Abstract
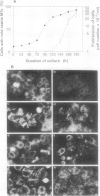
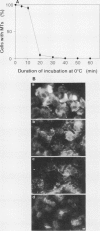
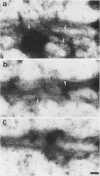
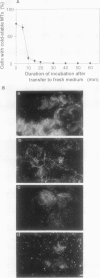
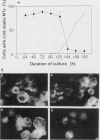
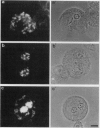
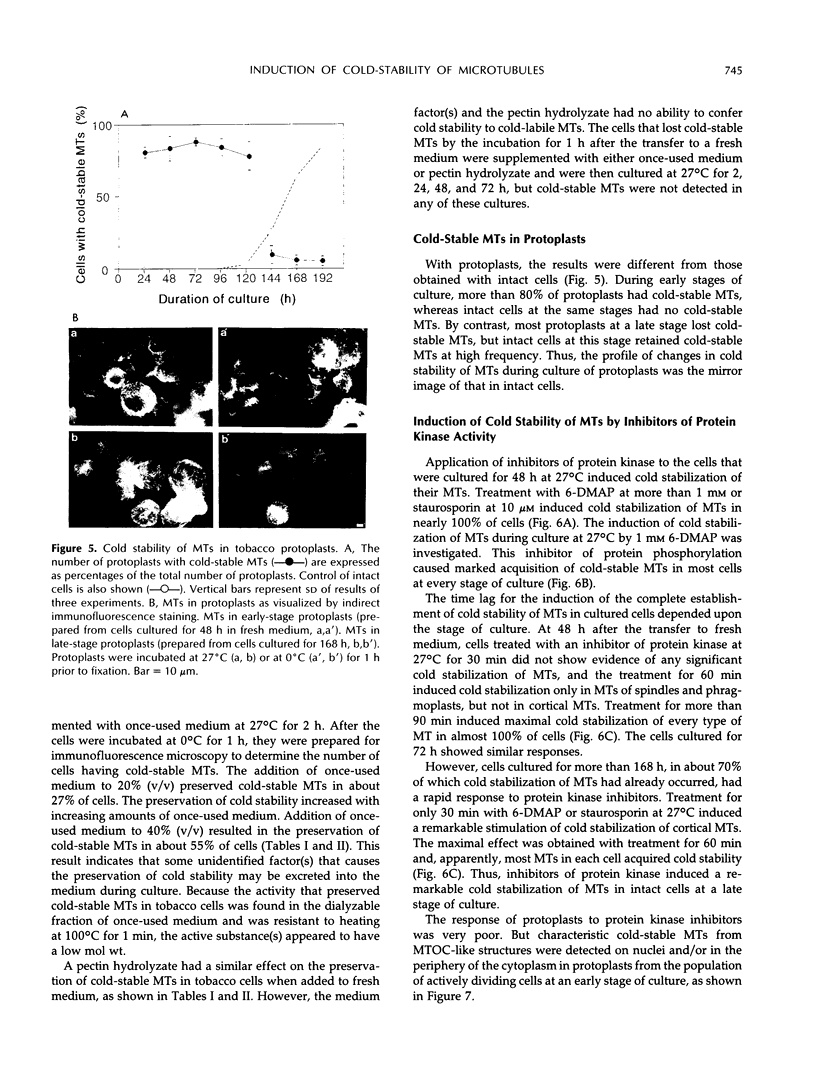
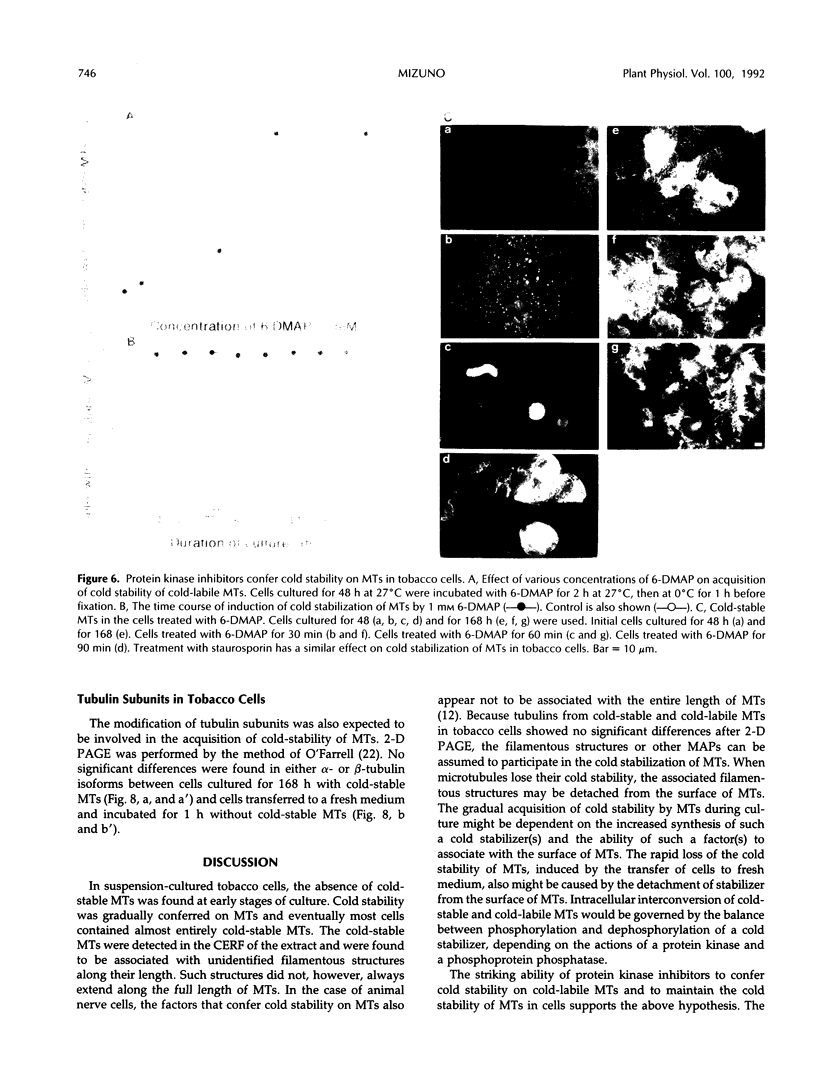
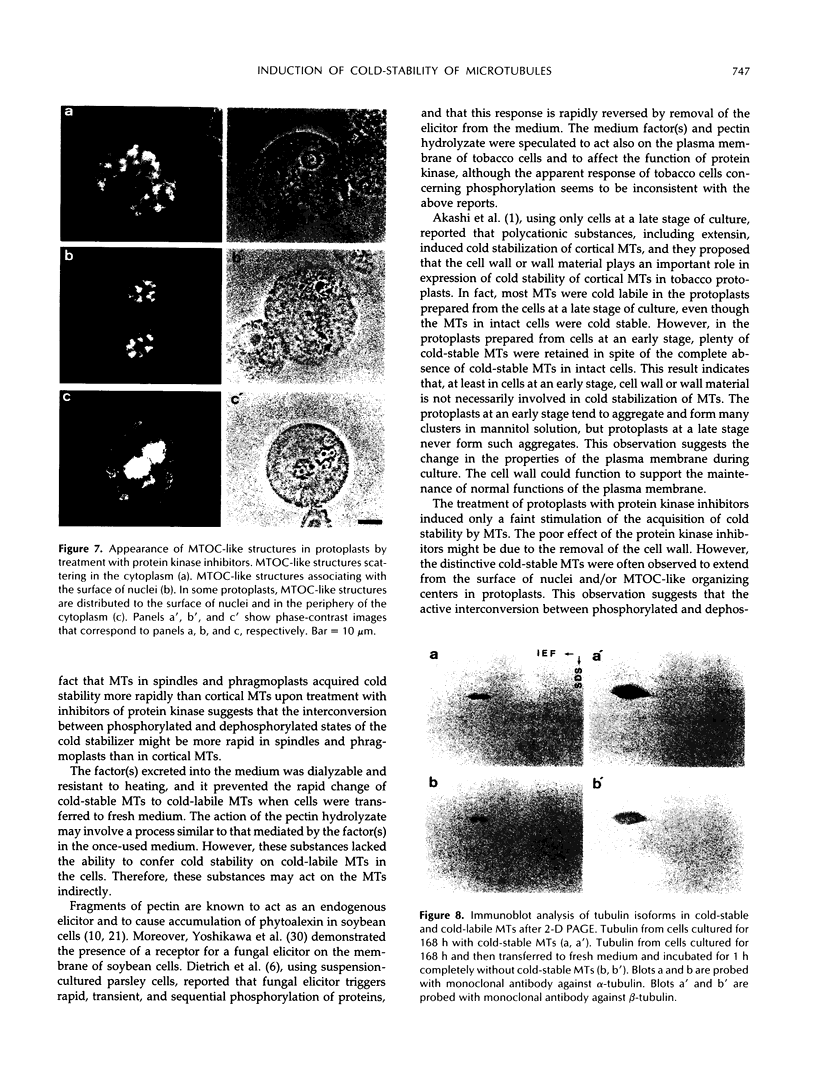
In suspension-cultured tobacco (Nicotiana tabacum) cells, we have often encountered cold-stable microtubules (MTs). The cold-stable MTs were found in the pelleted fraction of tobacco cell homogenates. These cold-stable MTs were shown to be accompanied by unidentified filamentous structures that extended along part of their length. However, during the early hours in culture such cold-stable MTs were never observed. They were detectable from 120 h after the beginning of subculture and then their numbers increased gradually. The number of cells with cold-stable MTs eventually accounted for more than 95% of the total population of cells at the stationary phase of culture. The rapid loss of cold stability of MTs occurred when such cells were transferred to fresh medium for subculture. However, if the fresh medium was supplemented with once-used medium, the cold stability of MTs was retained. The active agent in the medium appeared to be of low molecular weight and to be heat resistant. A similar activity was detected in a pectin hydrolyzate. When an inhibitor of protein kinase, either 6-dimethylaminopurine or staurosporin, was added to the cells at an early stage of culture, when cold-stable MTs were normally completely absent, most cells acquired cold-stable MTs. It appears that acquisition or loss of cold stability of MTs in tobacco cells is regulated by the action of a kinase/phosphatase or a phosphorylation/dephosphorylation system on some MT protein(s), such as a cold stabilizer of MTs, some unidentified MT-associated filamentous structure, or even tubulin itself.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartolo M. E., Carter J. V. Microtubules in mesophyll cells of nonacclimated and cold-acclimated spinach : visualization and responses to freezing, low temperature, and dehydration. Plant Physiol. 