Abstract
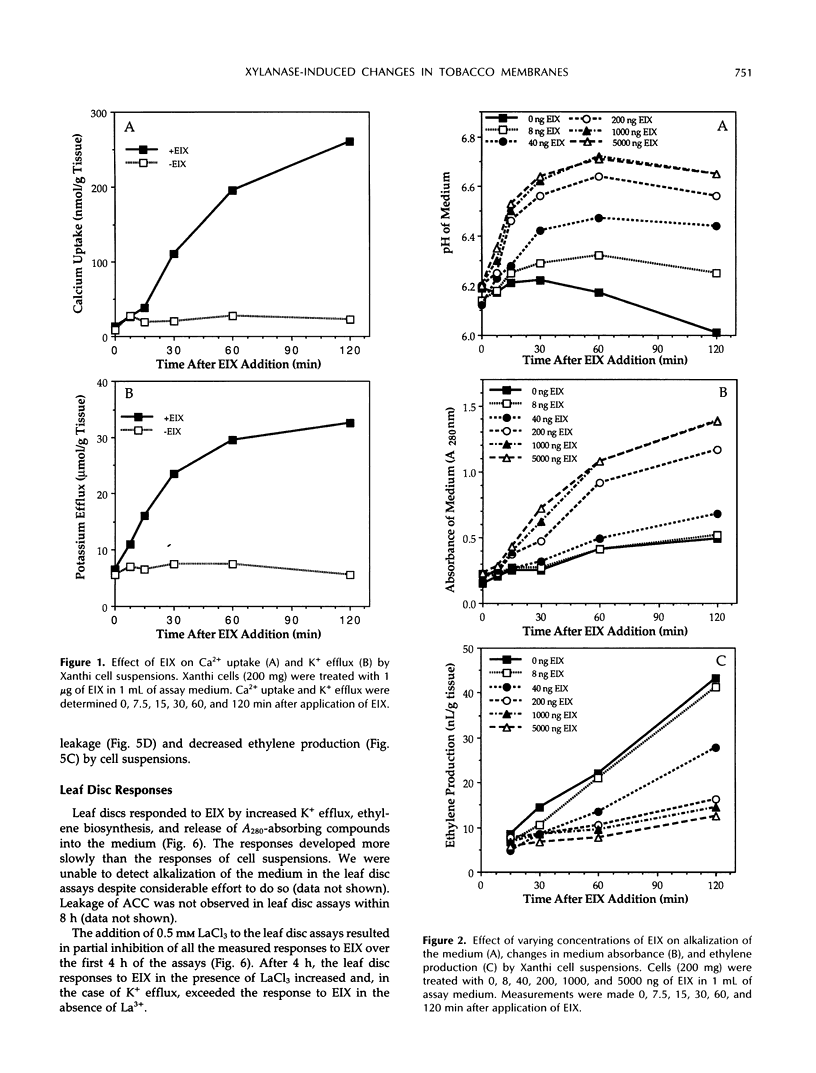
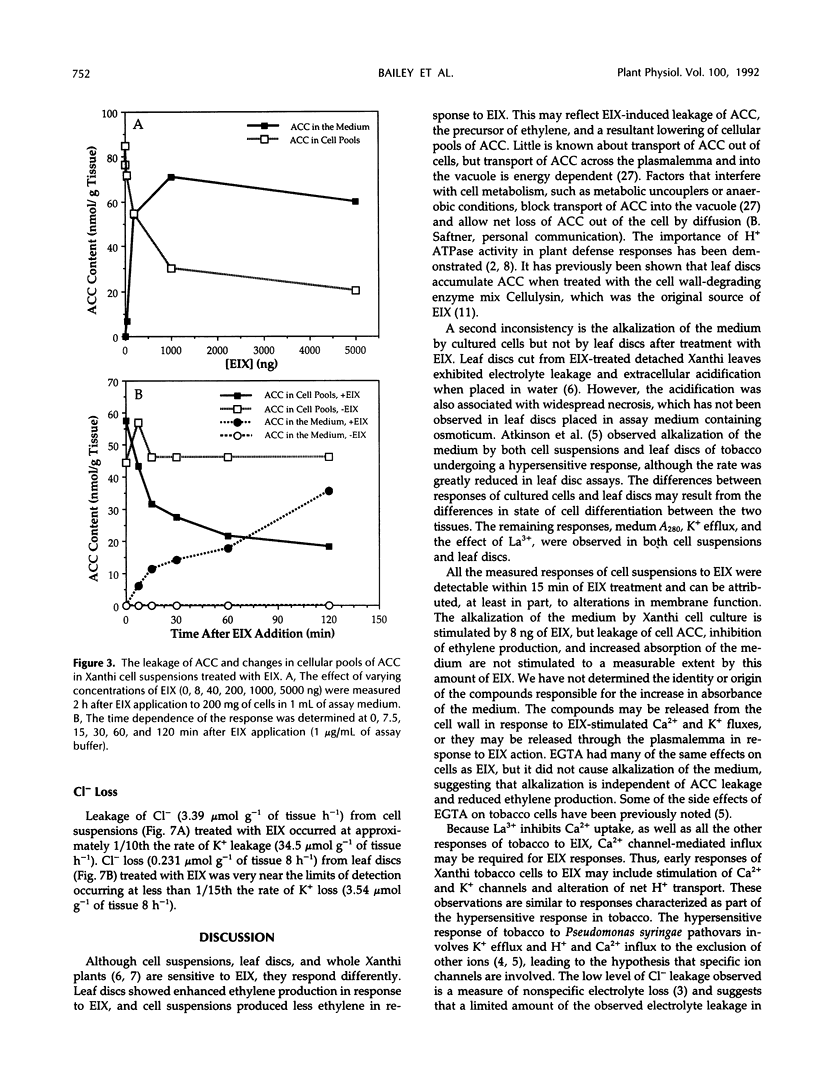
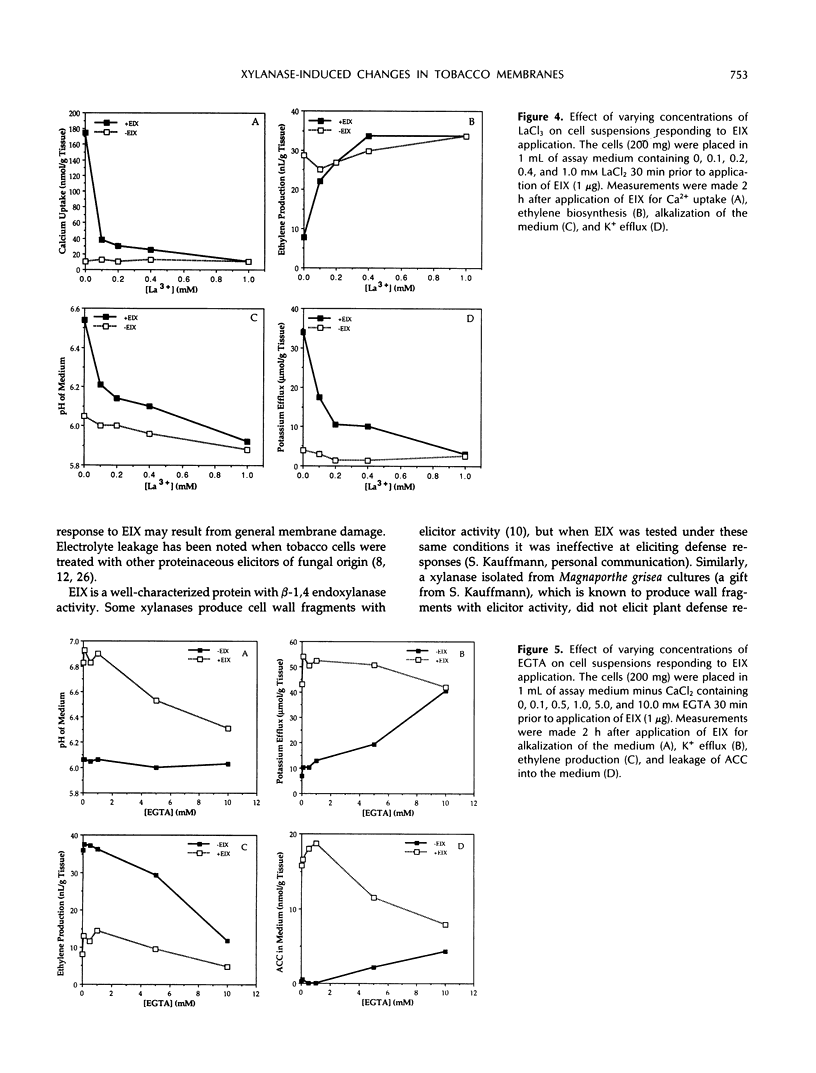
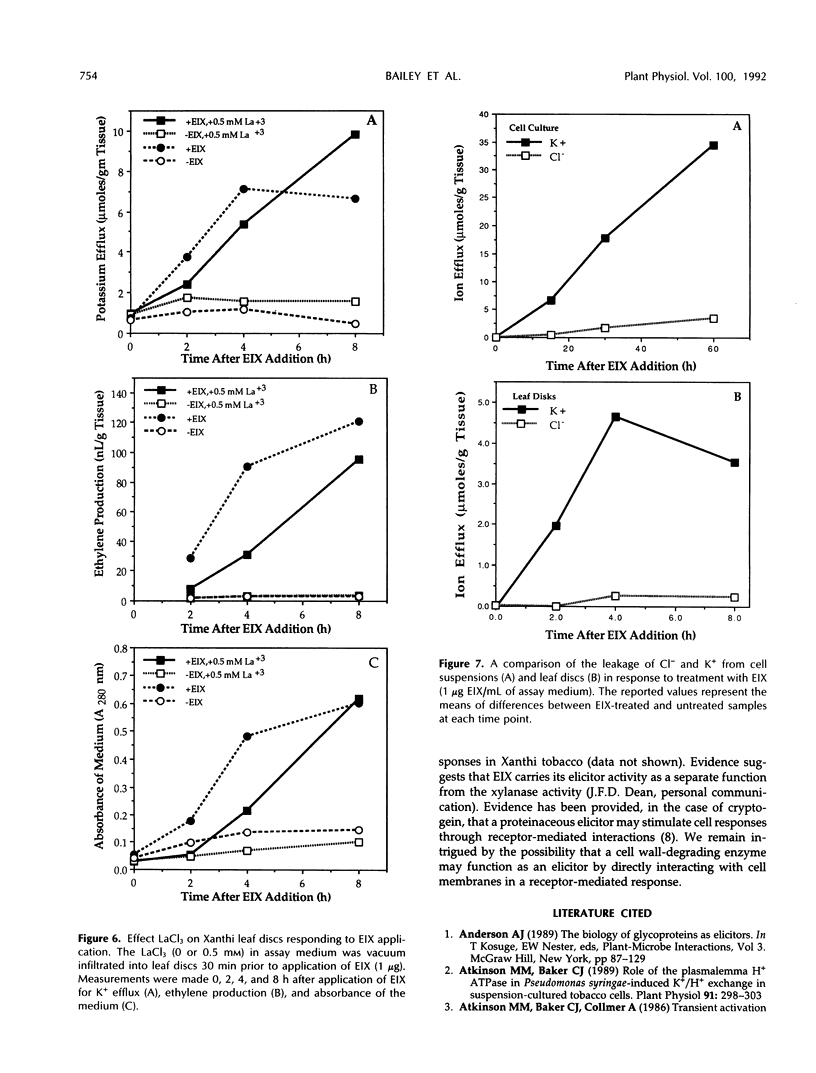
An ethylene biosynthesis-inducing xylanase (EIX) produced by the fungus Trichoderma viride elicited enhanced ethylene biosynthesis and leakage of potassium and other cellular components when applied to leaf disks of tobacco (Nicotiana tabacum L. cv Xanthi). Suspension-cultured cells of Xanthi tobacco responded to EIX by rapid efflux of potassium, uptake of calcium, alkalization of the medium, inhibition of ethylene biosynthesis, and increased leakage of cellular components. EIX-treated cell suspensions released 1-aminocyclopropane-1-carboxylate (ACC) into the surrounding medium, resulting in a reduction of cellular pools of ACC. The responses of both cell suspensions and leaf disks were inhibited (50-80%) by the preincubation of the tissues with the calcium channel blocker La3+. High concentrations of EGTA inhibited the alkalization of the medium by cell suspensions responding to EIX, but EGTA alone caused extensive loss of K+ and ACC and inhibited ethylene biosynthesis by tobacco cells. Alterations in membrane function appear to be important in the mode of action of EIX in Xanthi cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. M., Baker C. J. Role of the Plasmalemma H-ATPase in Pseudomonas syringae-Induced K/H Exchange in Suspension-Cultured Tobacco Cells. Plant Physiol. 1989 Sep;91(1):298–303. doi: 10.1104/pp.91.