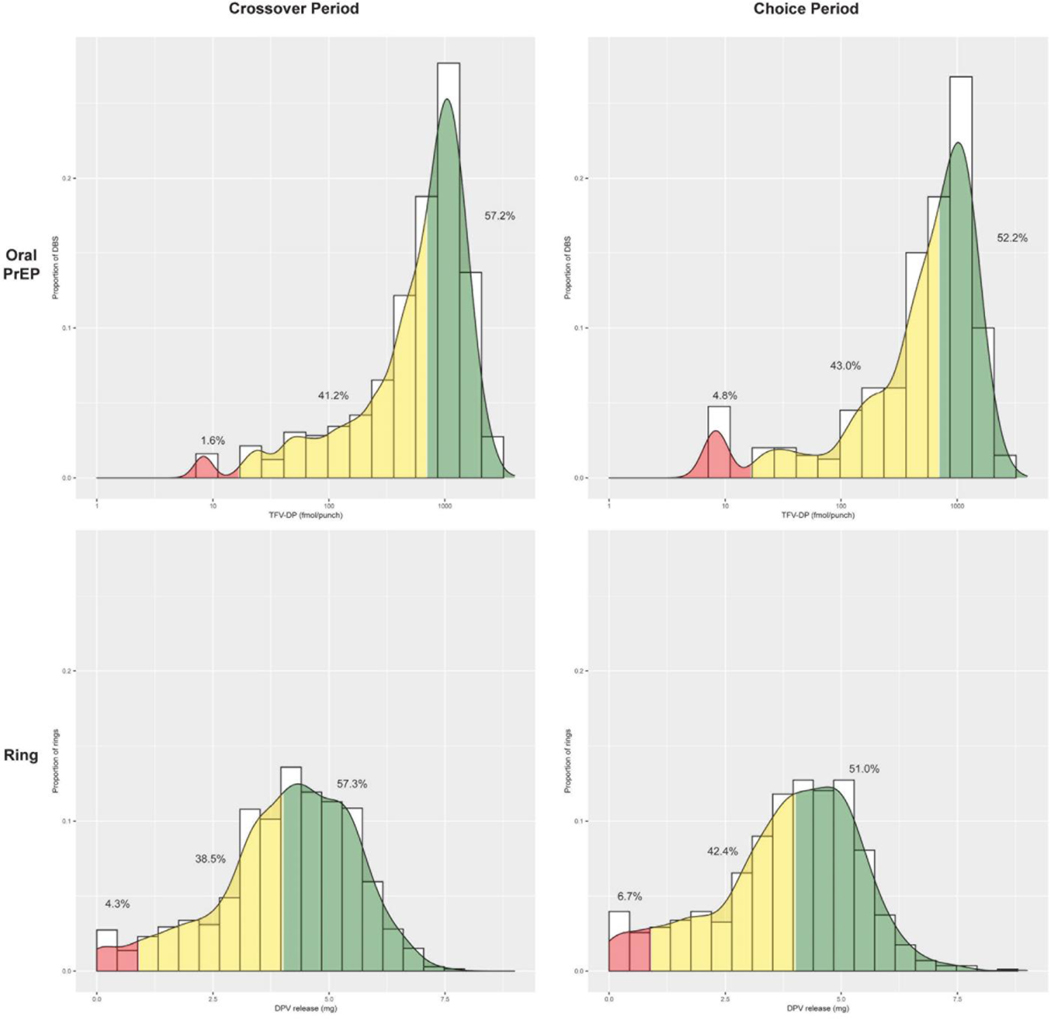
A total of 1316 DBS were tested for intracellular TFV/DP levels to assess oral FTC/TDF use in the prior month, representing 89% of expected DBS. The median TFV-DP level was 789 fmol/punch; 295 (22%) of DBS indicated very high use (>1200 fmol/punch associated with 6–7 tablets/week), 458 (35%) high use (700–1200 fmol/punch associated with an average of 4–6 tablets per week), and 21 (2%) no use (<16.6 fmol/punch). The median time from DBS collection to counseling about TFV-DP levels was 41.5 days (IQR 29–70 days).
A total of 1407 used rings were returned from participants to measure residual dapivirine levels, representing 95% of expected used rings collected and analyzed. The median dapivirine release rate was 4.3 mg/month (IQR 3.3–5.2). Based on residual drug levels, 806 (57%) indicating continuous use (dapivirine ring release rate >4.0 mg/month) and 60 (4%) had levels of dapivirine suggesting that the rings were not used (<0.9 mg/month released). The median time between return of used dapivirine rings and adherence counseling about dapivirine levels was 56 days (IQR 29–76 days).
