Abstract
Introduction
Neuromuscular electrical stimulation (NMES) is a treatment to prevent or reverse acquired disability in hospitalised adults. We conducted a systematic review and meta-analysis of its effectiveness.
Method
We searched MEDLINE, EMBASE, Cumulative Index to Nursing & Allied Health (CINAHL) and the Cochrane library. Inclusion criteria: randomised controlled trials of hospitalised adult patients comparing NMES to control or usual care. The primary outcome was muscle strength. Secondary outcomes were muscle size, function, hospital length of stay, molecular and cellular biomarkers, and adverse effects. We assessed risk of bias using the Cochrane risk-of-bias tool. We used Review Manager (RevMan) software for data extraction, critical appraisal and synthesis. We assessed certainty using the Grading of Recommendations Assessment, Development and Evaluation tool.
Results
A total of 42 papers were included involving 1,452 participants. Most studies had unclear or high risk of bias. NMES had a small effect on muscle strength (moderate certainty) (standardised mean difference (SMD) = 0.33; P < 0.00001), a moderate effect on muscle size (moderate certainty) (SMD = 0.66; P < 0.005), a small effect on walking performance (moderate certainty) (SMD = 0.48; P < 0.0001) and a small effect on functional mobility (low certainty) (SMD = 0.31; P < 0.05). There was a small and non-significant effect on health-related quality of life (very low certainty) (SMD = 0.35; P > 0.05). In total, 9% of participants reported undesirable experiences. The effects of NMES on length of hospital stay, and molecular and cellular biomarkers were unclear.
Conclusions
NMES is a promising intervention component that might help to reduce or prevent hospital-acquired disability.
Keywords: neuromuscular electrical stimulation, hospital-acquired disability, muscle strength, physical function, systematic review, older people
Key Points
Neuromuscular electrical stimulation is a potential intervention to reduce hospital-acquired disability.
Neuromuscular electrical stimulation improves muscle strength, size, walking and functioning performance in hospitalised adults.
Further applied research should optimise the stimulation parameters and evaluate its contribution to rehabilitation programmes.
Introduction
People admitted to hospital frequently develop hospital-acquired disability [1]. This is partly due to loss of muscle mass and function, in turn due to factors including immobilisation, inflammation and malnutrition [2].
Early rehabilitation using exercise improves outcomes in hospital patients [3–5]. In practice, however, many patients are medically unstable or experience symptoms that render exercise unfeasible [6, 7]. An alternative or additional intervention is neuromuscular electrical stimulation (NMES), in which involuntary muscle contraction occurs from non-invasive, low-frequency current transmitted through electrodes typically placed over thigh and leg muscles. Patients can use NMES in bed or seated, with or without voluntary effort [8, 9].
Previous systematic reviews of NMES have shown inconsistent effects in conditions including elective surgery [10], neurological disorders [11, 12], osteoarthritis [13], chronic obstructive pulmonary disease (COPD) [14, 15], heart failure [16], advanced diseases (chronic respiratory disease, chronic heart failure, cancer or HIV/AIDS) [17], cancer [18] and intensive care unit (ICU) patients [15, 19, 20]. However, no review has focused on the effectiveness of lower limb NMES in hospitalised adults. We report a systematic review and meta-analysis to examine evidence for the effects of NMES in hospitalised adults.
Methods
The protocol followed PRISMA-P guidelines [21] and was registered at the International Prospective Register of Systematic Reviews (PROSPERO; registration number: CRD42021259763).
Eligibility criteria
Inclusion criteria
Participants: adults (aged ≥18 years) hospitalised with acute medical or acute or elective surgical conditions.
Intervention: NMES applied to a limb, whether given as a single intervention or in combination with other interventions.
Control: no, sham treatment or other usual care.
-
Outcomes: including one or more of the outcomes of interest.
Primary outcomes: muscle strength: chosen as the most immediate and direct benefit of NMES.
-
Secondary outcomes, including the following outcomes and categories:
-
▪
Muscle size: sarcopenia is the combination of reduced muscle strength and mass.
-
▪
Function: to examine whether any benefits of NMES translate into functional gains.
-
▪
Hospital length of stay: economic importance.
-
▪
Molecular and cellular (fibre type composition; inflammatory mediators; muscle protein synthesis and breakdown; bone; lipid and lipoprotein markers): to illuminate the mechanism of action of NMES and identify biomarkers.
-
▪
Adverse effects: the decision to use NMES is a balance between benefits and harms.
-
▪
Study design: randomised controlled trials (RCTs) and quasi-randomised controlled trials.
Exclusion criteria
Participants: patients selected due to psychiatric, speech, swallowing or facial disorders.
Intervention: NMES superimposed onto movement or not applied to a limb (e.g. solely applied to treat facial, swallowing or speech problems); electrical stimulation used for its afferent effect such as for pain or spasticity rather than to produce muscular stimulation (transcutaneous elections stimulation); or pulsed electrical stimulation to augment normal movement such as functional electrical stimulation.
Control: where any control conditions, treatments or interventions other than NMES were different from the intervention group, for example if NMES was given with an exercise programme that was not given to the control group, or where the control group had an exercise programme not given to the NMES group; where the control comparison only a difference between NMES parameters.
Reporting: not published in English, for researcher resource reasons.
Information sources
MEDLINE, EMBASE, Cumulative Index to Nursing & Allied Health (CINAHL) and Cochrane library electronic databases were searched from inception to 18 February 2023. Trial registers were not searched for unpublished studies, which may be less reliable than studies that have been through peer review.
Search strategy
Keywords used to perform the search were adults AND hospitalised AND critically ill AND neuromuscular electrical stimulation (supplementary file). Reference lists of selected studies were searched for additional studies.
Selection process
Two reviewers independently screened titles and abstracts against eligibility criteria, using Rayyan software. From those included at this stage, two reviewers independently examined full-text articles against eligibility criteria. Any disagreements were resolved by discussion or by a third reviewer.
Data extraction
Two reviewers independently performed data extraction for all included studies using a standardised data extraction table. Information extracted included study information (first author, publication year, country, design, study period and setting), participant characteristics (conditions, total sample size, gender, age), intervention group (sample size, gender, age, detailed protocol parameters, additional intervention), control group (sample size, gender, age, intervention type), outcome measures, follow-up, results, dropout and limitations.
Risk-of-bias assessment
Two reviewers independently performed risk-of-bias assessments (RoB) using a third reviewer to resolve disagreements. The Cochrane Collaboration RoB assessment tool for randomised trials [22] was used, which takes account of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases. Each aspect was graded using three levels (low, unclear or high risk of bias).
Quality assessment
The certainty level of the result for each outcome of interest was determined with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool [23]. This assessed risk of bias, inconsistency of results, indirectness of evidence, imprecision and reporting bias. Certainty was classified as high (no serious concerns found in the five domains), moderate (serious concerns found in one of five domains), low (serious concerns found in two of five domains) or very low (serious concerns found in three or more of five domains).
Data analysis and synthesis
Meta-analyses were conducted, using Review Manager software (RevMan version 5.4; The Cochrane Collaboration, 2020), if three or more studies with similar interventions investigated the same outcome domain using comparable measures. Standardised mean difference (SMD), 95% confidence intervals (CI) and two-sided P values were calculated to measure treatment effects for each outcome. The effect sizes (d) were classified using Cohen’s classification, whereby d <0.2 was ‘no effect’, d = 0.2–0.49 was ‘small’, d = 0.5–0.79 was ‘moderate’ and d ≥0.8 was ‘large effect’ [24].