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. M., Huang J. S., Knopp J. A. The Hypersensitive Reaction of Tobacco to Pseudomonas syringae pv. pisi: Activation of a Plasmalemma K/H Exchange Mechanism. Plant Physiol. 1985 Nov;79(3):843–847. doi: 10.1104/pp.79.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. M., Keppler L. D., Orlandi E. W., Baker C. J., Mischke C. F. Involvement of plasma membrane calcium influx in bacterial induction of the k/h and hypersensitive responses in tobacco. Plant Physiol. 1990 Jan;92(1):215–221. doi: 10.1104/pp.92.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey B. A., Dean J. F., Anderson J. D. An Ethylene Biosynthesis-Inducing Endoxylanase Elicits Electrolyte Leakage and Necrosis in Nicotiana tabacum cv Xanthi Leaves. Plant Physiol. 1990 Dec;94(4):1849–1854. doi: 10.1104/pp.94.4.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey B. A., Taylor R., Dean J. F., Anderson J. D. Ethylene Biosynthesis-Inducing Endoxylanase Is Translocated through the Xylem of Nicotiana tabacum cv Xanthi Plants. Plant Physiol. 1991 Nov;97(3):1181–1186. doi: 10.1104/pp.97.3.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blein J. P., Milat M. L., Ricci P. Responses of Cultured Tobacco Cells to Cryptogein, a Proteinaceous Elicitor from Phytophthora cryptogea: Possible Plasmalemma Involvement. Plant Physiol. 1991 Feb;95(2):486–491. doi: 10.1104/pp.95.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Chalutz E., Mattoo A. K., Solomos T., Anderson J. D. Enhancement by ethylene of cellulysin-induced ethylene production by tobacco leaf discs. Plant Physiol. 