The proportions with high, some and no use of ring and oral FTC/TDF did not differ by sequence A or B and thus are combined in Figure 2.
Figure 2: Adherence to monthly dapivirine vaginal ring (DVR) and daily oral emtricitabine-tenofovir disoproxyl fumarate (oral PrEP) during the randomized periods.
The upper panels represents the proportion of dried blood spots (DBS) with semi-quantitative thresholds for adherence in the randomized oral PrEP period on the left (Figure 2a) and among those who chose oral PrEP in the choice period on the right (Figure 2b). The green zone for oral PrEP use depicts the threshold for high adherence with intracellular TFV-DP levels ≥700 fmol/DBS punch, which correlates with ≥4 doses/per week on average in directly-observed dosing studies and was associated with 100% efficacy in an open-label extension study of FTC/TDF among men who have sex with men (efficacy for cisgender women with less than 6–7 doses of FTC/TDF per week is not known and currently being studied).The yellow zone depicts any to moderate oral PrEP adherence, defined as 16.6–700 fmol/punch, and the red zone depicts no use (<16.6 fmol/punch, the lower limit of detection). The lower panels represent dapivirine (DPV) ring use based on calculated release of DPV in returned rings in the randomized DPV ring user period on the left (Figure 2c) and among those who chose the DPV ring in the choice period (Figure 2d). The green zone for high use (>4.0 mg DPV released per month DPV with 28 days of use), yellow zone as some to moderate use (0.9 mg–4.0 mg DPV released per month associated with 1–27 days of use), and red zone as no use (<0.9 mg DPV released), based on the rate of DPV release over 28 days and the correlation of >4 mg released per month with reduced risk of HIV acquisition in the HOPE trial. To provide a comparable level of daily use of monthly use of the ring with the highest level of adherence to oral PrEP, 6–7 doses of oral PrEP per week is correlated with TFV-DP levels >1200 fmol/punch, which was observed in 374 (22%) of DBS samples.
Safety of dapivirine ring and oral FTC/TDF tablets
A total of 1450 AEs were reported during the randomized crossover periods, 827 during the oral PrEP period and 623 during the ring period, of which 456 and 442, respectively, were grade 2 or higher (Table 2). Of the total AEs during the randomized periods, 347 and 30 AEs were determined by the clinician as related to oral PrEP and the ring, respectively, with 54 grade 2 and higher AEs related to oral PrEP and five to the ring. The most common product-related grade 2 and higher AEs attributed to oral PrEP were decreased creatinine clearance, headache, nausea, diarrhea, and vomiting. Median baseline creatinine clearance was 102 ml/min. All but one adverse event related to creatinine clearance were grade 2 which includes a 10% change from the baseline creatinine clearance. The median creatinine clearance was 99.6 ml/min (IQR 90.3–110.7 ml/min) at the end of the six months of oral PrEP use and 101.8 ml/min (IQR 90.0–114.1 ml/min) at the end of 6 months of ring use. No participants discontinued oral PrEP during the randomized period and one participant discontinued oral PrEP in the product choice period due to tolerability.
Table 2:
Adverse events and Serious Adverse Events during dapivirine ring and oral PrEP use, by period of use
| Dapivirine Ring | Oral PrEP | |||||
|---|---|---|---|---|---|---|
| First period | Second period | Overall | First period | Second period | Overall | |
| Total Adverse Events (AEs) | 318 | 305 | 623 | 436 | 391 | 827 |
| Grade 2 and higher AEs | 202 | 240 | 442 | 223 | 233 | 456 |
| Grade 2 and higher AEs related to study product | 3 | 2 | 5 | 12 | 42 | 54 |
| Product holds due to AEs | 0 | 0 | 0 | 0 | 0 | 0 |
| Most common grade 2 and higher AEs related to product | ||||||
| Decreased creatinine clearancê | 0 | 0 | 0 | 6 | 18 | 24 |
| Headache | 0 | 0 | 0 | 3 | 5 | 8 |
| Nausea | 0 | 0 | 0 | 0 | 8 | 8 |
| Diarrhea | 0 | 0 | 0 | 2 | 3 | 5 |
| Vomiting | 0 | 0 | 0 | 0 | 3 | 3 |
| Intermenstrual bleeding | 2 | 0 | 2 | 0 | 0 | 0 |
| Bacterial vaginosis | 1 | 0 | 1 | 0 | 0 | 0 |
| Vaginal odour | 0 | 1 | 1 | 0 | 0 | 0 |
| Vulvovaginal discomfort | 0 | 1 | 1 | 0 | 0 | 0 |
| Decreased creatinine clearance regardless of association with study product | ||||||
| Grade 2 AE | 34 | 41 | 75 | 27 | 57 | 84 |
| Grade 3 AE | 0 | 0 | 0 | 0 | 1 | 1 |
| Grade 4 AE | 0 | 0 | 0 | 0 | 0 | 0 |
| Total Serious Adverse Events (SAEs) | 0 | 0 | 0 | 2 | 1 | 3 |
| Product-related SAEs | 0 | 0 | 0 | 0 | 0 | 0 |
| SAEs resulting in hospitalization or death | 0 | 0 | 0 | 2 | 1 | 3 |
Includes all decreased creatinine clearance values from baseline, following DAIDS grading criteria which clinician attributed to that study product.
