Significance
Human social development from foragers to citizens was linked to amplified agricultural production to feed the growing settlement populations. The first settlements in Europe with large numbers of inhabitants (up to 15,000) were built some 6,100 B.P. by Trypillia societies in modern-day Moldova and Ukraine. Each of these “mega-sites” existed for multiple generations. The isotopic composition of bones and plants tells us that cattle were intensively pastured to provide manure for the labor-intensive growing of pulses and that the human diet was based mostly on pulses and cereals. There is no discernible economic reason for the demise of these mega-sites. Developing sociopolitical inequalities likely caused people to leave the mega-sites and re-establish smaller settlements.
Keywords: Chalcolithic, Trypillia economy, paleodiet, manure production, legumes
Abstract
After 500 y of colonizing the forest-steppe area northwest of the Black Sea, on the territories of what is today Moldova and Ukraine, Trypillia societies founded large, aggregated settlements from ca. 4150 BCE and mega-sites (>100 ha) from ca. 3950 BCE. Covering up to 320 ha and housing up to 15,000 inhabitants, the latter were the world’s largest settlements to date. Some 480 δ13C and δ15N measurements on bones of humans, animals, and charred crops allow the detection of spatio-temporal patterns and the calculation of complete agricultural Bayesian food webs for Trypillia societies. The isotope data come from settlements of the entire Trypillia area between the Prut and the Dnieper rivers. The datasets cover the development of the Trypillia societies from the early phase (4800–4200/4100 BCE), over the agglomeration of mega-sites (4200/4100–3650 BCE), to the dispersal phase (3650–3000 BCE). High δ15N values mostly come from the mega-sites. Our analyses show that the subsistence of Trypillia mega-sites depended on pulses cultivated on strongly manured (dung-)soils and on cattle that were kept fenced on intensive pastures to easy collect the manure for pulse cultivation. The food web models indicate a low proportion of meat in human diet (approximately 10%). The largely crop-based diet, consisting of cereals plus up to 46% pulses, was balanced in calories and indispensable amino acids. The flourishing of Europe’s first mega-populations depended on an advanced, integral mega-economy that included sophisticated dung management. Their demise was therefore not economically, but socially, conditioned [Hofmann et al., PLoS One. 14, e0222243 (2019)].
The world’s first proto-urban settlements, covering up to 320 ha and with some 15,000 inhabitants, developed in the modern-day territory of Ukraine and Moldova more than 6 millennia ago, that is, hundreds of years before urban settlements in Mesopotamia, such as Uruk (1, 2). These mega-sites (defined as >100 ha) were created by Trypillia (Tripolye) societies, during a so-called agglomeration phase (4200/4100–3650 BCE), and individual settlements often persisted for more than 150 y (3–5).
The agglomeration phase, with its numerous mega-sites, large total settlement area, new styles of pottery painting, elaborated pottery kilns, and miniature ceramic house and sledge models, represents the cultural blooming of Trypillia societies (6). The never-before-seen typical concentric layout of the agglomeration phase sites, characterized by rows of houses along ring corridors arranged around an open central place, developed earlier in the middle Trypillia area (4200 BC) than in the eastern Trypillia area (4100 BC), and in both cases some hundred years after the start of the Trypillia colonization of the hitherto sparsely populated forest-steppe ecotone northwest of the Black Sea (the early phase, 4800–4200/4100 BCE) (Fig. 1).
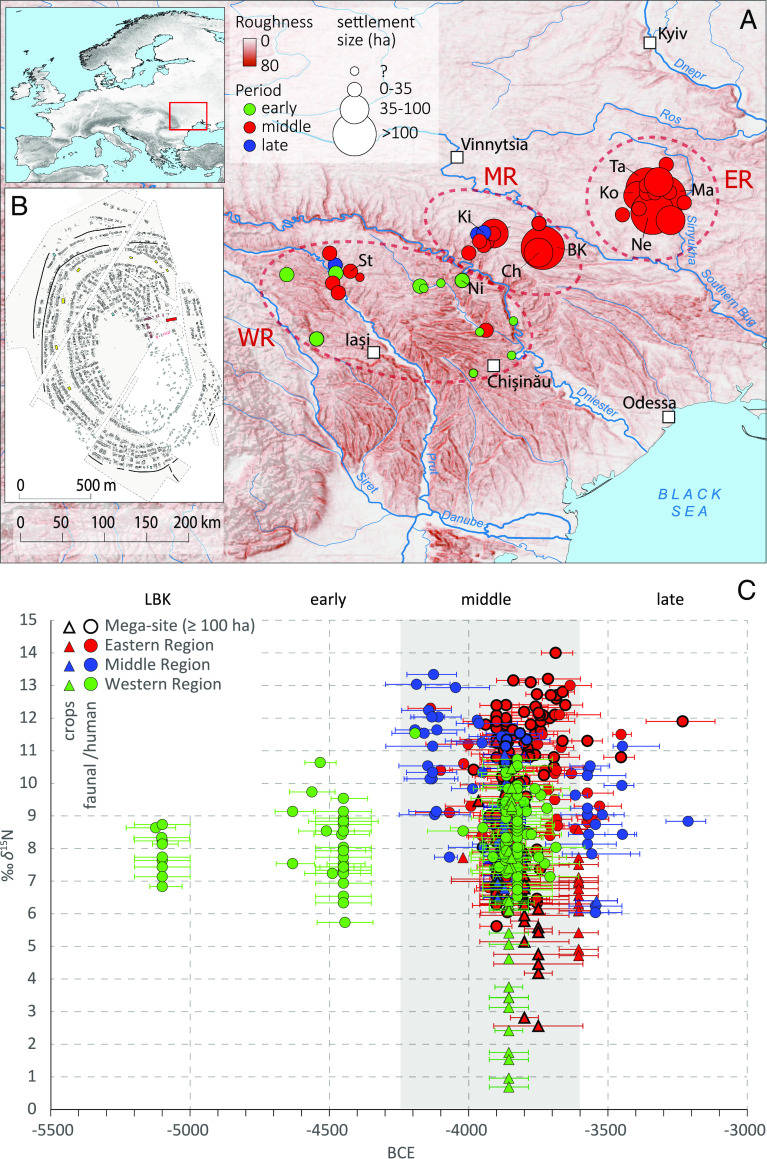
Fig. 1.
