Abstract
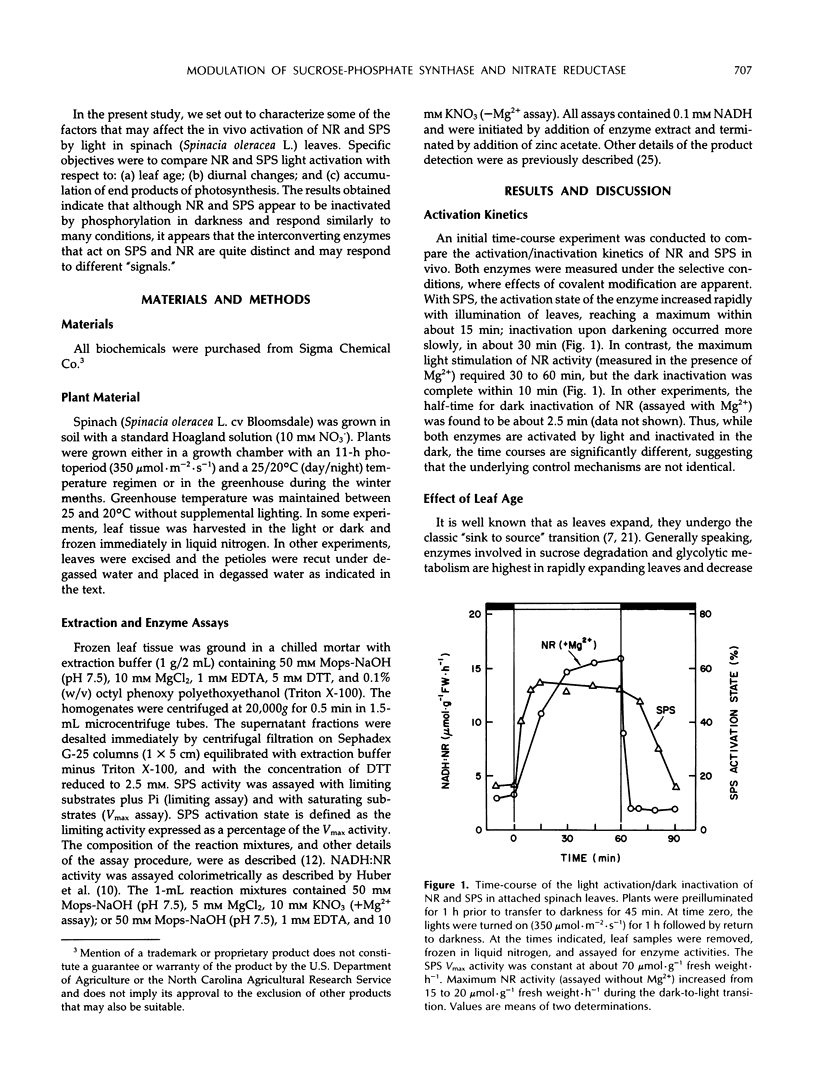
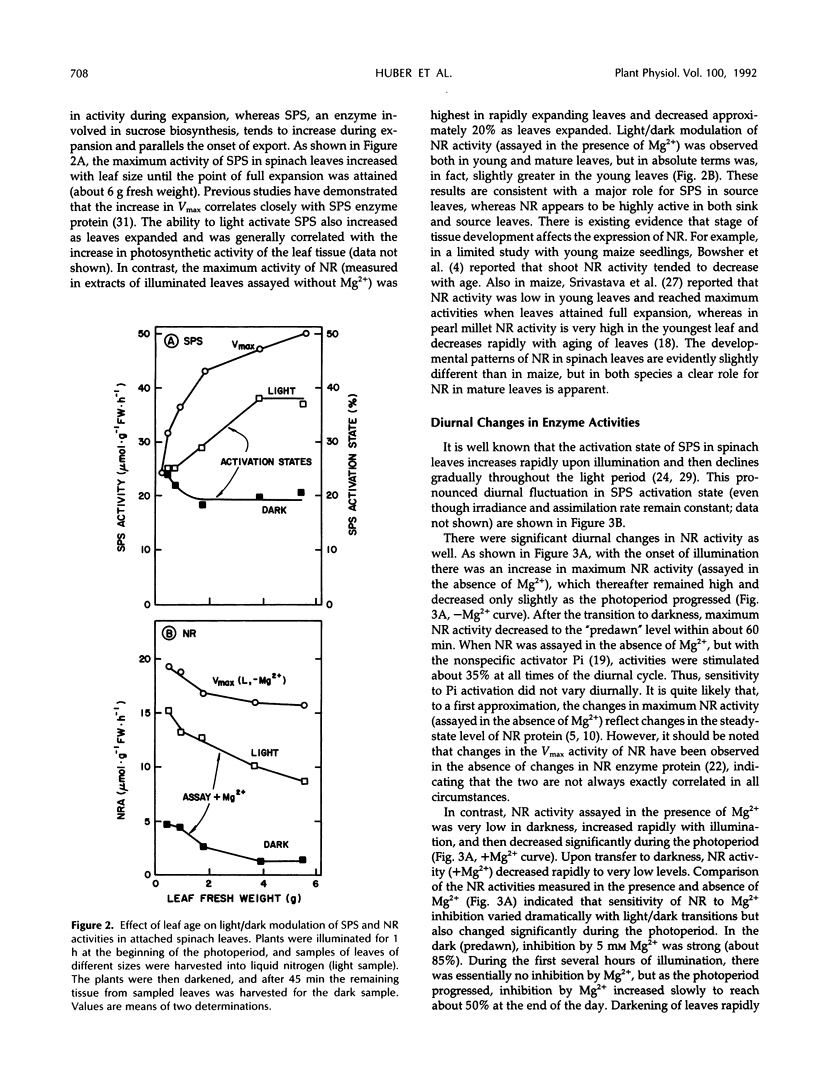
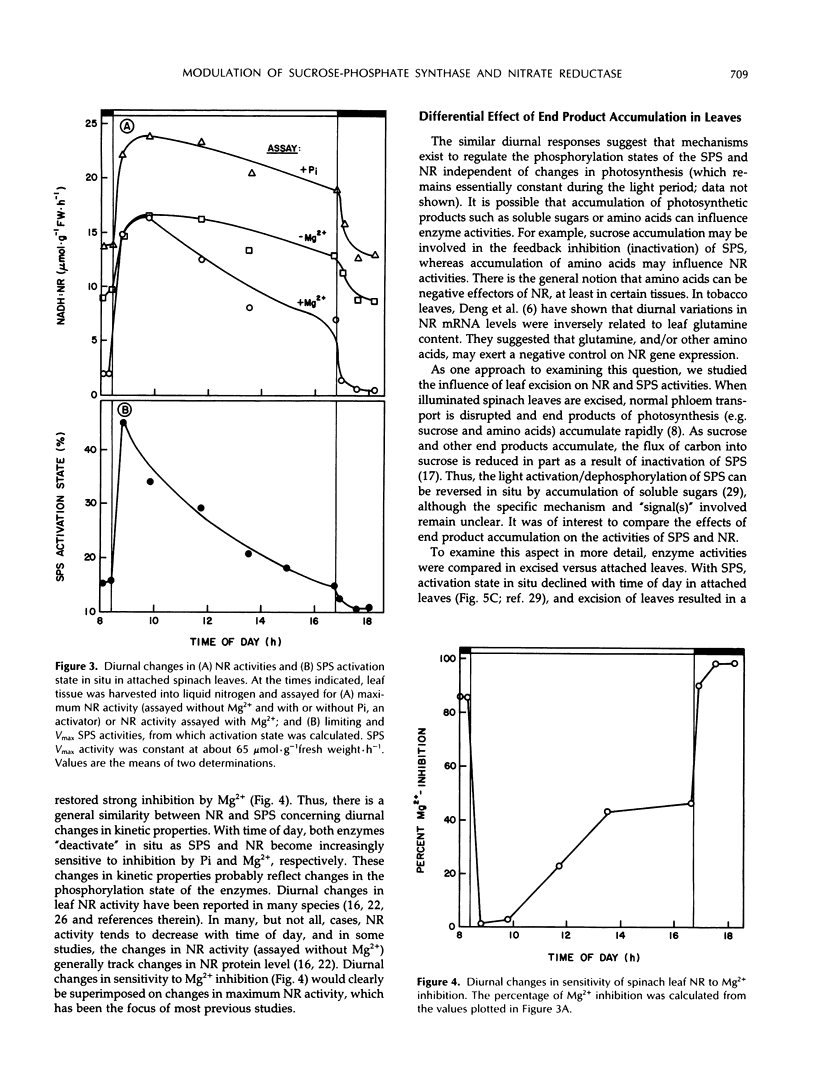
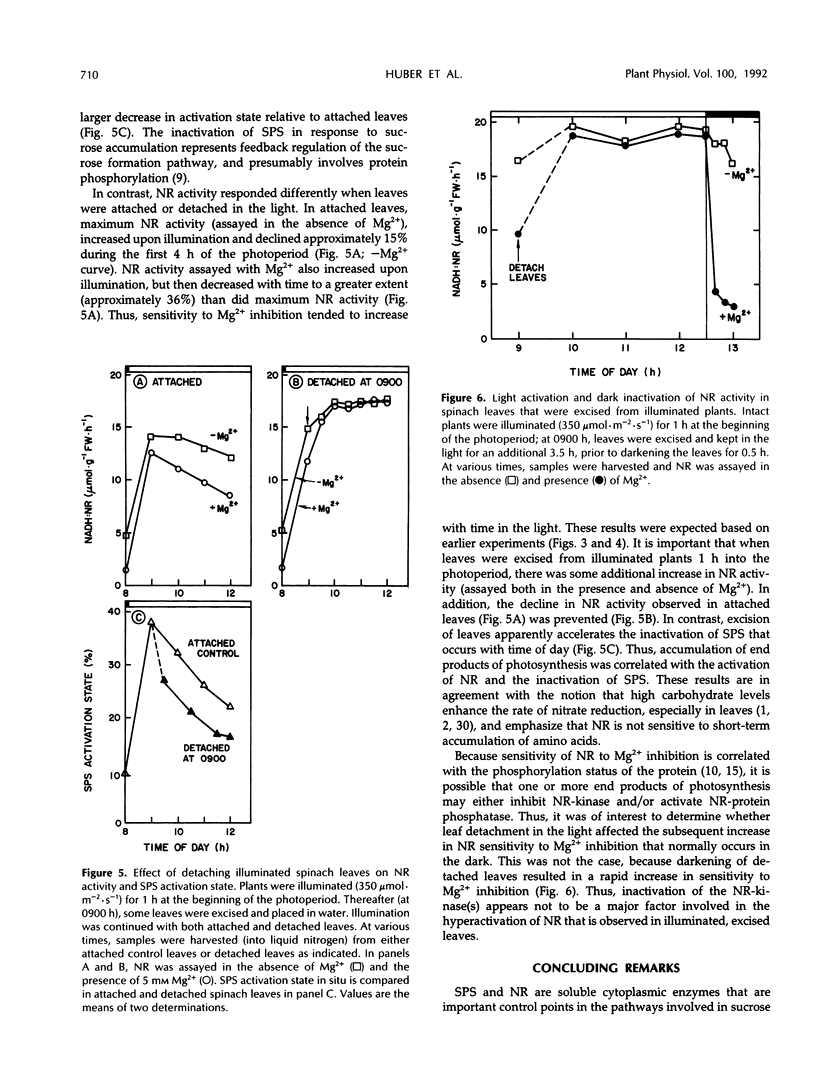
We recently obtained evidence that the activity of spinach (Spinacia oleracea L.) leaf nitrate reductase (NR) responds rapidly and reversibly to light/dark transitions by a mechanism that is strongly correlated with protein phosphorylation. Phosphorylation of the NR protein appears to increase sensitivity to Mg2+ inhibition, without affecting activity in the absence of Mg2+. In the present study, we have compared the light/dark modulation of sucrose-phosphate synthase (SPS), also known to be regulated by protein phosphorylation, and NR activities (assayed with and without Mg2+) in spinach leaves. There appears to be a physiological role for both enzymes in mature source leaves (production of sucrose and amino acids for export), whereas NR is also present and activated by light in immature sink leaves. In mature leaves, there are significant diurnal changes in SPS and NR activities (assayed under selective conditions where phosphorylation status affects enzyme activity) during a normal day/night cycle. With both enzymes, activities are highest in the morning and decline as the photoperiod progresses. For