For missing data, means and standard deviations (SD) were approximated from available data, such as medians, interquartile ranges and minimum–maximum values using previously reported methods [25–27], and included in meta-analyses unless significant skewness was detected [28]. Standard errors or 95% CIs were converted to SDs using RevMan and Cochrane calculations. If more than one outcome measure was recorded for an outcome in the same study, the most valid and reliable measure was used. Heterogeneity was assessed using the I2 statistic, where a value between 25 and 50% corresponds to low heterogeneity, 50–75% to moderate heterogeneity and >75% to high heterogeneity. A random-effects model was used, as heterogeneity was expected. Sensitivity analyses were performed when approximated values were used. Funnel plot and Egger’s regression test were used to investigate publication bias [29]. Post hoc secondary analyses to compare non-ICU with ICU patients were performed to explore possible heterogeneity due to this setting and patient group.
Results
Study selection
The search strategy yielded 2,026 unique titles: 1,914 were excluded based on title and abstract. Of the remaining 112 papers, 70 were excluded on full-text review (supplementary file), leaving 42 eligible papers from 38 studies [30–71]: all 38 studies were included in the qualitative synthesis [30–49, 51–61, 66–71], and 39 papers from 35 studies were included in meta-analyses [30, 31, 33–44, 46–60, 62–71] (Figure 1).
Figure 1.
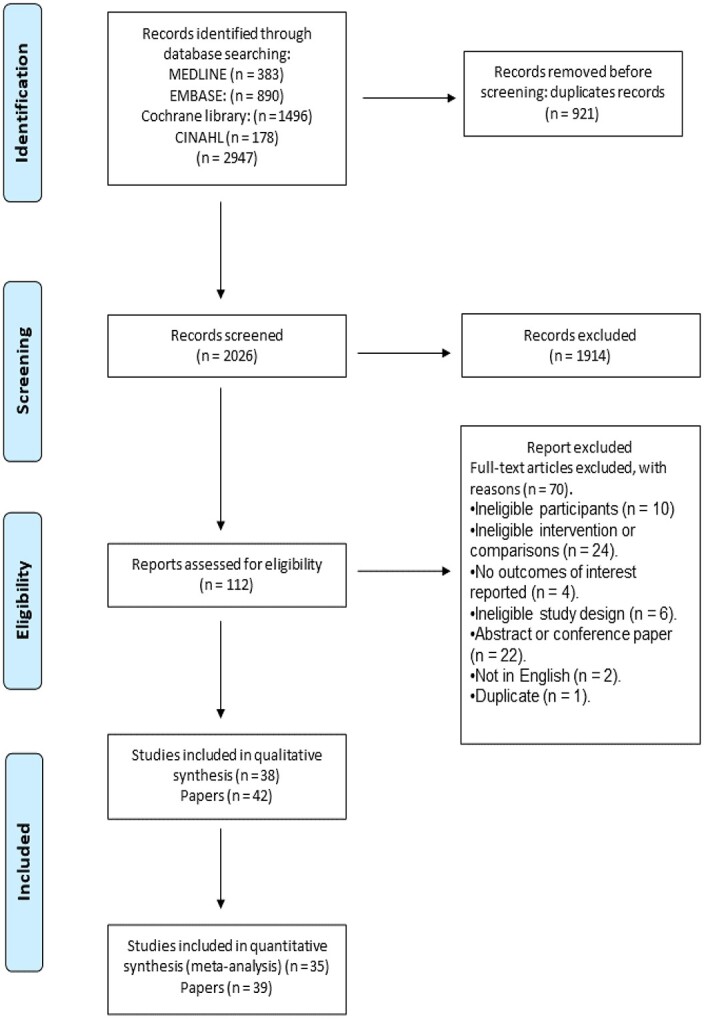
Results of search and study selection process.
Characteristics of the studies included
Sample sizes ranged from 6 to 180 participants, with a total sample size of 1,473 participants in all 42 papers. Data from 1,452 participants were used for analysis because in two studies [32, 62] only two of three participant groups were eligible for inclusion. The trials included 1,452 participants but, because study design for 151 participants involved a comparison between their treated and untreated legs, the number of data points for comparison between NMES and control was 1,603.
In total, 894/1,452 (61%) participants were male: two studies included only males [34, 56]. The age range was 19–86 years. Twenty-two studies were conducted in Europe [30, 32–37, 43, 45, 47–51, 55, 56, 58, 59, 61, 62, 66, 70], eight in South America [38–40, 42, 44, 46, 57, 60], five in Asia [41, 53, 54, 67, 68], two in North America [52, 71] and one in Africa [31]. Studies were published between 2003 and 2023. Included studies involved a variety of patient conditions: critical illness [31, 38, 43–45, 47, 49, 52, 54, 58–60]; post-surgery [35–37, 39, 40, 50, 51, 53, 61, 66, 67]; COPD [30, 32, 48, 70]; heart failure [33, 42, 46, 55, 68, 69]; spinal injury [34]; sepsis [56, 57]; COVID-19 [71]; frailty [68]. Table 1 summarises the studies’ characteristics (for full studies characteristics, see supplementary file).
Table 1.
Characteristics of the included studies
| Author | Conditions and setting | N (CON/NMES) | Interventions | Outcomes |
|---|---|---|---|---|
| Abdellaoui, 2011 [30] France |
COPD patients Setting: ICU |
17 (6/9) | Sham vs NMES | Muscle strength Muscle oxidation 6-minute walk test Muscle structure |
| Abu-khaber, 2013 [31] Egypt |
Patients on mechanical ventilation Setting: ICU |
80 (40/40) | CON (no treatment) vs NMES | Muscle strength |
| Akar, 2015 [32] Turkey |
Patients on mechanical ventilation Setting: ICU |
20 (10/10) | Mobilisation vs NMES + mobilisation | Muscle strength Mobilisation function Inflammatory response |
| Arenja, 2021 [33] Switzerland |
Acute heart failure old patients Setting: Hospital/home |
13 (5/4/4) | CON vs low NMES vs high NMES | 6-minute walk test Gait speed Health-related QoL |
| Arija-Blázques, 2014 [34] Spain |
Spinal cord injury patients Setting: Hospital |
8 (3/5) | Sham vs NMES | Muscle CSA Bone markers |
| Avramidis, 2011 [35] Greece |
Patients with total knee arthroplasty Setting: Hospital/home |
70 (35/35) | CON (PT) vs NMES + PT | Knee function 3-minute walk test QoL |
| Avramidis, 2003 [36] UK |
Patients with total knee arthroplasty Setting: Hospital/home |
30 (15/15) | CON (PT) vs NMES + PT | Knee pain 3-minute walk test |
| Braid, 2007 [37] UK |
Femoral fracture patients Setting: Hospital/home |
26 (11/15) | CON (PT) vs NMES + PT | Functional mobility Health-related QoL |
| Campos, 2022 [38] Brazil |
Critically ill patients Setting: ICU |
74 (40/34) | CON (EM) vs NMES + EM | Functional status Muscle strength Adverse events |
| Cerqueira, 2018 [39] Brazil |
Patients after cardiac valve surgery Setting: ICU |
59 (33/26) | CON (PT) vs NMES + PT | Walking test Muscle strength Functional independence Health-related QoL |
| Cerqueira, 2018 [40] Brazil |
Patients undergoing cardiac surgery Setting: ICU |
45 (22/23) | CON (PT) vs NMES + PT | 6-minute walking test Lactate level Muscle strength Functional independence |
| Chen, 2019 [41] Tiawan |
Patients undergoing prolonged mechanical ventilation Setting: ICU |
33 (17/16) | Sham vs NMES | Muscle thickness and circumference Muscle strength Physical function |
| de Araújo, 2012 [42] Brazil |
Heart failure patients Setting: Hospital |
20 (10/10) | CON (rehabilitation) vs NMES + rehabilitation | 6-minute walking test Blood lactate Oxygen saturation |
| Dirks, 2015 [43] Belgium |
Critically ill comatose patients Setting: ICU |
6 (within subject) | Sham vs NMES | Muscle fibre CSA mRNA and protein expression of selected genes |
| Falavigna, 2013 [44] Brazil |
Patients on mechanical ventilation Setting: ICU |
11 (within subject) | CON vs NMES | Muscle strength ROM Muscle mass |
| Fischer, 2016 [45] Austria |
Critically ill patients after cardiothoracic surgery Setting: ICU |
54 (27/27) | Sham vs NMES | Muscle thickness Muscle strength Functional independence |
| Forestieri, 2017 [46] Brazil |
Advanced heart failure patients Setting: Hospital |
49 (25/24) | CON vs NMES | 6-minute walking test |
| Gerovasili, 2009 [47] Greece |
Critically ill patients Setting: ICU |
26 (13/13) | CON vs NMES | Muscle mass |