Trypillia site location and isotope data. (A) Map showing site locations scaled to spatial extent of the settlement for the western region (WR, Prut–Dniester), the middle region (MR, Dniester–Southern Bug), and the eastern region (ER, Southern Bug–Dnepr), by archaeological period. (B) Plan of the mega-site of Maidanetske, with ring corridor, rows of houses, and open central place. (C) δ15N values of crops and bone collagen by regions and early to late phases of the Trypillia societies, as well as the preceding Linear Pottery phase (LBK) of the multi-phase settlement of Nicolaevca. The age data points are the median values determined by Oxcal v. 4.4 for 14C dated samples; the error bars indicate the 1-sigma range (68.3%). For samples not directly dated, dates for the respective feature or site are given (see also SI Appendix, Fig. S1). Sites mentioned: Bilyi Kamin (BK), Chechelnyk (Ch), Kisnytia (Ki), Kosenivka (Ko), Maidanetske (Ma), Nebelivka (Ne), Nicolaevca (Ni), Stolniceni (St), and Talianki (Ta).
In terms of the spatial extent of the agglomeration, three geographical regions can be distinguished. In the western region (WR), encompassing the Prut–Dniester interfluve, sites reached up to 35 ha. In the middle region (MR), between the Dniester and Southern Bug rivers, some mega-sites of approximately 100 ha are known. But in terms of the large number and size of the mega-sites (up to 320 ha), it is the eastern region (ER, encompassing the Southern Bug–Dnepr interfluve), south of modern-day Kyiv, that stands out.
The mega-sites had a novel, well-planned design with houses along a ring corridor and an empty central place (Fig. 1B). Several smaller assembly houses on the level of neighborhoods and quarters as well as a giant assembly house for representatives of the whole settlement ensured equal participation in communal decision-making processes. The giant assembly houses represent some of the largest buildings in prehistoric Europe (7–10). This equality made these proto-urban settlements enormously attractive and this attractiveness, in turn, increased the size of these massive agglomerations. A simultaneous reduction in variability in house size indicates the existence of effective mechanisms for lessening social inequality (11). At the beginning of the sequence of Trypillia mega-sites, we see a widely distributed participation of the population in sequential political decision-making processes and effective mechanisms of social levelling. Correspondingly, decreasing differences in house sizes demonstrate a decrease in social inequality in the early agglomeration phase. Later, decision-making by increasingly smaller groups of people is indicated by the disappearance of low-level assembly houses. Linked to this, the differences in house sizes and social inequality increased significantly. This points to failure of levelling mechanisms and ran counter to the founder ideology of social equality, which is manifested in the planned concentric layouts of the settlements. In our view, these social dynamics led to intra-community conflicts and first resulted in the gradual disintegration of mega-site communities and decline of innovative dynamics, and later (c. 3650 BCE) culminated in their collapse (4, 6).
In addition to trying to address questions about the social and political makeup of the Trypillia settlements, researchers have long attempted to clarify their economic basis. Up until now, the Trypillia economy, especially the subsistence of the mega-site populations, has been poorly understood. Food web analyses from the region were focused exclusively on isotopes from human and animal bone collagen, omitting any of the crop plants, and were based on biplots of the δ13C and δ15N. Moreover, these analyses sometimes incorporated undated bones from sites spanning several archaeological periods (12–19).
We compiled a dataset of more than 480 measurements of δ13C and δ15N on animal bones, charred crops (cereal grains and chaff, peas), and feather grass, as well as palaeosoils, to understand animal herding and crop production strategies. We also compiled measurements from human bones found within the settlements. We then created food webs, using Bayesian modeling, to identify the relative importance of crops and meat in the human diet.
Results and Discussion
Indications from the δ15N patterns are here discussed by taxon, followed by general remarks on the δ13C patterns.
Spatio-Temporal Patterns and Animal Husbandry Practices Inferred from Stable Isotopes.
Cattle are the best-represented group in the stable isotope dataset, occurring in most of the three chronological phases in each of the three geographical regions (Fig. 2 and Dataset S1). The agglomeration phase is the best represented, with 173 measured bones (n), while the preceding early phase (29 n) and the succeeding dispersal phase (33 n) are less well represented. The biggest agglomeration phase dataset (86 n) is from the ER. It exhibits the highest variety (SD 1.89) and widest range (6.1–14.0‰) in δ15N (SI Appendix, Table S1).