SPS, diurnal changes are largely the result of phosphorylation/dephosphorylation, whereas with NR, the covalent modification is super-imposed on changes in the level of NR protein. Accumulation of end products of photosynthesis in excised illuminated leaves increased maximum NR activity, reduced its sensitivity of Mg2+ inhibition, and prevented the decline in activity with time in the light seen with attached leaves. In contrast, SPS was rapidly inactivated in excised leaves. Overall, NR and SPS share many common features of control but are not identical in terms of regulation in situ.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Huffaker R. C. Dependency of Nitrate Reduction on Soluble Carbohydrates in Primary Leaves of Barley under Aerobic Conditions. Plant Physiol. 1984 Jul;75(3):623–628. doi: 10.1104/pp.75.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Huffaker R. C., Rains D. W., Rao K. P. Influence of light and ambient carbon dioxide concentration on nitrate assimilation by intact barley seedlings. Plant Physiol. 1979 Jun;63(6):1205–1209. doi: 10.1104/pp.63.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher C. G., Long D. M., Oaks A., Rothstein S. J. Effect of light/dark cycles on expression of nitrate assimilatory genes in maize shoots and roots. Plant Physiol. 1991 Jan;95(1):281–285. doi: 10.1104/pp.95.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Source and sink leaf metabolism in relation to Phloem translocation: carbon partitioning and enzymology. Plant Physiol. 1978 Mar;61(3):380–385. doi: 10.1104/pp.61.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. L., Huber S. C., Campbell W. H., Redinbaugh M. G. Reversible light/dark modulation of spinach leaf nitrate reductase activity involves protein phosphorylation. Arch Biochem Biophys. 1992 Jul;296(1):58–65. doi: 10.1016/0003-9861(92)90544-7. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Bickett D. M. Evidence for control of carbon partitioning by fructose 2,6-bisphosphate in spinach leaves. Plant Physiol. 1984 Feb;74(2):445–447. doi: 10.1104/pp.74.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Brendle-Behnisch E. Rapid Modulation of Spinach Leaf Nitrate Reductase Activity by Photosynthesis : I. Modulation in Vivo by CO(2) Availability. Plant Physiol. 1991 Jun;96(2):363–367. doi: 10.1104/pp.96.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Spill D. Rapid Modulation of Spinach Leaf Nitrate Reductase by Photosynthesis : II. In Vitro Modulation by ATP and AMP. Plant Physiol. 1991 Jun;96(2):368–375. doi: 10.1104/pp.96.