Includes all decreased creatinine clearance values from baseline, following DAIDS grading criteria during that product use period, regardless of clinician attribution to study product. Grade 2 AE for creatinine clearance includes a change in baseline of 10%. The median creatinine clearance at the end of the randomized FTC/TDF period and DPV ring periods were 99.6 ml/min (IQR 90.3–110.7 ml/min) and 101.8 ml/min (IQR 90–114.1 ml/min), respectively.
The product-related grade 2 and higher AEs reported during use of the ring were intermenstrual bleeding, vaginal discharge, vaginal odor, and vulvovaginal discomfort. No participants discontinued the ring due to tolerability issues. There were no product-related SAEs with either product.
Product choice in six months choice period and predictors of initial product choice
In Period 3, 155 (65%) initially chose the ring, 72 (30%) initially chose oral PrEP and 11 (5%) chose neither product. During the subsequent six months, those choices were relatively stable; 137 (58%) of participants continued with the ring, 62 (26%) with oral PrEP, 30 (13%) switched products during the product choice period, and 9 (4%) did not use either product. Adherence levels to chosen products in the third period was similar to adherence in the randomized six month periods (Figure 2).
Three predictors were selected for the final multivariable product choice model (Table 3), with two significant factors being reporting vaginal sex in the 3 months prior to enrollment which was associated with an odds ratio of 2.91 in favor of choosing the ring (95% CI: 1.29, 6.69; p=0.011) and always receiving feedback of high adherence (i.e., the green zone in Supplement Figure 2) to oral PrEP associated with an odds ratio of 6.15 in favor of choosing oral PrEP (95% CI: 3.27, 12.07, p<0.001).
Table 3:
Predictors of participant’s initial choice of oral PrEP or dapivirine ring, among the 227 participants who reached the product choice period
| Characteristic | Proportion choosing ring | Odds Ratio (95% CI) | p-value |
|---|---|---|---|
| Vaginal sex, 3 months prior to enrollment | |||
| No | 19/36 (53%) | ||
| Yes | 136/189 (72%) | 2.91 (1.29,6.69) | 0.011 |
| Oral PrEP feedback always green during crossover period | |||
| No | 106/126 (84%) | ||
| Yes | 49/99 (49%) | 0.16 (0.08, 0.31) | <0.001 |
| One or more grade 2 or higher AEs related to ring during crossover period | |||
| No | 153/220 (70%) | ||
| Yes | 2/5 (40%) | 0.29 (0.03, 1.96) | 0.2 |
HIV seroconversion
Four participants seroconverted to HIV during follow-up: one seroconverted at month 6 of the randomized oral PrEP period, one at month five of the randomized dapivirine ring period, one during the choice period in which she had chosen oral PrEP, and one during the choice period in which she had chosen the ring. The four seroconversions were associated with low or no product use based on TFV-DP levels and dapivirine release rates prior to seroconversion (Supplement Table 3).
Discussion
This randomized crossover open label study of the monthly dapivirine ring and daily oral PrEP for HIV preexposure prophylaxis among sexually-active AGYW from South Africa, Uganda and Zimbabwe demonstrated high safety, adherence and persistence with oral PrEP and the ring. Notably, 57% had objective evidence of high adherence to the ring and oral PrEP during the randomized periods, 40% showing some use, and <5% no product use. Participants’ motivation for HIV prevention was evident in the final product choice period; only 5% chose to use neither product, 66% of women chose the ring and 30% oral PrEP. Encouragingly, young women’s adherence to their preferred HIV prevention product and persistence remained high during the choice period, supporting the concept of providing women a menu with choice of PrEP formulation, which in this study was informed by their prior use of both oral PrEP and the ring.