Fig. 2.
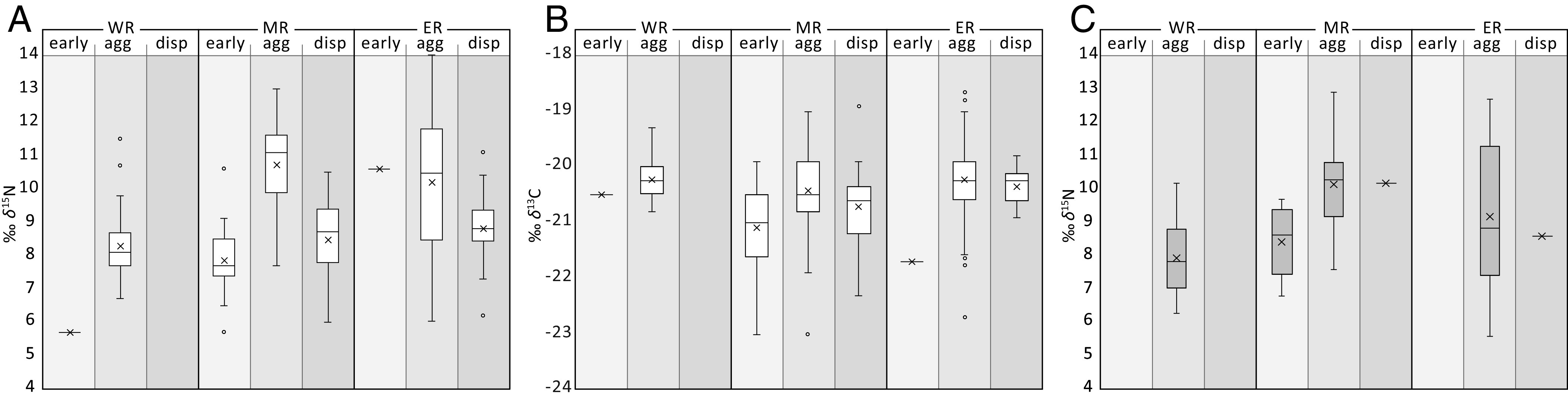
Faunal stable isotope data of bone collagen by region and phase, for the western (WR), middle (MR), and eastern (ER) regions and the early, agglomeration (agg), and dispersal (disp) phases. (A) Cattle δ15N. (B) Cattle δ13C. (C) Sheep/goat δ15N.
The three chronological phases are the best represented by cattle bone collagen isotope values from the geographical center of the Trypillia societies. Here, in the MR, the mean cattle δ15N is lowest in the early phase (7.8‰), reaches its maximum during the agglomeration phase (10.7‰), and decreases in the dispersal phase (8.5‰). In the ER, mean cattle δ15N is also at its maximum in the agglomeration phase (10.2‰) and declines in the dispersal phase (8.8‰). In the WR, mean cattle δ15N is relatively low in the agglomeration phase (8.3‰), even though sample coverage is good (56 n). Indeed, the agglomeration δ15N is approximately the same as the pre- and post-agglomeration δ15N of the two more eastern regions.
The cattle bone δ13C is also higher during the agglomeration phase (MR −20.4‰; ER −20.2‰), but, in contrast to the situation for δ 15N, is on the same, high level in the WR (−20.2‰). This uniform δ13C level, which is decoupled from the δ15N pattern, may reflect a supra-regional climate signal, such as somewhat warmer temperatures.
At the site level, mean δ15N values above ~10‰ are also mostly restricted to the agglomeration phase of the MR and ER, where the largest mega-sites occur. An overall strong positive correlation between cattle δ15N and settlement size is demonstrated by values increasing from the smallest sites to the largest mega-sites (Fig. 3A). As exemplified for Maidanetske, high δ15N can be the result of intensive pasturing practices (20).
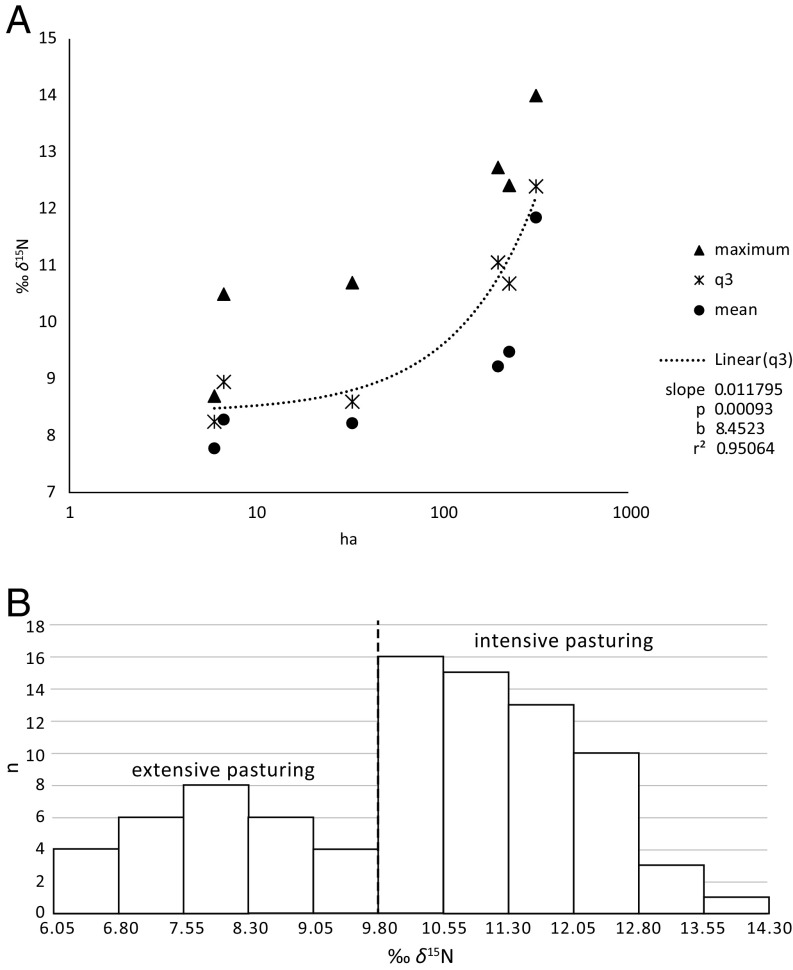
Fig. 3.