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oji Y., Ryoma Y., Wakiuchi N., Okamoto S. Effect of Inorganic Orthophosphate on in Vitro Activity of NADH-Nitrate Reductase Isolated from 2-Row Barley Leaves. Plant Physiol. 1987 Mar;83(3):472–474. doi: 10.1104/pp.83.3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmler J. L., Campbell W. H. Regulation of Corn Leaf Nitrate Reductase : II. Synthesis and Turnover of the Enzyme's Activity and Protein. Plant Physiol. 1986 Feb;80(2):442–447. doi: 10.1104/pp.80.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riens B., Heldt H. W. Decrease of Nitrate Reductase Activity in Spinach Leaves during a Light-Dark Transition. Plant Physiol. 1992 Feb;98(2):573–577. doi: 10.1104/pp.98.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Fondy B. R., Li B., Geiger D. R. Sources of Carbon for Export from Spinach Leaves throughout the Day. Plant Physiol. 1989 Jul;90(3):1168–1174. doi: 10.1104/pp.90.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell A. C., Bruneau J. M., Summerfelt K., Boersig M., Voelker T. A. Expression of a maize sucrose phosphate synthase in tomato alters leaf carbohydrate partitioning. Plant Cell. 1991 Oct;3(10):1121–1130. doi: 10.1105/tpc.3.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]