1984 Jan;74(1):99–103. doi: 10.1104/pp.74.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U., Jeblick W., Kauss H. The protein kinase inhibitor, K-252a, decreases elicitor-induced Ca2+ uptake and K+ release, and increases coumarin synthesis in parsley cells. FEBS Lett. 1991 Feb 11;279(1):141–144. doi: 10.1016/0014-5793(91)80269-9. [DOI] [PubMed] [Google Scholar]
- Dean J. F., Anderson J. D. Ethylene Biosynthesis-Inducing Xylanase : II. Purification and Physical Characterization of the Enzyme Produced by Trichoderma viride. Plant Physiol. 1991 Jan;95(1):316–323. doi: 10.1104/pp.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J. F., Gross K. C., Anderson J. D. Ethylene Biosynthesis-Inducing Xylanase : III. Product Characterization. Plant Physiol. 1991 Jun;96(2):571–576. doi: 10.1104/pp.96.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Basse C. W., Boller T. Elicitor-induced ethylene biosynthesis in tomato cells: characterization and use as a bioassay for elicitor action. Plant Physiol. 1991 Sep;97(1):19–25. doi: 10.1104/pp.97.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Anderson J. D. Purification and characterization of ethylene inducing proteins from cellulysin. Plant Physiol. 1987 Jul;84(3):732–736. doi: 10.1104/pp.84.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Saxena A., Gamble H. R., Anderson J. D. Ethylene biosynthesis-inducing protein from cellulysin is an endoxylanase. Plant Physiol. 1989 Jan;89(1):138–143. doi: 10.1104/pp.89.