Cattle bone collagen δ15N data by settlement size. (A) Mean, third quartile (q3), and maximum values by settlement size, for settlements with at least 14 δ15N values (Nicolaevca 6 ha, Kisnytia 7 ha, Stolniceni 33 ha, Maidanetske 200 ha, Nebelivka 230 ha, and Talianki 320 ha), and regression line of q3, with equation, coefficient of determination (r2), and probability (p). (B) Cattle δ15N values (n = 86) from the agglomeration phase in the ER, exhibiting a sharp rise from extensively (<9.8‰) to intensively pastured cattle; bins are adapted to meet the 9.8‰ subpopulation delimitation calculated by k-means.
A statistical analysis (k-means) of the biggest agglomeration dataset, of 86 δ15N values from the ER, indicates that a δ15N of 9.8‰ forms the threshold between two pasturing subgroups (Fig. 3B). The extensively pastured subgroup (<9.8‰) shows a clear normal distribution confirmed through statistical testing (Shapiro–Wilk W = 0.97, P = 0.46). The normal distribution may reflect cattle herds roaming free, with access to natural habitats from forests to steppes, containing vegetation varying in δ15N. The intensively pastured cattle (>9.8‰) make up 66% of the 86 bones. Their δ15N values are clearly non-normally distributed (W = 0.95, P = 0.028, skewness = 0.5) and are separated by a steep step from the normally distributed, extensively grazed cattle group. The values indicate that the intensively grazed cattle were not able to access low δ15N vegetation. This implies that they were either fenced in or partly tethered to stakes. Fencing likely sped up dung collection for fertilizing arable land and allowed for pastures and cultivated fields to be located in direct proximity, saving the inhabitants time and space while simultaneously protecting the crops from devastation by the herds.
We chose here the third quartile (q3) as the statistical parameter for differences in the δ15N of the intensive pastured cattle between settlements. The q3 is the median of the upper 50% of all values of a site and covers therefore preferably the high δ15N values of a settlement. As a median, q3 is robust against outliers. As a gauge of animal density on the intensively grazed pastures, we therefore chose the increase of the q3 of cattle δ15N with settlement size. The slope of the linear regression model (r2 = 0.95; P = 0.001) for q3 indicates a δ15N increase of 0.01‰ per 1-ha increase in settlement size (Fig. 3A). From Stolniceni (33 ha), with nearly no intensive pasturing, to the largest mega-site, Talianki (320 ha), with no δ15N value below 10‰, the regression of q3 rises by 3.4‰. The detailed analyses of refs. 21 and 22 on a broad variety of different farming systems from middle Germany into the Alps show that an increase in stocking rate by 1 livestock unit (LSU, which stands for a cattle live weight of 500 kg) per ha can raise the δ15N of the pasture’s top soil, the pasture plants, and the grazing animals by around 1‰. Thus, an increase of 3.4‰ might point to some 3.4 LSU/ha.
However, since Neolithic cattle were smaller and approximately half the weight of cattle today (23), that is approximately 250 kg, a δ15N increase of 3.4‰ could reflect approximately 7 adult cattle per ha. A density of 3.4 LSU/ha today is considered very intensive livestock husbandry. Our calculated value may to some degree be an overestimation due to the high soil quality and high microbial activity of loess soils in the Trypillia area (24) compared with the pastures on less favorable substrates studied by refs. 21 and 22. Nevertheless, our results indicate that elaborate and labor-intensive animal management was taking place some centuries after the start of the transformation to a Neolithic subsistence at least in the MR and ER. Such intensive pasturing may not have been necessary at non-mega-sites (<100 ha), such as Stolniceni and others.