| Giavedoni, 2012 [48] UK |
COPD patients Setting: Hospital/home |
11 (within subject) | CON vs NMES | Muscle strength |
| Gruther, 2010 [49] Austria |
Critically ill patients Setting: ICU |
33 (17/16) | Sham vs NMES | Muscle thickness |
| Harbo, 2018 [50] Denmark |
Guillain–Barre syndrome Setting: Hospital |
16 (within subject) | CON vs NMES | Muscle CSA Muscle strength |
| Hardy, 2022 [51] UK |
Patients undergoing abdominal surgery Setting: Hospital |
15 (within subject) | CON vs NMES | Muscle CSA Muscle thickness Muscle architecture Muscle strength Physical activity level |
| Kho, 2015 [52] USA |
Critically ill patients on mechanical ventilation Setting: ICU |
34 (18/16) | CON vs NMES | Muscle strength Functional status Maximum walking distance test Hospital LoS |
| Kitamura, 2019 [53] Japan |
Patients after cardiovascular surgery | 119 (59/60) | CON vs NMES | Knee muscle strength Concentration of 3-methylhistidine corrected for urinary creatinine 10-minute walk test |
| Nakanishi, 2020 [54] Japan |
Critically ill patients on mechanical ventilation Setting: ICU |
36 (19/17) | CON (mobilisation) vs NMES + mobilisation | Muscle mass Muscle strength ICU mobility Hospital LoS Amino acid |
| Poltavskaya, 2022 [55] Russia |
Heart failure patients Setting: Hospital |
45 (23/22) | Sham vs NMES | 6-minute walking test QoL Adverse events |
| Poulsen, 2011 [56] Denmark |
Septic shock patients Setting: ICU |
16 (8/8) | CON vs NMES | Muscle volume |
| Rodriguez, 2012 [57] Argentina |
Septic patients requiring mechanical ventilation Setting: ICU |
14 (within subject) | CON vs NMES | Arm and leg circumference Biceps thickness Muscle strength |
| Routsi, 2010 [58] Greece |
Critically ill patients Setting: ICU |
52 (28/24) | CON vs NMES | Muscle strength |
| Segers, 2021 [59] Belgium |
Critically ill patients Setting: ICU |
47 (within subject) | CON vs NMES | Muscle mass Muscle strength Morphological and molecular markers |
| Silva, 2019 [60] Brazil |
Traumatic brain injury patients on mechanical ventilation Setting: ICU |
60 (30/30) | CON (PT) + NMES + PT | Muscle architecture Plasma level of systematic inflammation Catabolic responses Hospital LoS |
| Strasser, 2009 [61] Austria |
Patients who underwent abdominal surgery Setting: Hospital |
18 (within subject) | Sham vs NMES | mRNA level of IGF-1Ea mRNA level of MGF Total RNA content Total protein content Ubiquitin-conjugated proteins Proteasome activity |
| Suetta, 2004, 2008, 2010 [62–65] Denmark |
Patients scheduled for unilateral hip replacement surgery Setting: Hospital/home |
19 (9/10) | CON vs NMES | Muscle CSA Muscle thickness Muscle strength Hospital LoS Walking test Stair climbing test Sit-to-stand test IGF-I |
| Sumin, 2020 [66] Russia |
Patients with postoperative complications after cardiovascular surgery Setting: ICU/hospital |
37 (19/18) | CON vs NMES | Knee extensor strength Knee flexor strength Muscle CSA 6-minute walking test |
| Takino, 2023 [67] Japan |
Patients with diabetes after cardiovascular surgery Setting: Hospital |
180 (90/90) | Sham vs NMES | Knee extensors strength 10-minute walking speed |
| Tanaka, 2022 [68, 69] Japan |
Frail old patients with acute decompensated heart failure Setting: Hospital |
31 (16/15) | CON (mobilisation) vs NMES + mobilisation | Muscle strength 6-minute walking test Clinical function Adverse events |
| Vivodtzev, 2006 [70] France |
COPD patients Setting: Hospital |
17 (8/9) | CON vs NMES | QoL Muscle strength Muscle mass 6-minute walking test |
| Zulbaran-Rojas, 2022 [71] USA |
COVID-19 patients Setting: ICU |
16 (8/8) | Sham vs NMES | Ankle strength Oxygen saturation Safety |
Abbreviation: N, sample size; CON, control; NMES; neuromuscular electrical stimulation; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; CSA, cross-sectional area; PT, physiotherapy; EM, early mobilisation; LoS, length of stay; IGF-I, insulin-like growth factor I; IGF-1Ea, insulin-like growth factor-1EA; MGF, mechano growth factor; QoL, quality of life.
The intervention
Location of the delivery of NMES varied between studies: twenty-one studies were in ICU [30–32, 38–41, 43–45, 47, 49, 52, 54, 56–60, 66, 71], in two of which NMES continued for the remainder of the hospital stay [41, 66]. In seventeen studies, NMES was delivered in hospital wards [33–37, 42, 46, 48, 50, 51, 53, 55, 61, 62, 67, 68, 70], in six of which NMES continued at home after discharge [33, 35–37, 48, 62]. Nine studies used within-subject comparison, comparing one side of the body to the other [43, 44, 48, 50, 51, 56, 57, 59, 61].
Intervention duration differed among studies. Nine studies performed NMES for ≤30 min a day [33, 37, 44, 48, 51, 53, 54, 60, 61], nineteen studies for 30–60 min [30, 31, 34, 38, 41, 43, 45, 47, 50, 52, 56–59, 62, 67, 68, 70, 71] and seven studies for ≥60 min [35, 36, 39, 40, 42, 46, 66]. One study reported progression from 30 to 60 min [49], and another progression from 30 to 90 min [55]. The total period over which NMES was delivered ranged from 3 days to 14 weeks. Frequency of NMES stimulation ranged from 10 to 200 Hz, with pulse duration ranging from 100 to 1,400 μs. Session frequency was 5 days/week in 10 studies [30, 32–34, 37, 38, 41, 49, 55, 68], and 4 days/week in one study [70], whilst the majority of the studies reported daily sessions. Twelve studies reported that NMES was delivered twice per day [35, 36, 39–43, 45, 46, 51, 52, 57].
Risk-of-bias assessment
Seventeen studies were rated high risk of bias [31, 33, 36–38, 41, 44, 48, 50, 52, 53, 56–58, 60, 66, 68, 69], thirteen with unclear risk of bias [30, 32, 43, 45, 47, 49, 51, 54, 59, 61, 62, 70, 71] and eight low risk of bias [34, 35, 39, 40, 42, 46, 55, 67] (Figure 2). Only 13 studies clearly described both sequence random generation and allocation concealment [35–42, 45, 46, 56, 60, 62]. Few studies achieved blinding of staff providing NMES and participants due to the nature of the intervention. Outcome assessors were blinded in more than half of the studies [34, 35, 39, 40, 42–44, 46, 47, 49, 52–57, 59, 61, 67].