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki F., Tsurusawa Y., Nishi A. Breakdown of Phosphatidylinositol during the Elicitation of Phytoalexin Production in Cultured Carrot Cells. Plant Physiol. 1987 Nov;85(3):601–604. doi: 10.1104/pp.85.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966 Mar;41(3):376–382. doi: 10.1104/pp.41.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Lotan T., Fluhr R. Xylanase, a novel elicitor of pathogenesis-related proteins in tobacco, uses a non-ethylene pathway for induction. Plant Physiol. 1990 Jun;93(2):811–817. doi: 10.1104/pp.93.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. P. How much editing does a manuscript need? Can Med Assoc J. 1984 Aug 1;131(3):181–181. [PMC free article] [PubMed] [Google Scholar]
- Ojalvo I., Rokem J. S., Navon G., Goldberg I. P NMR Study of Elicitor Treated Phaseolus vulgaris Cell Suspension Cultures. Plant Physiol. 1987 Nov;85(3):716–719. doi: 10.1104/pp.85.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci P., Bonnet P., Huet J. C., Sallantin M., Beauvais-Cante F., Bruneteau M., Billard V., Michel G., Pernollet J. C. Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur J Biochem. 1989 Aug 15;183(3):555–563. doi: 10.1111/j.1432-1033.1989.tb21084.x. [DOI] [PubMed] [Google Scholar]
- Saftner R. A., Baker J. E. Transport and Compartmentation of 1-Aminocyclopropane-1-Carboxylic Acid and Its Structural Analog, alpha-Aminoisobutyric Acid, in Tomato Pericarp Slices. Plant Physiol. 1987 Jun;84(2):311–317. doi: 10.1104/pp.84.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]