Taking a figure of 1 cattle (~0.5 LSU) per house (23, 25), the most populated mega-sites, Maidanetske and Talianki, with approximately up to 2,300 and 1,830 contemporaneously occupied houses each, would have had cattle herds equivalent to some 1,150 and 915 LSU, respectively. If all cattle were pastured intensively (3.4 LSU per ha), approximately 340 and 270 ha of pastureland would have been needed. Such an area would be only a small fraction of the 7,850 ha within an hour’s walking distance from the settlements (25, 26). We know that there are sites where all cattle were being pastured intensively: At Talianki, none of the cattle bones had a δ15N value below 9.8‰. In the WR, nearly all of the cattle were kept extensively managed during the agglomeration phase, but in the MR approximately only a quarter, and in the ER a third, which would have entailed a larger area requirement for the extensively pastured herds, which were moving around at some distance from the settlement.
The intensively pastured cattle may have needed additional foddering with grass, hay, leafy hay, pea straw, or cereal straw. These kinds of plant materials very rarely survive archaeologically. However, foddering management involving the transport of grasses and herbs from the steppe may be indicated here by the increase in the number of feather grass (Stipa) finds during the settlement agglomeration phase (27). If we take the Stipa δ13C (−24.3‰) as a proxy for steppe vegetation, we see that the difference from the overall mean δ13C of agglomeration cattle (−20.3‰) is close to the often-assumed offset of 4.8‰ between diet and herbivore bone collagen (28, 29).
The stable isotope values of sheep/goats separate into four statistically valid subsets. The number of datapoints (44 n), variance (2.0), and range (5.6–12.7‰) of the δ15N values are highest during the agglomeration phase of the ER, whereas the mean is highest in the MR (10.2‰). The δ15N of the sheep/goats shows the same trend as that of the cattle, in that the highest mean occurs during the agglomeration phase, in the MR (Fig. 2C). The δ15N of the sheep/goats in the WR is the same during the agglomeration phase as it is during the non-agglomeration phases of the more eastern regions, in this case the early phase of the MR.
While the δ15N ranges of the sheep/goats resembles those of the cattle, most sheep/goat subsets show a trend to somewhat lower means and medians as the corresponding cattle subsets. This trend is strongest in the ER, where with only 36% of sheep/goats intensively pastured during the settlement agglomeration phase, the distribution is opposite to that of the cattle (66%). Assuming a live weight of approximately 30 kg (23), 1 LSU equals approximately 17 sheep or goats. Therefore, if only a small proportion of the increase in δ15N (Fig. 3A) is caused by sheep/goat, a considerable number of sheep/goats must have grazed on high δ15N pastures.
Pigs are represented by just five small subsets, with 2 to 11 values each. Again, the agglomeration phase in the ER and MR is marked by the highest mean values of δ15N (SI Appendix, Fig. S2), and again, the δ15N of the agglomeration phase in the WR (11 n) is close to the pre- and post-agglomeration values of the MR and ER. Due to pigs’ omnivory, their δ15N could, in theory, exceed that of herbivores, but here it does not so clear. The pig δ15N values are mostly in the upper ranges of those of cattle and sheep/goats, probably due to the pigs being fed on domestic waste while ranging around in the settlement space (20). The trend in their δ15N values, which are highest during the agglomeration phase, indicates that pigs must have been an integral component of agricultural food production like cattle and sheep/goat, although less important numerically.
Gardens of Pulses and Fields of Cereals.
Through symbiotic bacteria, pulses, such as peas, receive their nitrogen from the soil atmosphere around the roots, resulting in a plant δ15N close to 0‰. Even when pulses are fertilized, their atmospheric nitrogen signal remains relatively dominant. Thus, even a small increase in δ15N, of approximately 1‰, can point to manuring (30). When manured on the same level, the δ15N of pulses can be more than 5‰ lower than that of cereals (31).
The δ15N values of pea seeds from Trypillia sites show considerable variability (Fig. 4). The peas from Stolniceni range from 0.7 to 1.7‰ (3 n), while those from the mega-site of Maidanetske range from (2.8 to) 4.2 to 7.0‰ (n 15) (27). Broad bean (Vicia faba), a pulse not known from Trypillia sites, shows values of 3 to 5‰ when cultivated on dung-soils (31) formed by labor-intensive improvement with high levels of manuring, over long periods, on small plots close to houses and stables. Broad bean shows values of 2.6‰ from short-term cultivation on middens of dung and compost, whereas the substrate shows values of 8.1‰ (27, 32). From these pulse data, we can infer that the 15N-rich peas from Maidanetske must have been labor-intensively manured, possibly in garden plots. To reduce the amount of effort required to transport the manure, these plots may have been in close vicinity to either the intensively managed pastures or the houses (if human feces were used as well). Areas of higher magnetic activity close to houses (3) may identify manured plots similar to terra preta, including burned material from hearths and another household waste (33). The δ13C values of the pulses point to favorable water conditions during summer (27); the good water storage capabilities of the humus-rich soils may also have played a role in this.