The randomized crossover design enabled a direct comparison of the adherence and safety for oral PrEP and the ring. Although more AEs were reported in the randomized oral PrEP period, the safety and tolerability of both oral PrEP and the ring were high. Consistent with safety and tolerability findings from the placebo-controlled efficacy trials of these products, safety profiles were favorable and no new safety concerns were identified for either product, and only one participant discontinued product (oral PrEP) due to tolerability. Study participants reported more systemic AEs during oral PrEP use and more vaginal complaints during use of the ring, which is consistent with safety findings from the placebo-controlled efficacy trials of these products.3, 4, 6, 7 No significant nephrotoxicity was observed during oral PrEP use in this young population; changes in creatinine clearance were minor and observed with both products. The median creatinine clearance was 99.6 ml/min after six months of oral PrEP use, which was very similar to the baseline median creatinine clearance (104.2 ml/min) and after six months of the ring (101.8 ml/min), highlighting the lack of nephrotoxicity of oral PrEP with FTC/TDF in this young population.
Adherence to the ring and oral PrEP were higher than in the placebo-controlled efficacy trials and in projects with open label use.16–18, 25 Prior studies of oral PrEP and the ring have not included monthly visits or offered AGYW a choice of products, and supportive adherence counseling based on objective markers of product use. Although it is not possible to determine the precise influence of these factors on the high adherence, persistence and retention during the trial, the higher adherence to the ring and oral PrEP in this cohort of AGYW compared to prior efficacy trials, open label extensions and demonstration projects is encouraging in terms of a multi-pronged effort to provide a welcoming environment, more frequent visits, and supportive and flexible adherence strategies. Although receiving drug level feedback could have contributed to the adherence levels observed in this study (i.e., the Hawthorne effect), the provision of drug level feedback for both oral PrEP based on DBS and residual dapivirine in used rings should have been similar between the randomized product use periods.
Almost all (95%) participants chose to use either the ring or oral PrEP after six months of use of each product in the randomized periods. Two-thirds chose the ring and the strongest predictor for choosing the ring was vaginal sex in the three months prior to enrollment, perhaps due to a preference for a longer-acting discrete or topical PrEP formulation. However, one-third chose oral PrEP indicating that an important subset preferred the oral option. The association of choosing oral PrEP with having consistently received drug level feedback indicative of high adherence during the randomized periods could reflect that participants who found strategies for high adherence to oral PrEP were motivated to continue to use it. Similar to the effect of contraceptive method mix on meeting women’s preferences and needs and increasing contraceptive coverage,26, 27 a growing number of HIV prevention options, including with longer acting, lower user burden, and less user-adherence dependent formulations, are anticipated to meet the needs of a larger proportion of young women.28
Limitations of the study include a modest reduction in the sample size due to the impact of COVID-19 during national lock-downs in each country which restricted recruitment efforts. In spite of these lock-downs, retention through 18 months was 94%. During these periods, participants could be dispensed more than one ring or bottle of tablets to reduce clinic visits; there was potential misclassification of rings to a specified use period and a greater likelihood of rings not being returned, and fewer DBS samples were obtained due to increased spacing of visits. The lack of a washout period due to ethical concerns about withholding effective HIV prevention products to AGYW at risk of HIV confounds attribution of AEs in the first months of the second crossover period when side effects could be residual from the prior product or the new randomized product. A cumulative measure of AEs over each product use period was analyzed, and to address the potential of carryover effect, AEs in the first month of the second crossover period were analyzed separately from the remaining five months in that period. In the context of a crossover randomized trial with careful implementation and monitoring, a strategy of sequential product use was useful. However, this study has limitations in generalizability given potential selection bias in enrolling AGYW who were willing to have monthly visits for 18 months and the study was not designed to inform programmatic implementation of HIV prevention product choice with briefer product use periods, which would require a different study design.
HIV prevention remains a priority for African AGYW who have persistently high HIV incidence and previously had lower adherence to oral PrEP and the ring. The study population was at high risk of sexually transmitted HIV and other infections; which is similar to other PrEP demonstration projects among young African women.16, 25, 29 This observation highlights the need for etiologic STI testing, ideally point-of-care testing, and STI treatment as part of integrated sexual and reproductive health care with PrEP delivery.
