Abstract
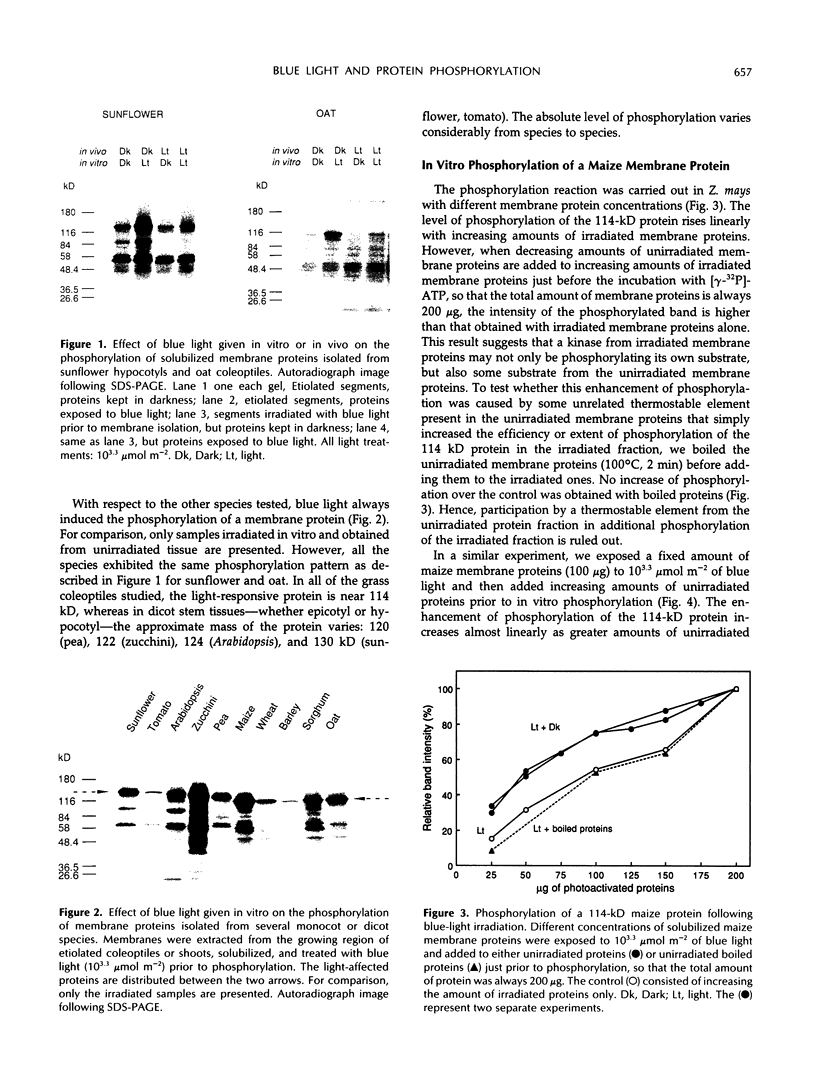
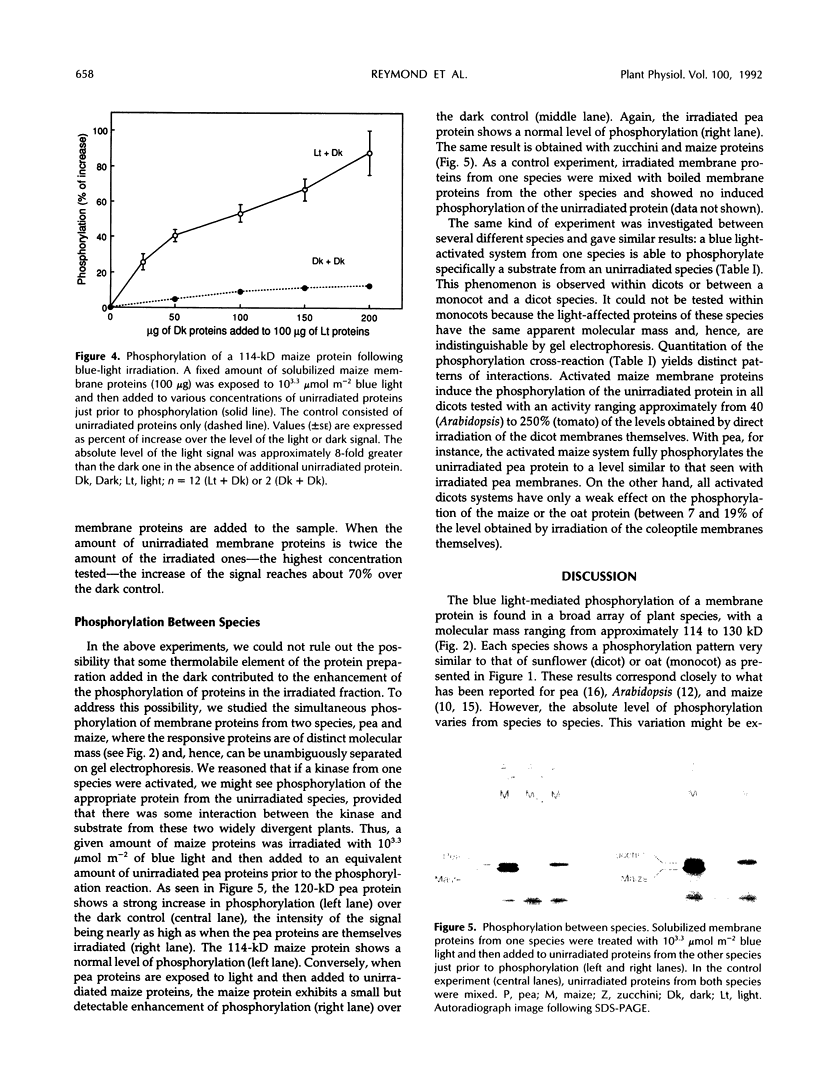
Blue light mediates the phosphorylation of a membrane protein in seedlings from several plant species. When crude microsomal membrane proteins from dark-grown pea (Pisum sativum L.), sunflower (Helianthus annuus L.), zucchini (Cucurbita pepo L.), Arabidopsis (Arabidopsis thaliana L.), or tomato (Lycopersicon esculentum L.) stem segments, or from maize (Zea mays L.), barley (Hordeum vulgare L.), oat (Avena sativa L.), wheat (Triticum aestivum L.), or sorghum (Sorghum bicolor L.) coleoptiles are illuminated and incubated in vitro with [γ-32P]ATP, a protein of apparent molecular mass from 114 to 130 kD is rapidly phosphorylated. Hence, this system is probably ubiquitous in higher plants. Solubilized maize membranes exposed to blue light and added to unirradiated solubilized maize membranes show a higher level of phosphorylation of the light-affected protein than irradiated membrane proteins alone, suggesting that an unirradiated substrate is phosphorylated by a light-activated kinase. This finding is further demonstrated with membrane proteins from two different species, where the phosphorylated proteins are of different sizes and, hence, unambiguously distinguishable on gel electrophoresis. When solubilized membrane proteins from one species are irradiated and added to unirradiated membrane proteins from another species, the unirradiated protein becomes phosphorylated. These experiments indicate that the irradiated fraction can store the light signal for subsequent phosphorylation in the dark. They also support the hypothesis that light activates a specific kinase and that the systems share a close functional homology among different higher plants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budde R. J., Randall D. D. Light as a signal influencing the phosphorylation status of plant proteins. Plant Physiol. 1990 Dec;94(4):1501–1504. doi: 10.1104/pp.94.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S., Short T. W., Ray P. M., Pratt L. H., Briggs W. R. Light-mediated changes in two proteins found associated with plasma membrane fractions from pea stem sections. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8003–8007. doi: 10.1073/pnas.85.21.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P., Short T. W., Briggs W. R., Poff K. L. Light-induced phosphorylation of a membrane protein plays an early role in signal transduction for phototropism in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1992 May;89(10):4718–4721. doi: 10.1073/pnas.89.10.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short T. W., Briggs W. R. Characterization of a Rapid, Blue Light-Mediated Change in Detectable Phosphorylation of a Plasma Membrane Protein from Etiolated Pea (Pisum sativum L.) Seedlings. Plant Physiol. 1990 Jan;92(1):179–185. doi: 10.1104/pp.92.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]