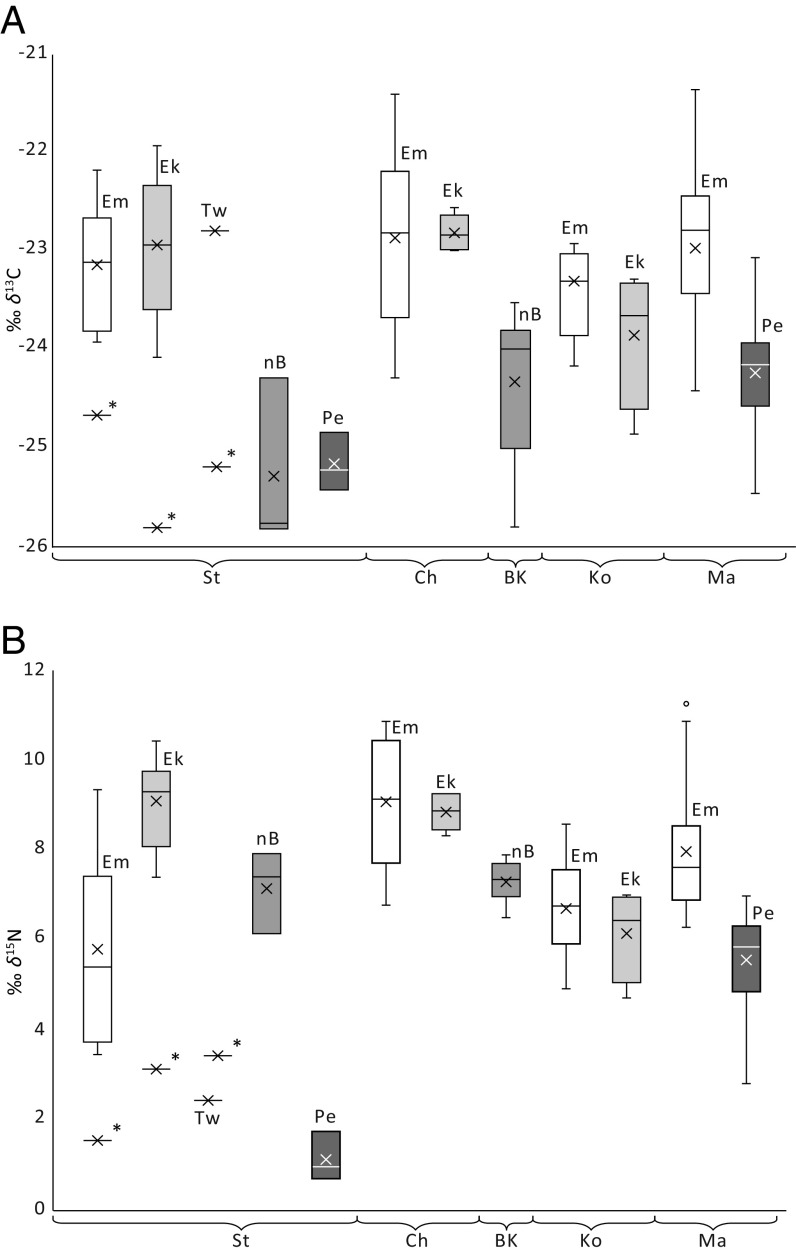
Fig. 4.
Stable isotope values of the cereals emmer (Em), einkorn (Ek), Timopheev’s wheat (Tw), naked barley (nB), and pea (Pe) from the Trypillia settlements of Stolniceni (St), Chechelnyk (Ch), Bilyi Kamin (BK), Kosenivka (Ko), and Maidanetske (Ma), sequenced West–East. (A) δ13C. (B) δ15N. δ15N adjusted by −0.5‰ for charring; *glume bases.
The δ15N values of the grains range from 3.4 to 11.3‰, in emmer; to 4.7–10.4‰, in einkorn; to 6.5–7.9‰, in naked barley. Applying published δ15N thresholds for manuring, from mostly non-Chernozem soils, would classify all cereals as moderately to highly manured (31, 34). However, such thresholds seem unlikely for the Trypillia agriculture, due to the high natural fertility of their loess soils. For any interpretation of Trypillia δ15N in terms of anthropogenic management, the difficult-to-deduce natural δ15N baseline is therefore a crucial assumption.
Often the δ15N of wild animals serves as natural baseline, but animal δ15N decreases by some permille when animals live in forests. Wild animal δ15N therefore does not reliably reflect the open steppe habitats, extensive pastures and field areas (35, 36). In pre- and post-agglomeration times, the mostly extensively pastured domesticated animals exhibit a mean δ15N value of 8‰. Using the δ15N offset of 5.5‰ from human food webs, we end up with a humble landscape baseline of 2.5‰, probably due to fundamental differences in human and ruminant digestion and absorption of nitrogen compounds.
Samples from a (pre-)Trypillia palaeosoil buried downslope from the Maidanetske site exhibit a mean δ15N of 7.3‰ (37). Samples from the lower part of an overlying later Trypillia colluvium, possibly incorporating soil from the decayed settlement, show δ15N values up to 8.9‰ (SI Appendix, Fig. S3 and Dataset S2). Grassland plants on a 7.3‰ δ15N soil can show a δ15N of around 5.4‰ (21). Applying an intra-plant enrichment of some 0.3‰ (38) results in a δ15N of approximately 5.7‰ for cereal grains cultivated on such soils unaltered by people.
The Stipa awns from Trypillia times exhibit a broad variety in δ15N (2.6–6.5‰), seemingly regardless of settlement size. They may reflect more or less natural conditions, ranging from at that time young, early soils on slopes to well-developed, mature soils on the plain (39). Judging from the offsets between husk and grains (38), the δ15N of the awns (mean 5.3‰) can be expected to be relatively depleted by 1.2‰ compared to grains. Therefore, judged from the Stipa awns, cereal grains of 6.5‰ would demarcate for unmanured conditions. Based on the derivations from the palaeosoil (5.7‰) and Stipa (6.5‰), a natural δ15N baseline for cereal grains of around 6.1‰ is assumed here.