Figure 2.
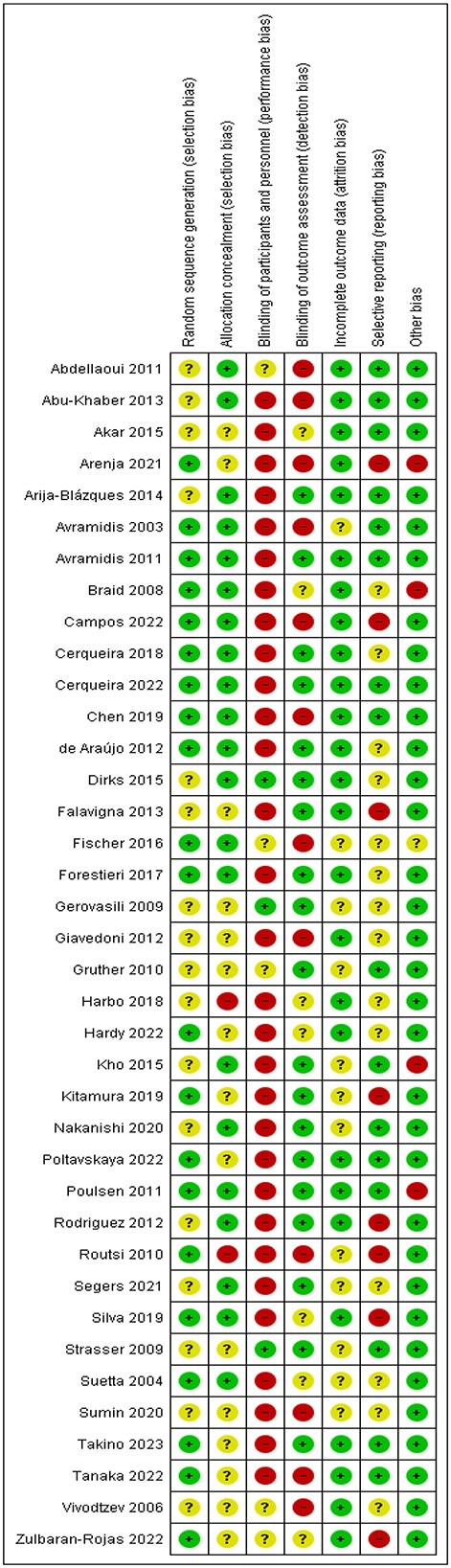
Risk-of-bias summary: review authors’ judgements about each risk-of-bias item for each included study.
Muscle strength
Twenty-four studies reported effect of NMES on muscle strength. Results were pooled from 21 RCTs with 816 participants: one study was excluded because of insufficient data [45] and two studies due to skewness of non-parametric data [32, 41]. The meta-analysis showed a small treatment effect of NMES compared to control (SMD 0.33; 95% CI [0.20, 0.46]; P < 0.00001) with no heterogeneity (I2 = 0%) (Figure 3A). GRADE rating of this small effect was ‘moderate’ certainty (Table 2). Visual inspection of the funnel plot showed nearly symmetrical distribution (Figure S1, supplementary file), and Egger’s regression test showed no significant evidence of asymmetry (P > 0.05).
Figure 3.
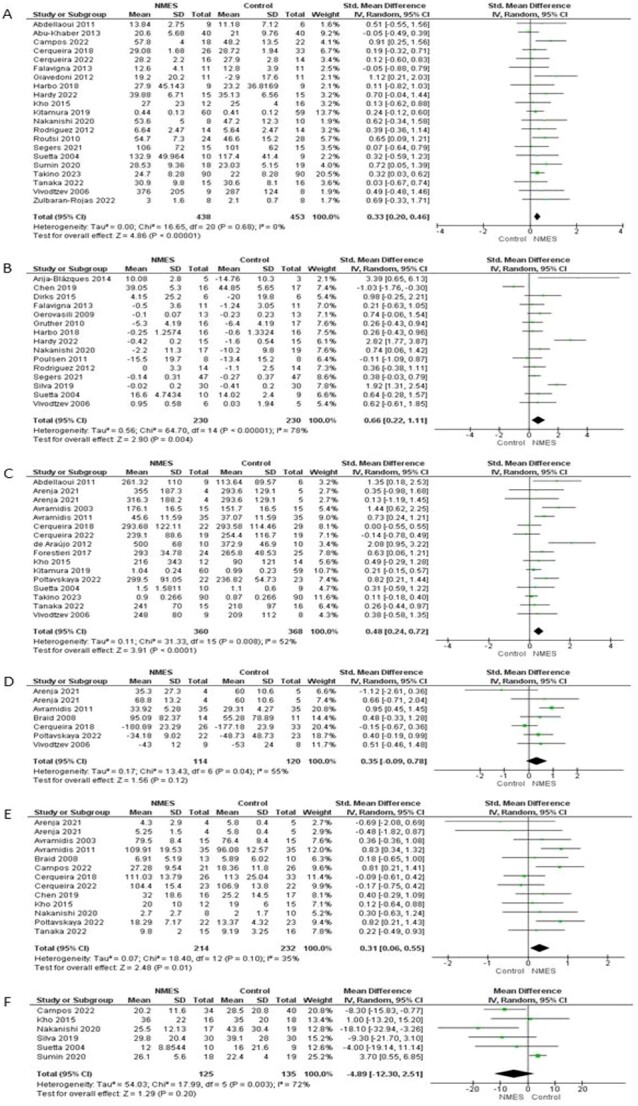
Forest plot: effects of NMES on (A) muscle strength; (B) muscle size; (C) walking performance; (D) health-related QoL; (E) functional mobility; (F) hospital length of stay.
Table 2.
Summary of findings
| NMES compared to control for hospitalised patients | ||||||
|---|---|---|---|---|---|---|
|
Patient or population: Hospitalised patients Intervention: NMES Comparison: Control | ||||||
| Outcomes | Anticipated absolute effects a (95% CI) |
Relative effect
(95% CI) |
No. of participants
(studies) |
Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with NMES | |||||
| Muscle strength | SMD 0.3 SD higher (0.20 higher to 0.46 higher) |
– | 816 (21 RCTs) |
⊕ ⊕ ⊕ ⊝ Moderateb |
NMES probably results in slight preserving muscle strength | |
| Muscle size | SMD 0.66 SD higher (0.22 higher to 1.11 higher) |
– | 343 (15 RCTs) |
⊕ ⊕ ⊕ ⊝ Moderateb |
NMES likely results in preserving muscle size | |
| Walking performance | SMD 0.48 SD higher (0.24 higher to 0.72 higher) |
– | 723 (15 RCTs) |
⊕ ⊕ ⊕ ⊝ Moderateb |
NMES probably increases walking performance | |
| Health-related QoL | SMD 0.35 SD higher (0.09 lower to 0.78 higher) |
– | 229 (6 RCTs) |
⊕ ⊝ ⊝ ⊝ Very lowc |
NMES may result in little to no difference in health-related QoL | |
| Functional mobility | SMD 0.31 SD higher (0.06 higher to 0.55 higher) |
– | 441 (12 RCTs) |
⊕ ⊕ ⊝ ⊝ Lowd |
NMES may result in little to no difference in functional mobility | |
| Hospital length of stay | MD 4.89 Days fewer (12.30 fewer to 2.51 more) |
– | 260 (6 RCTs) |
⊕ ⊝ ⊝ ⊝ Very lowc |
NMES may reduce/have little to no effect on hospital length of stay but the evidence is very uncertain | |
CI, confidence interval; OR, odds ratio; RR, risk ratio.
GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations
aThe risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
bDowngraded one level due to risk of bias (blinding was unlikely to be achieved because of the nature of NMES).
cDowngraded one level due to risk of bias (blinding was unlikely to be achieved because of the nature of NMES), one level due to inconsistency and one level due to imprecision.
dDowngraded one level due to the risk of bias (blinding was unlikely to be achieved because of the nature of NMES) and one level due to inconsistency.
In subgroup analysis, both ICU and non-ICU studies showed a significant benefit of NMES over control. For ICU studies, there was a small effect size (SMD 0.31; 95% CI [0.09, 0.52]; P < 0.01) with no heterogeneity (I2 = 6%). For non-ICU studies, there was also a small effect size (SMD 0.30; 95% CI [0.10, 0.50]; P < 0.005) with no heterogeneity (I2 = 0%) (Figure S2, supplementary file).
Muscle size
Seventeen studies reported effect of NMES on muscle size. Standardised mean differences were calculated because studies reported different variables (muscle thickness and cross-sectional area (CSA), muscle fibre CSA, arm and leg circumferences, cross-section diameter, muscle volume and muscle mass). One study was not included because of skewed non-parametric data [66] and one because of insufficient data [45]. Fifteen studies including 343 participants were included in the meta-analysis. The meta-analysis showed a significant benefit of NMES over control with high heterogeneity (I2 = 78%) and moderate effect size (SMD 0.66; 95% CI [0.22, 1.11]; P < 0.005, Figure 3B). The GRADE rating of this moderate effect was ‘moderate certainty’ (Table 2).
In subgroup analysis, both ICU and non-ICU studies showed a significant benefit of NMES over control. For ICU studies, there was a moderate effect size (SMD 0.62; 95% CI [0.21, 1.04]; P < 0.005) with moderate heterogeneity (I2 = 66%). For non-ICU studies, there was a large effect size (SMD 1.56; 95% CI [0.05, 3.06]; P < 0.05) with high heterogeneity (I2 = 84%) (Figure S3, supplementary file).
Function
Walking performance
Sixteen studies reported effect of NMES on walking performance, using different measures (3-, 6- and 10-minute walk tests, gait speed and 1,000 feet walking distance). One study was not included because of insufficient data [66]. One study was included as two trials because they used two different NMES techniques [33]. The pooled data from 15 studies including 723 participants showed significant benefit of NMES over control, with a small effect size (SMD 0.48; 95% CI [0.24, 0.72]; P < 0.0001, Figure 3C) and moderate heterogeneity (I2 = 52%). The GRADE rating of this small effect was ‘moderate’ certainty (Table 2).
In subgroup analysis, ICU studies showed a non-significant difference between groups with no heterogeneity (I2 = 0%) and no/negligible effect (SMD 0.06; 95% CI [−0.31, 0.43]; P > 0.05), but in non-ICU settings there was a significant difference with moderate heterogeneity (I2 = 61%) and a small effect size (SMD 0.49; 95% CI [0.15, 0.83]; P < 0.01) (Figure S4, supplementary file).
Health-related quality of life
Seven studies reported the effect of NMES on health-related quality of life (HRQoL) using different measures (EuroQoL five dimensions, Short Form 36, Minnesota Living with Heart Failure Questionnaire, Nottingham health profile and the 28-item Maugeri Foundation Respiratory Failure questionnaire). One study was not included because of skewed non-parametric data [38]. One study was included as two trials because they used two different NMES techniques [33]. The pooled data from six studies including 229 participants showed a non-significant benefit of NMES over control group with a small effect size (SMD 0.35; 95% CI [−0.09, 0.78]; P > 0.05, Figure 3D) and moderate heterogeneity (I2 = 55%). The GRADE rating of this small treatment effect was ‘very low’ certainty (Table 2).
Functional mobility
Twelve studies reported effect of NMES on activities related to mobility using different measures (Katz Index of Activity of Daily Living, Functional Independence Measure, American Knee Society score, Hospital for Special Surgery Knee-rating score, Functional Status in the ICU, Duke Activity Status Index, Elderly mobility scale, ICU mobility scale and Short Physical Performance Battery). One study was included as two trials because they used two different NMES techniques [33]. The pooled data from 12 studies including 441 participants showed a significant benefit of NMES over control with a small effect size (SMD 0.31; 95% CI [0.06, 0.55]; P < 0.05, Figure 3E) and low heterogeneity (I2 = 35%). The GRADE rating of this small treatment effect was ‘low’ certainty (Table 2).
Hospital length of stay
Six studies investigating effect of NMES on hospital length of stay, where NMES was delivered in hospital ward and ICU (260 participants). There was no significant difference between NMES and control groups (mean difference − 4.89 days; 95% CI [−12.30, 2.51]; P > 0.05, Figure 3F), with moderate heterogeneity (I2 = 72%). The GRADE rating for this effect was ‘very low’ certainty (Table 2).
Molecular and cellular outcomes
The nine studies that examined cellular and molecular biomarkers were not suitable for meta-analysis. We grouped molecular and cellular biomarkers into five categories: fibre type composition; inflammatory mediators; muscle protein synthesis and breakdown; bone; lipid and lipoprotein markers.
Four studies [30, 43, 59, 65] examined muscle fibre type composition. Overall, NMES produced a moderate shift towards fibre type I and a small reduction in type II. However, this evidence was rated to be at low certainty.
Two small studies measured inflammatory mediators. Their results were inconsistent: one [32] showed a reduction in Interleukin 6 (IL-6), whilst the other [60] reported no effect. The study showing a reduction in IL-6 [32] showed no reduction in Interleukin 10 (IL-10) or Tumor necrosis factor alpha (TNF-α).
Five studies investigated muscle protein synthesis and breakdown markers (muscle protein expression [43, 60], mRNA expression [43, 59–61] and 3-methylhistidine concentration corrected for urinary creatinine content [53]) with inconsistent findings. Three studies [43, 53, 60] reported that NMES has no significant effect on muscle protein synthesis and degradation, whereas two other studies [59, 61] reported that NMES had a significant effect on some variables (MyHC-I and proteasome activity) but no effect on others (myofibrillar protein content, MyHC-II and atrogin-1). On the basis that the two studies of moderate quality showed no effect, we judged this to represent evidence of no effect, albeit at low certainty because of risk of bias and inconsistency.
Only one study reported the effect of NMES on bone turnover biomarkers (testosterone, cortisol, growth hormone, insulin-growth factor I, osteocalcin, serum type I collagen C-telopeptide) and lipid and lipoprotein profiles [34]. The study was of low risk of bias, but had a very small sample size (n = 8). It found that NMES has no effect on bone, lipid and lipoprotein markers (P > 0.05), but in view of the limited sample size, we rated this as ‘inadequate evidence’.
Adverse events
Twenty-five studies measured adverse events, 13 of which reported no adverse event related to NMES, and 45/553 (9%) participants experienced undesirable experiences (a prickling sensation, hypotension, intolerable stimulation, muscle discomfort, pain and superficial burn).
Sensitivity analyses
Sensitivity analyses excluding studies using data approximated from non-parametric statistics, studies reporting change score for muscle strength and studies reporting post-intervention score for muscle size produced similar results to the primary analyses for muscle strength, muscle size and walking performance. However, the small significant benefit on functional mobility seen in the primary analysis was not significant in the sensitivity analysis (supplementary file).