The findings from the REACH study are encouraging in that they highlight that AGYW can achieve high adherence and persistence with oral PrEP and the ring for up to 18 months, with client-centered, non-judgmental, flexible adherence support strategies and monthly visits. Over half of participants had high adherence to oral PrEP and the ring and only 5% did not use their assigned product during the randomized periods. Safety and tolerability of both products was high and no new safety signals were identified. Participants in this study made informed choices about HIV prevention after six months of use of oral PrEP and the dapivirine ring with two-thirds choosing the dapivirine ring. Longer-acting formulations of PrEP may be more acceptable to African AGYW even if the efficacy is lower, and higher adherence and persistence with a chosen product make increase coverage and partially offset the lower efficacy of the ring in this population. Implementation science studies are needed to determine whether a sequential form of product choice in which AGYW can experience use of different PrEP modalities for a brief period provide women with a greater opportunity to choose a product that meets their needs.
Supplementary Material
Panel: Research in context.
Systematic review
Daily oral emtricitabine/tenofovir (FTC/TDF) tablets and the monthly dapivirine vaginal ring (ring) have been shown to be safe and effective as HIV pre-exposure prophylaxis (PrEP) among women and recommended by WHO as part of combination HIV prevention. We searched PubMed and EMBASE for studies on the safety, adherence, and preferences related to oral emtricitabine/tenofovir (FTC/TDF) and the dapivirine vaginal ring between January 2018 and September 2022 with the terms “HIV” and “Africa South of the Sahara” and (“safety” OR “adherence” OR “preferences” OR “choice”). Findings from randomized clinical trials and demonstration projects have shown that African adolescent girls and young women (AGYW) <21 years of age have lower product use, adherence and persistence through six months with oral FTC/TDF than women >21 years of age. Randomized placebo-controlled trials and open label extension studies of the ring in Africa have shown that young women <21 years had lower use of the ring which was associated with lack of effectiveness.
Added value of this study
Given the need for more consistent and higher effective use of HIV prevention products by young African women who continue to have high rates of HIV acquisition, a randomized open label cross-over study of oral PrEP and the ring followed by a product choice period provides a direct comparison of adherence and safety to oral PrEP and the ring and product choice after use of both products. Study participants had monthly visits, and were provided flexible adherence support and drug-level feedback in adherence counseling. The study provides evidence that the majority of African AGYW were able to use both oral FTC/TDF and ring with high adherence, that both products were safe, well-tolerated, and that two-thirds of AGYW chose the ring in the final product choice period.
Implications of all the available evidence
African AGYW achieved higher adherence to oral PrEP and the ring than has been previously reported. Experience with these PrEP products informed AGYW’s choice of products. A majority of African AGYW chose the ring, which is a PrEP option that is less burdensome to the user. Sexual activity and high adherence to oral PrEP during the randomized periods were associated with choice of product in the product choice period.
Acknowledgments
The study was designed and implemented by the Microbicide Trials Network (MTN). The MTN was fully funded by the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases through individual grants (UM1AI068633, UM1AI068615 and UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. Dr. Lydia Soto-Torres is an employee of NIAID. Drs. Anderson, Baeten, Bekker, Celum, Brown, Hendrix, Hillier, Hosek, Ngure, Panahlee-Phillips and van der Stratten have received NIH grants. The International Partnership for Microbicides developed the dapivirine ring and supplied rings for this trial. FTC/TDF tablets were supplied by Gilead Sciences, Inc. We thank the women who participated in this study for their motivation and dedication, the communities that supported this work, and the leadership and staff at the clinical research sites which conducted the study.
Funding:
National Institutes of Health
Footnotes
Data sharing
Individual participant data that underlie the results reported in this Article, after de-identification, are available, beginning after publication, as well as the study protocol, data dictionary, statistical analysis plan, and informed consent. Data are available for researchers who provide a methodologically sound proposal in accordance with policies of the Microbicide Trials Network (MTN).
Declaration of interests
Connie Celum has received consulting fees from Gilead Sciences and Merck, and has provided expert witness for Gilead. Sharon Hillier has received consulting fees and funds to her institution from Merck. Kenneth Ngure has received research funds from Merck (MSD). Jared Baeten and Jim Rooney are employees of Gilead Sciences. All other authors declare no competing interests.