With regard to a general increase in δ15N by approximately 3‰ per trophic (40) and manure (34) level, cereal grains higher than 9‰ in δ15N can be considered to have been manured. The δ15N of several emmer and einkorn grains from Maidanetske, Stolniceni, and Chechelnyk range from 9.2 to 11.3‰ and probably originate from manured fields. At least occasional manuring will have been necessary to compensate for the loss of nutrients that occurs even on (sandy) loess soils when these have been cultivated over decades (41). A few of the high δ15N cereal grains of Maidanetske and all cereals from Stolniceni are more than 5‰ above the respective local pea mean and thus may have been cultivated on highly fertile garden dung-soils, either together with peas as climbing aids or alternating with peas in crop rotation. Emmer grains low in δ15N may stem from green manuring of cereal fields by incorporating low δ15N fixed by pulses or from just recently cultivated forest soils.
The higher 13C depletion of barley (−25.5 to −23.8‰) compared with emmer (−24.4 to −23.0‰) and einkorn (−24.5 to −22.3‰) is a result of barley’s greater water use efficiency (27, 42, 43). The δ13C ranges of all three taxa point to good water availability (27). The 13C values of the barley point to potential yields of some 1.6–2.0 tons/ha (SI Appendix, Table S2). This is within the range of barley yields in early nineteenth-century Germany, of 1.1–4.2 tons/ha (44). Nevertheless, the true Trypillia amounts are difficult to estimate due to for instance ecological differences between landraces and varieties and our lack of knowledge of the details of Trypillia cultivation practices. The values of the measured glume base samples are comparable to those of the grains depleted in 13C (emmer 1.6‰; einkorn 2.5‰) and 15N (emmer 4.3‰; einkorn 5.7‰). The δ13C offsets fall within the variability of wheats (45), but the δ15N offsets are far above the range of 2.4 ± 0.8‰ expected from recent experiments (31, 46). Such high δ15N offsets, of around 5‰, have been found occasionally between grains and rachis segments, especially in the upper parts of ears (47). One reason for the high offset could be stress from direct exposure to solar radiation. Under this assumption, the glumes investigated might be from the upper parts of ears that somehow became accumulated during harvesting and processing or from ears that rose above the others, as happens in landraces, which are regularly rich in different morphotypes (48).
Pulses can incorporate nitrogen via symbiotic root nodules from the atmosphere and therefore are not dependent on manuring. Nevertheless, manuring can be of great importance for both quality and quantity. Pulses can put more effort into leaves, stems, and seeds when they do not need to feed the symbiotic bacteria (49). A high amount of plant-available nitrogen in the substrate increases the protein content, and the phosphorus in the dung can improve the cooking properties of the seeds (50). In addition, dung-soils warm up quickly because they are dark, which accelerates germination and extends the growing season, leading to higher yields.
Cultivating peas in manure beds was a common practice in the 18th century (51). The manuring results in a disproportional increase in pea straw, which is a highly valued fodder for cattle and sheep (51–53). We can surmise from high δ15N values in the literature (34, 54–56), that knowledge about the benefits of manuring pulses was already widespread in the Neolithic. In view of the mega-populations to be fed, high productivity combined with low space requirements will also have been a decisive advantage of the intensive manuring (55, 57).
The cultivation of a wide variety of cereals (emmer, einkorn, hulled barley, naked barley) plus pulses (pea, lentil, bitter vetch) (27) allows farmers to significantly extend the periods of sowing and harvesting, leading to an equalized and efficient allocation of the restricted work force. At the same time, the risk of high crop losses due to certain unfavorable seasonal weather conditions and species-specific pests was reduced.
Human health is predicated on a sufficient intake of indispensable amino acids (IAA). Although humans can gain all IAA from a balanced vegetable diet, cereal-dominated diets are deficient in some IAA, such as lysine (58), which is the first limiting amino acid for humans and its adequate availability is therefore indispensable for the healthy growth of children and sub-adults (59). Pulses are not only approximately twice as high in proteins as cereals but are also rich in lysine, making them a needed complement (60). The composition and digestibility of these proteins are also much better in pulses than in cereals (60). Consumption of protein from pulses and cereals together provides a healthy and sustainable balance between amino acid composition and adequate caloric intake (61) and fulfills the need for IAA in crop-dominated diets without exceeding the daily caloric needs (62). It requires less energy and effort to produce a meal from pulses than from meat (63). Research on archival sources has shown that pulses and cereals were a dominant part of human diet in Europe from at least medieval times until industrialization (44, 52, 64). Another limiting nutrient is Vitamin B12, which humans can only acquire through the consumption of animal products.
The combination of labor-intensive growing of pulses on dung-soil beds and intensive animal husbandry on fenced pastures, which allowed the large and dense human population to have a healthy diet, sets the mega-economy of these proto-urban Trypillia societies apart and underlines that they developed independently from early urbanism in Mesopotamia and the Aegean (2, 3, 65).
Food Webs.