Discussion
In adults hospitalised for a wide range of conditions, NMES produced a small benefit in muscle strength, a moderate benefit in muscle size, a small improvement in walking performance, a small improvement in functional mobility, no effect on HRQoL, no effect on length of stay and inconsistent effects on muscle metabolism. NMES was safe although associated with a small number of minor discomforting symptoms.
Our findings are consistent with previous positive reviews [10, 14, 16–18] of NMES in other populations. There is some discrepancy between our findings and those from reviews of ICU [15, 20] and COPD patients [15, 72]. This inconsistency could be because these reviews were confined to a specific population (ICU and COPD patients) and they included fewer studies (<10 studies) than our review.
These findings show that NMES is a promising intervention to reduce hospital-acquired disability. We were unable to identify an optimal treatment protocol because of the numerous parameters. Further research should identify NMES parameters (electrical stimulation parameters, frequency and duration of NMES) that optimise its effectiveness, convenience and tolerability. Nevertheless, rehabilitation practitioners are justified in offering this intervention for selected individuals, aiming to stimulate as much muscle as possible and as close to maximal contraction as is tolerated, for as long as would be seen in a voluntary exercise programme. Further research should establish the optimal role of this intervention in routine clinical care, including the feasibility of NMES in patient most at risk of hospital-acquired disability such as those with frailty [73]. Treatment packages that blend NMES into best existing rehabilitation practice and train therapists in its use are needed. Future studies should detail the treatment protocols, not only the electrical parameters but also the practical and contextual elements of the intervention, for example by using the TiDIER framework [74].
Our findings are trustworthy because we adhered to PRISMA-P guidelines, valuable because we were able to conduct numerical synthesis (meta-analyses) and robust because the results for our primary outcome (muscle strength) were consistent and stood up to sensitivity analyses. However, there were limitations. Not all studies were at low risk of bias, and most were small, which could have exaggerated the estimated effect sizes. The moderate heterogeneity of the secondary outcomes of muscle size and walking performance may reflect the fact that different studies used different measures of these variables and that our methods could not fully correct for this. The finding of a 5-day reduction in length of hospital stay (an outcome only indirectly related to the direct effects of NMES) had high heterogeneity, and this contributed to our conclusion that this apparent benefit was of low certainty. We excluded studies conducted in languages other than English and although only two studies were excluded on these grounds, this may have reduced the levels of precision of our findings. We did not search unpublished studies although the bias this may have introduced is uncertain.
In conclusion, NMES is a promising technique to contribute to reduction of hospital-acquired disability through improvement or preservation of muscle function, muscle size and functioning.
Supplementary Material
Contributor Information
Helal B Alqurashi, University of Nottingham, Nottingham, UK; Department of Physical Therapy, Faculty of Applied Medical Science, Taif University, Taif, Saudi Arabia; NIHR Nottingham Biomedical Research Centre (BRC), UK.
Katie Robinson, University of Nottingham, Nottingham, UK; NIHR Nottingham Biomedical Research Centre (BRC), UK; Nottingham University Hospitals NHS Trust, Nottingham, UK.
Dominic O’Connor, University of Nottingham, Nottingham, UK.
Mathew Piasecki, University of Nottingham, Nottingham, UK; NIHR Nottingham Biomedical Research Centre (BRC), UK.
Adam L Gordon, University of Nottingham, Nottingham, UK; NIHR Nottingham Biomedical Research Centre (BRC), UK; NIHR Applied Research Collaboration (ARC) East Midlands, UK; University Hospitals of Derby and Burton NHS Foundation Trust, Derby, UK.
Tahir Masud, NIHR Nottingham Biomedical Research Centre (BRC), UK; Nottingham University Hospitals NHS Trust, Nottingham, UK.
John R F Gladman, University of Nottingham, Nottingham, UK; NIHR Nottingham Biomedical Research Centre (BRC), UK; Nottingham University Hospitals NHS Trust, Nottingham, UK; NIHR Applied Research Collaboration (ARC) East Midlands, UK.
Declaration of Sources of Funding
This study was supported by the NIHR Nottingham Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. H.A. was supported by a grant from Taif University, Saudi Arabia. It is important to note that the sponsors had no involvement in the study’s design, execution, analysis and interpretation of data, or manuscript preparation.
Declaration of Conflicts of Interest
None.
References
- 1. Martínez-Velilla N, Sáez de Asteasu ML, Ramírez-Vélez R, Zambom-Ferraresi F, García-Hermoso A, Izquierdo M. Recovery of the decline in activities of daily living after hospitalization through an individualized exercise program: secondary analysis of a randomized clinical trial. J Gerontol A Biol Sci Med Sci 2021; 76: 1519–23. [DOI] [PubMed] [Google Scholar]
- 2. Zinglersen AH, Halsteen MB, Kjaer M, Karlsen A. Can electrical stimulation enhance effects of a functional training program in hospitalized geriatric patients? Exp Gerontol 2018; 106: 101–8. [DOI] [PubMed] [Google Scholar]
- 3. Miranda Rocha AR, Martinez BP, Maldaner da Silva VZ, Forgiarini Junior LA. Early mobilization: why, what for and how? Med Intensiva 2017; 41: 429–36. [DOI] [PubMed] [Google Scholar]
- 4. José A, Dal Corso S. Inpatient rehabilitation improves functional capacity, peripheral muscle strength and quality of life in patients with community-acquired pneumonia: a randomised trial. J Physiother 2016; 62: 96–102. [DOI] [PubMed] [Google Scholar]
- 5. Borges RC, Carvalho CR. Impact of resistance training in chronic obstructive pulmonary disease patients during periods of acute exacerbation. Arch Phys Med Rehabil 2014; 95: 1638–45. [DOI] [PubMed] [Google Scholar]
- 6. Camp PG, Reid WD, Chung Fet al. Clinical decision-making tool for safe and effective prescription of exercise in acute exacerbations of chronic obstructive pulmonary disease: results from an interdisciplinary Delphi survey and focus groups. Phys Ther 2015; 95: 1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rice H, Harrold M, Fowler R, Watson C, Waterer G, Hill K. Exercise training for adults hospitalized with an acute respiratory condition: a systematic scoping review. Clin Rehabil 2020; 34: 45–55. [DOI] [PubMed] [Google Scholar]
- 8. Nussbaum EL, Houghton P, Anthony J, Rennie S, Shay BL, Hoens AM. Neuromuscular electrical stimulation for treatment of muscle impairment: critical review and recommendations for clinical practice. Physiother Can 2017; 69: 1–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo Y, E Phillips B, Atherton PJ, Piasecki M. Molecular and neural adaptations to neuromuscular electrical stimulation; implications for ageing muscle. Mech Ageing Dev 2021; 193: 111402. 10.1016/j.mad.2020.111402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X, Peng Y, Zhong Fet al. Effects of neuromuscular electrical stimulation on functional capacity and quality of life among patients after cardiac surgery: a systematic review and meta-analysis. J Cardiol 2022; 79: 291–8. [DOI] [PubMed] [Google Scholar]