Clinicaltrials.gov registration: NCT03593655
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Joshi K, Lessler J, Olawore O, Loevinsohn G, Bushey S, Tobian AAR, et al. Declining HIV incidence in sub-Saharan Africa: a systematic review and meta-analysis of empiric data. J Int AIDS Soc. 2021;24(10):e25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ECHO Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet. 2019;394(10195):303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375(22):2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375(22):2133–43. [DOI] [PubMed] [Google Scholar]
- 5.Gray GE, Bekker LG, Laher F, Malahleha M, Allen M, Moodie Z, et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N Engl J Med. 2021;384(12):1089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of preexposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. [DOI] [PubMed] [Google Scholar]
- 10.Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. AIDS. 2015;29(7):819–24. [DOI] [PubMed] [Google Scholar]
- 11.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanscom B, Janes HE, Guarino PD, Huang Y, Brown ER, Chen YQ, et al. Brief Report: Preventing HIV-1 infection in women using oral preexposure prophylaxis: a meta-analysis of current evidence. J Acquir Immune Defic Syndr. 2016;73(5):606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Straten A, Montgomery ET, Hartmann M, Minnis A. Methodological lessons from clinical trials and the future of microbicide research. Curr HIV/AIDS Rep. 2013;10(1):89–102. [DOI] [PubMed] [Google Scholar]
- 15.Stoner MCD, Rucinski KB, Giovenco D, Gill K, Morton JF, Bekker LG, et al. Trajectories of PrEP adherence among young women aged 16 to 25 in Cape Town, South Africa. AIDS Behav. 2021;25(7):2046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celum C, Hosek S, Tsholwana M, Kassim S, Mukaka S, Dye BJ, et al. PrEP uptake, persistence, adherence, and effect of retrospective drug level feedback on PrEP adherence among young women in southern Africa: Results from HPTN 082, a randomized controlled trial. PLoS Med. 2021;18(6):e1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baeten JM, Palanee-Phillips T, Mgodi NM, Mayo AJ, Szydlo DW, Ramjee G, et al. Safety, uptake, and use of a dapivirine vaginal ring for HIV-1 prevention in African women (HOPE): an open-label, extension study. Lancet HIV. 2021;8(2):e87–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nel A, van Niekerk N, Van Baelen B, Malherbe M, Mans W, Carter A, et al. Safety, adherence, and HIV-1 seroconversion among women using the dapivirine vaginal ring (DREAM): an open-label, extension study. Lancet HIV. 2021;8(2):e77–e86. [DOI] [PubMed] [Google Scholar]
- 19.Brown ER, Hendrix CW, van der Straten A, Kiweewa FM, Mgodi NM, Palanee-Philips T, et al. Greater dapivirine release from the dapivirine vaginal ring is correlated with lower risk of HIV-1 acquisition: a secondary analysis from a randomized, placebo-controlled trial. J Int AIDS Soc. 2020;23(11):e25634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. WHO recommends the dapivirine vaginal ring as a new choice for HIV prevention for women at substantial risk of HIV infection 2021 [Internet]. [cited 2022 December 20] from: https://www.who.int/news/item/26-01-2021-who-recommends-the-dapivirine-vaginal-ring-as-a-new-choice-for-hiv-prevention-for-women-at-substantial-risk-of-hiv-infection.
- 21.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother. 2018;62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts ST, Mancuso N, Williams K, Kalule HN, Mposula H, Mugocha C, et al. Strategies to support effective use of the vaginal ring and oral PrEP among adolescent girls and young women in sub-Saharan Africa: qualitative findings from MTN-034/REACH. AIDS 2022; 29 July - 2 August; Montreal, CA 2022. [Google Scholar]
- 24.Division of AIDS. DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1, July 2017. and Addendum 1. National Institute of Allergy and Infectious Diseases.
- 25.Celum CL, Bukusi EA, Bekker LG, Delany-Moretlwe S, Kidoguchi L, Omollo V, et al. PrEP use and HIV seroconversion rates in adolescent girls and young women from Kenya and South Africa: the POWER demonstration project. J Int AIDS Soc. 2022;25(7):e25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haakenstad A, Angelino O, Irvine CMS, Bhutta ZA, Bienhoff K, Bintz C, et al. Measuring contraceptive method mix, prevalence, and demand satisfied by age and marital status in 204 countries and territories, 1970–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400(10348):295–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaffield ME, Kiarie J. Setting global standards: The paramount importance of considering contraceptive values and preferences of clients and providers. Contraception. 2022;111:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bekker LG, Pike C, Hillier SL. HIV prevention: better choice for better coverage. J Int AIDS Soc. 2022;25(1):e25872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celum CL, Gill K, Morton JF, Stein G, Myers L, Thomas KK, et al. Incentives conditioned on tenofovir levels to support PrEP adherence among young South African women: a randomized trial. J Int AIDS Soc. 2020;23(11):e25636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.