1991 Sep;97(1):175–181. doi: 10.1104/pp.97.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S. T., Tytell M., Lasek R. J. Axonal tubulin and axonal microtubules: biochemical evidence for cold stability. J Cell Biol. 1984 Nov;99(5):1716–1724. doi: 10.1083/jcb.99.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Cartwright J., Jr Cold-labile and cold-stable microtubules in the mitotic spindle of mammalian cells. Ann N Y Acad Sci. 1975 Jun 30;253:428–439. doi: 10.1111/j.1749-6632.1975.tb19218.x. [DOI] [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Hahn M. G., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XIX. THE ENDOGENOUS ELICITOR, A FRAGMENT OF A PLANT CELL WALL POLYSACCHARIDE THAT ELICITS PHYTOALEXIN ACCUMULATION IN SOYBEANS. Plant Physiol. 1981 Nov;68(5):1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Fischer E. H., Margolis R. L. Rapid disassembly of cold-stable microtubules by calmodulin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4679–4682. doi: 10.1073/pnas.78.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Rauch C. T., Fischer E. H., Margolis R. L. Recycling of cold-stable microtubules: evidence that cold stability is due to substoichiometric polymer blocks. Biochemistry. 1982 Feb 2;21(3):509–515. doi: 10.1021/bi00532a015. [DOI] [PubMed] [Google Scholar]
- Jones D. H., Gray E. G., Barron J. Cold stable microtubules in brain studied in fractions and slices. J Neurocytol. 1980 Aug;9(4):493–504. doi: 10.1007/BF01204838. [DOI] [PubMed] [Google Scholar]
- Kerr G. P., Carter J. V. Relationship between Freezing Tolerance of Root-Tip Cells and Cold Stability of Microtubules in Rye (Secale cereale L. cv Puma). Plant Physiol. 1990 May;93(1):77–82. doi: 10.1104/pp.93.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr G. P., Carter J. V. Tubulin Isotypes in Rye Roots Are Altered during Cold Acclimation. Plant Physiol. 1990 May;93(1):83–88. doi: 10.1104/pp.93.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Rauch C. T. Characterization of rat brain crude extract microtubule assembly: correlation of cold stability with the phosphorylation state of a microtubule-associated 64K protein. Biochemistry. 1981 Jul 21;20(15):4451–4458. doi: 10.1021/bi00518a033. [DOI] [PubMed] [Google Scholar]