- 11. Bekhet AH, Bochkezanian V, Saab IM, Gorgey AS. The effects of electrical stimulation parameters in managing spasticity after spinal cord injury: a systematic review. Am J Phys Med Rehabil 2019; 98: 484–99. [DOI] [PubMed] [Google Scholar]
- 12. Lee JH, Baker LL, Johnson RE, Tilson JK. Effectiveness of neuromuscular electrical stimulation for management of shoulder subluxation post-stroke: a systematic review with meta-analysis. Clin Rehabil 2017; 31: 1431–44. [DOI] [PubMed] [Google Scholar]
- 13. Oliveira MM, Aragão FA, Vaz MA. Neuromuscular electrical stimulation for muscle strengthening in elderly with knee osteoarthritis - a systematic review. Complement Ther Clin Pract 2013; 19: 27–31. [DOI] [PubMed] [Google Scholar]
- 14. Wu X, Hu X, Hu W, Xiang G, Li S. Effects of neuromuscular electrical stimulation on exercise capacity and quality of life in COPD patients: a systematic review and meta-analysis. Biosci Rep 2020; 40: 1–12. 10.1042/BSR20191912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gutiérrez-Arias R, Jalil Y, Fuentes-Aspe R, Seron P. Effectiveness of neuromuscular electrostimulation in COPD subjects on mechanical ventilation. A systematic review and meta-analysis. Clinics (Sao Paulo) 2022; 77: 100108. 10.1016/j.clinsp.2022.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gomes Neto M, Oliveira FA, Reis HF, Sousa Rodrigues E Jr, Bittencourt HS, Oliveira Carvalho V. Effects of neuromuscular electrical stimulation on physiologic and functional measurements in patients with heart failure: a systematic review with meta-analysis. J Cardiopulm Rehabil Prev 2016; 36: 157–66. [DOI] [PubMed] [Google Scholar]
- 17. Jones S, Man WD, Gao Wet al. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst Rev 2016; 2016: 1–59. 10.1002/14651858.CD009419.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Connor D, Caulfield B, Lennon O. The efficacy and prescription of neuromuscular electrical stimulation (NMES) in adult cancer survivors: a systematic review and meta-analysis. Support Care Cancer 2018; 26: 3985–4000. [DOI] [PubMed] [Google Scholar]
- 19. Trethewey SP, Brown N, Gao F, Turner AM. Interventions for the management and prevention of sarcopenia in the critically ill: a systematic review. J Crit Care 2019; 50: 287–95. [DOI] [PubMed] [Google Scholar]
- 20. Zayed Y, Kheiri B, Barbarawi Met al. Effects of neuromuscular electrical stimulation in critically ill patients: a systematic review and meta-analysis of randomised controlled trials. Aust Crit Care 2020; 33: 203–10. [DOI] [PubMed] [Google Scholar]
- 21. Page MJ, Moher D, Bossuyt PMet al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160: 1–36. 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gøtzsche PCet al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guyatt GH, Oxman AD, Vist GEet al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coe R, editor It’s the effect size, stupid. What effect size is and why it is important. Paper Presented at the British Educational Research Association Annual Conference, Exeter, University of Exter; 2002. http://www.leeds.ac.uk/educol/documents/00002182.htm. [Google Scholar]
- 25. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi J, Luo D, Weng Het al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods 2020; 11: 641–54. [DOI] [PubMed] [Google Scholar]
- 27. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018; 27: 1785–805. [DOI] [PubMed] [Google Scholar]
- 28. Shi J, Luo D, Wan Xet al. Detecting the skewness of data from the sample size and the five-number summary. arXiv2020. arXiv:2010.05749. 10.48550/arXiv.2010.05749. [DOI]
- 29. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abdellaoui A, Prefaut C, Gouzi Fet al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J 2011; 38: 781–8. [DOI] [PubMed] [Google Scholar]
- 31. Abu-Khaber HA, Abouelela AMZ, Abdelkarim EM. Effect of electrical muscle stimulation on prevention of ICU acquired muscle weakness and facilitating weaning from mechanical ventilation. Alexandria J Med 2013; 49: 309–15. [Google Scholar]
- 32. Akar O, Gunay E, Sarinc Ulasli Set al. Efficacy of neuromuscular electrical stimulation in patients with COPD followed in intensive care unit. Clin Respir J 2017; 11: 743–50. [DOI] [PubMed] [Google Scholar]
- 33. Arenja N, Mueller C, Tomilovskaya E, Koryak Y, Poltavskaya M, Saner H. Real-world experience of feasibility and efficacy of electrical muscle stimulation in elderly patients with acute heart failure: a randomized controlled study. Int J Cardiol 2021; 344: 113–9. [DOI] [PubMed] [Google Scholar]
- 34. Arija-Blazquez A, Ceruelo-Abajo S, Diaz-Merino MSet al. Effects of electromyostimulation on muscle and bone in men with acute traumatic spinal cord injury: a randomized clinical trial. J Spinal Cord Med 2014; 37: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Avramidis K, Karachalios T, Popotonasios K, Sacorafas D, Papathanasiades AA, Malizos KN. Does electric stimulation of the vastus medialis muscle influence rehabilitation after total knee replacement? Orthopedics 2011; 34: 175. 10.3928/01477447-20110124-06. [DOI] [PubMed] [Google Scholar]
- 36. Avramidis K, Strike PW, Taylor PN, Swain ID. Effectiveness of electric stimulation of the vastus medialis muscle in the rehabilitation of patients after total knee arthroplasty. Arch Phys Med Rehabil 2003; 84: 1850–3. [DOI] [PubMed] [Google Scholar]
- 37. Braid V, Barber M, Mitchell SL, Martin BJ, Granat M, Stott DJ. Randomised controlled trial of electrical stimulation of the quadriceps after proximal femoral fracture. Aging Clin Exp Res 2008; 20: 62–6. [DOI] [PubMed] [Google Scholar]
- 38. Campos DR, Bueno TBC, Anjos Jet al. Early neuromuscular electrical stimulation in addition to early mobilization improves functional status and decreases hospitalization days of critically ill patients. Crit Care Med 2022; 50: 1116–26. [DOI] [PubMed] [Google Scholar]
- 39. Cerqueira TCF, Cerqueira Neto ML, Cacau LAPet al. Ambulation capacity and functional outcome in patients undergoing neuromuscular electrical stimulation after cardiac valve surgery: a randomised clinical trial. Medicine 2018; 97: e13012. 10.1097/MD.0000000000013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cerqueira TCF, Cerqueira Neto ML, Cacau LAPet al. Effect of neuromuscular electrical stimulation on functional exercise capacity in patients undergoing cardiac surgery: a randomized clinical trial. Clin Rehabil 2022; 36: 789–800. [DOI] [PubMed] [Google Scholar]
- 41. Chen YH, Hsiao HF, Li LF, Chen NH, Huang CC. Effects of electrical muscle stimulation in subjects undergoing prolonged mechanical ventilation. Respir Care 2019; 64: 262–71. [DOI] [PubMed] [Google Scholar]
- 42. Araujo CJ, Goncalves FS, Bittencourt HSet al. Effects of neuromuscular electrostimulation in patients with heart failure admitted to ward. J Cardiothorac Surg 2012; 7: 124. 10.1186/1749-8090-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dirks ML, Hansen D, Van Assche A, Dendale P, Van Loon LJC. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci 2015; 128: 357–65. [DOI] [PubMed] [Google Scholar]
- 44. Falavigna LF, Silva MG, Freitas ALet al. Effects of electrical muscle stimulation early in the quadriceps and tibialis anterior muscle of critically ill patients. Physiother Theory Pract 2014; 30: 223–8. [DOI] [PubMed] [Google Scholar]
- 45. Fischer A, Spiegl M, Altmann Ket al. Muscle mass, strength and functional outcomes in critically ill patients after cardiothoracic surgery: does neuromuscular electrical stimulation help? The Catastim 2 randomized controlled trial. Crit Care 2016; 20: 30. 10.1186/s13054-016-1199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Forestieri P, Bolzan DW, Santos VBet al. Neuromuscular electrical stimulation improves exercise tolerance in patients with advanced heart failure on continuous intravenous inotropic support use-randomized controlled trial. Clin Rehabil 2018; 32: 66–74. [DOI] [PubMed] [Google Scholar]