- Neant I., Guerrier P. 6-Dimethylaminopurine blocks starfish oocyte maturation by inhibiting a relevant protein kinase activity. Exp Cell Res. 1988 May;176(1):68–79. doi: 10.1016/0014-4827(88)90121-8. [DOI] [PubMed] [Google Scholar]
- Nothnagel E. A., McNeil M., Albersheim P., Dell A. Host-Pathogen Interactions : XXII. A Galacturonic Acid Oligosaccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983 Apr;71(4):916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pirollet F., Job D., Fischer E. H., Margolis R. L. Purification and characterization of sheep brain cold-stable microtubules. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1560–1564. doi: 10.1073/pnas.80.6.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebhun L. I., White D., Sander G., Ivy N. Cleavage inhibition in marine eggs by puromycin and 6-dimethylaminopurine. Exp Cell Res. 1973 Mar 15;77(1):312–318. doi: 10.1016/0014-4827(73)90582-x. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Begg D. A. Functional implications of cold-stable microtubules in kinetochore fibers of insect spermatocytes during anaphase. J Cell Biol. 1980 Jun;85(3):853–865. doi: 10.1083/jcb.85.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Labbé J. C., Dorée M., Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature. 1990 Jan 18;343(6255):233–238. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- Webb B. C., Wilson L. Cold-stable microtubules from brain. Biochemistry. 1980 Apr 29;19(9):1993–2001. doi: 10.1021/bi00550a041. [DOI] [PubMed] [Google Scholar]
- Wick S. M., Seagull R. W., Osborn M., Weber K., Gunning B. E. Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol. 1981 Jun;89(3):685–690. doi: 10.1083/jcb.89.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Keen N. T., Wang M. C. A receptor on soybean membranes for a fungal elicitor of phytoalexin accumulation. Plant Physiol. 1983 Oct;73(2):497–506. doi: 10.1104/pp.73.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]