- 47. Gerovasili V, Stefanidis K, Vitzilaios Ket al. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care 2009; 13: R161. 10.1186/cc8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giavedoni S, Deans A, McCaughey P, Drost E, MacNee W, Rabinovich RA. Neuromuscular electrical stimulation prevents muscle function deterioration in exacerbated COPD: a pilot study. Respir Med 2012; 106: 1429–34. [DOI] [PubMed] [Google Scholar]
- 49. Gruther W, Kainberger F, Fialka-Moser Vet al. Effects of neuromuscular electrical stimulation on muscle layer thickness of knee extensor muscles in intensive care unit patients: a pilot study. J Rehabil Med 2010; 42: 593–7. [DOI] [PubMed] [Google Scholar]
- 50. Harbo T, Markvardsen LK, Hellfritzsch MB, Severinsen K, Nielsen JF, Andersen H. Neuromuscular electrical stimulation in early rehabilitation of Guillain-Barré syndrome: a pilot study. Muscle Nerve 2019; 59: 481–4. [DOI] [PubMed] [Google Scholar]
- 51. Hardy EJ, Hatt J, Doleman Bet al. Post-operative electrical muscle stimulation attenuates loss of muscle mass and function following major abdominal surgery in older adults: a split body randomised control trial. Age Ageing 2022; 51: 1–8. 10.1093/ageing/afac234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kho ME, Truong AD, Zanni JMet al. Neuromuscular electrical stimulation in mechanically ventilated patients: a randomized, sham-controlled pilot trial with blinded outcome assessment. J Crit Care 2015; 30: 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kitamura H, Yamada S, Adachi Tet al. Effect of perioperative neuromuscular electrical stimulation in patients undergoing cardiovascular surgery: a pilot randomized controlled trial. Semin Thorac Cardiovasc Surg 2019; 31: 361–7. [DOI] [PubMed] [Google Scholar]
- 54. Nakanishi N, Oto J, Tsutsumi Ret al. Effect of electrical muscle stimulation on upper and lower limb muscles in critically ill patients: a two-center randomized controlled trial. Crit Care Med 2020; 48: e997–1003. [DOI] [PubMed] [Google Scholar]
- 55. Poltavskaya M, Sviridenko V, Giverts Iet al. In-hospital electrical muscle stimulation for patients early after heart failure decompensation: results from a prospective randomised controlled pilot trial. open. Heart 2022; 9: 1–8. 10.1136/openhrt-2022-001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Poulsen JB, Moller K, Jensen CV, Weisdorf S, Kehlet H, Perner A. Effect of transcutaneous electrical muscle stimulation on muscle volume in patients with septic shock. Crit Care Med 2011; 39: 456–61. [DOI] [PubMed] [Google Scholar]
- 57. Rodriguez PO, Setten M, Maskin LPet al. Muscle weakness in septic patients requiring mechanical ventilation: protective effect of transcutaneous neuromuscular electrical stimulation. J Crit Care 2012; 27: 319.e1–8. 10.1016/j.jcrc.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 58. Routsi C, Gerovasili V, Vasileiadis Iet al. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care 2010; 14: R74. 10.1186/cc8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Segers J, Vanhorebeek I, Langer Det al. Early neuromuscular electrical stimulation reduces the loss of muscle mass in critically ill patients - a within subject randomized controlled trial. J Crit Care 2021; 62: 65–71. [DOI] [PubMed] [Google Scholar]
- 60. Silva PE, De Cassia MR, Livino-De-Carvalho Ket al. Neuromuscular electrical stimulation in critically ill traumatic brain injury patients attenuates muscle atrophy, neurophysiological disorders, and weakness: a randomized controlled trial. J Intensive Care 2019; 7: 59. 10.1186/s40560-019-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Strasser EM, Stättner S, Karner Jet al. Neuromuscular electrical stimulation reduces skeletal muscle protein degradation and stimulates insulin-like growth factors in an age- and current-dependent manner: a randomized, controlled clinical trial in major abdominal surgical patients. Ann Surg 2009; 249: 738–43. [DOI] [PubMed] [Google Scholar]
- 62. Suetta C, Aagaard P, Rosted Aet al. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol (Bethesda, Md: 1985)2004; 97: 1954–61. [DOI] [PubMed] [Google Scholar]
- 63. Suetta C, Magnusson SP, Rosted Aet al. Resistance training in the early postoperative phase reduces hospitalization and leads to muscle hypertrophy in elderly hip surgery patients—a controlled, randomized study. J Am Geriatr Soc 2004; 52: 2016–22. [DOI] [PubMed] [Google Scholar]
- 64. Suetta C, Andersen JL, Dalgas Uet al. Resistance training induces qualitative changes in muscle morphology, muscle architecture, and muscle function in elderly postoperative patients. J Appl Physiol 2008; 105: 180–6. [DOI] [PubMed] [Google Scholar]
- 65. Suetta C, Clemmensen C, Andersen JL, Magnusson SP, Schjerling P, Kjaer M. Coordinated increase in skeletal muscle fiber area and expression of IGF-I with resistance exercise in elderly post-operative patients. Growth Horm IGF Res 2010; 20: 134–40. [DOI] [PubMed] [Google Scholar]
- 66. Sumin AN, Oleinik PA, Bezdenezhnykh AV, Ivanova AV. Neuromuscular electrical stimulation in early rehabilitation of patients with postoperative complications after cardiovascular surgery: a randomized controlled trial. Medicine 2020; 99: e22769. 10.1097/MD.0000000000022769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Takino K, Kameshima M, Asai Cet al. Neuromuscular electrical stimulation after cardiovascular surgery mitigates muscle weakness in older individuals with diabetes. Ann Phys Rehabil Med 2022; 66: 101659: 1–7. 10.1016/j.rehab.2022.101659. [DOI] [PubMed] [Google Scholar]
- 68. Tanaka S, Kamiya K, Matsue Yet al. Effects of electrical muscle stimulation on physical function in frail older patients with acute heart failure: a randomized controlled trial. Eur J Prev Cardiol 2022; 29: e286–8. 10.1093/eurjpc/zwac022. [DOI] [PubMed] [Google Scholar]
- 69. Tanaka S, Kamiya K, Matsue Yet al. Efficacy and safety of acute phase intensive electrical muscle stimulation in frail older patients with acute heart failure: results from the ACTIVE-EMS trial. J Cardiovasc Dev Dis 2022; 9: 1–12. 10.3390/jcdd9040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vivodtzev I, Pepin JL, Vottero Get al. Improvement in quadriceps strength and dyspnea in daily tasks after 1 month of electrical stimulation in severely deconditioned and malnourished COPD. Chest 2006; 129: 1540–8. [DOI] [PubMed] [Google Scholar]
- 71. Zulbaran-Rojas A, Mishra R, Rodriguez Net al. Safety and efficacy of electrical stimulation for lower-extremity muscle weakness in intensive care unit 2019 novel coronavirus patients: a phase I double-blinded randomized controlled trial. Front Med (Lausanne) 2022; 9: 1017371. 10.3389/fmed.2022.1017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pan L, Guo Y, Liu X, Yan J. Lack of efficacy of neuromuscular electrical stimulation of the lower limbs in chronic obstructive pulmonary disease patients: a meta-analysis. Respirology 2014; 19: 22–9. [DOI] [PubMed] [Google Scholar]
- 73. Gladman JRF, Aloraibi S, Greenhaff PLet al. Feasibility RCT of neuromuscular electrical stimulation; an Intervention to Maintain and improve neuroMuscular function during periods of Immobility (IMMI): Protocol. East Midlands Research into Ageing Network (EMRAN) Discussion Paper Series. EMRAN, East Midlands, UK; 2021.
- 74. Hoffmann TC, Glasziou PP, Boutron Iet al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: 1–12. 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