To quantify the role of crops and animals as nutrient sources for Trypillia people, Bayesian mixing models (29) were set up for three isotopically well-represented settlements, Stolniceni (33 ha), Kosenivka (80 ha), and Maidanetske (200 ha). Macro remains of pulses are available from the mega-site of Maidanetske and the small site of Stolniceni. Considering the high importance of peas for human health, it seems likely that their apparent absence at Kosenivka (80 ha) results from the general underrepresentation of pulses in archaeobotanical assemblages (27). Nevertheless, we did not include any pulses in the Kosenivka model. Human remains are available for Kosenivka and Stolniceni. Mean isotope data of the well-preserved cranial bones of three individuals from Kosenivka are utilized also for the nearby Maidanetske, and the values of one local inhabitant are used for Stolniceni (Dataset S3). Cattle, sheep/goat, and pigs span most of the δ15N range, which makes grouping by taxon affiliation futile. The zoological data of each of the three sites are therefore divided into animals intensively (Ai; δ15N > 9.8%) or extensively (Ae; δ15N < 9.8%) pastured.
For Kosenivka, the model indicates a mean proportion of protein obtained from cereals of 81.5% (Fig. 5 and Table 1 and SI Appendix, Table S3), which is conspicuously less than the proportion output by the model when animal sources are not divided into Ai and Ae (SI Appendix, Table S4). The differentiation into Ae and Ai that we apply here increases the amount of animal protein in the model outputs for all three sites (SI Appendix, Table S5) and probably better reflects the human nutrition than do model outputs that do not incorporate this differentiation. By taking into consideration dietary components not reflected in the model (especially starch) and the caloric content of cereals and meat, we can calculate that meat makes up only some 8.1% of people’s caloric intake at Kosenivka.
Fig. 5.
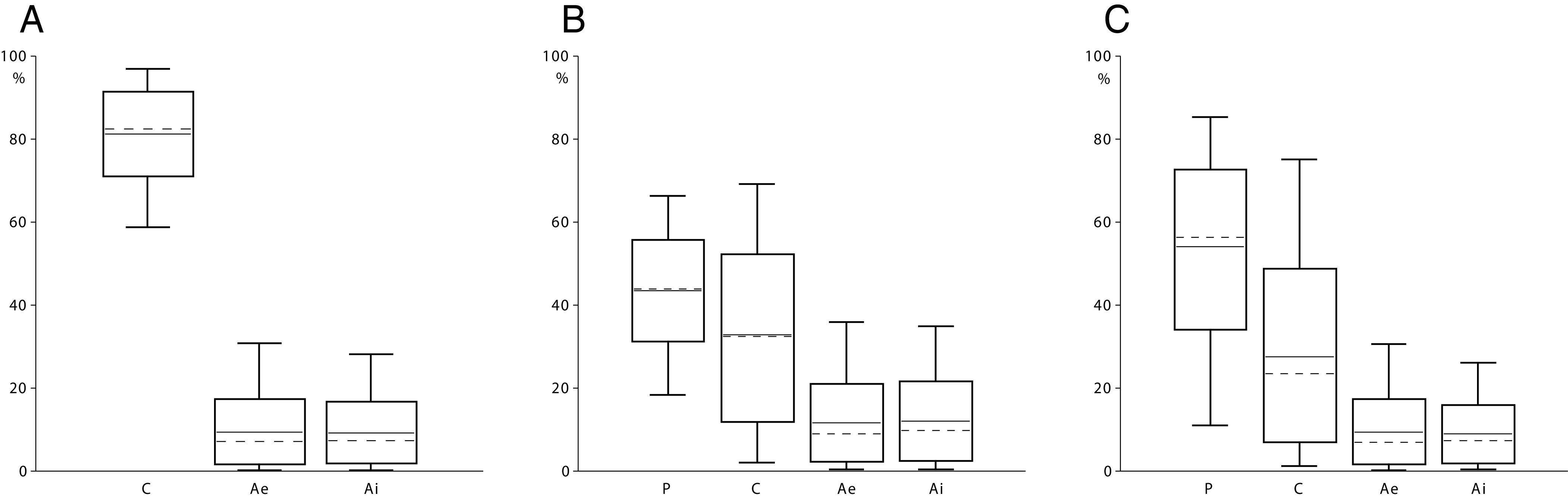
Results of food web analyses based on δ15N and δ13C performed in FRUITS v. 3.1. (29) for (A) Kosenivka, (B) Stolniceni, and (C) Maidanetske, representing the modeled protein contribution to human nutrition by the sources cereals (C), pulses (P), extensive pastured animals (Ae, δ15N < 9.8‰), and intensive pastured animals (Ai, δ15N > 9.8‰). Mean protein intake calculated for the δ15N and δ13C of (A) the mean values from three human individuals from Kosenivka and (B) one individual from Stolniceni. For Maidanetske (C), from where no human data exist, the values of Kosenivka were applied. Boxes span 1 SD from the mean (horizontal line); whiskers span 2 SD; medians are marked by discontinuous lines.
Table 1.
Mean protein proportion of crops and animals in the human diet of the models in Fig. 5
| Kosenivka | Stolniceni | Maidanetske | ||||
|---|---|---|---|---|---|---|
| Proteins | Calories | Proteins | Calories | Proteins | Calories | |
| P | – | – | 43.6 | 35.8 | 54.1 | 45.6 |
| C | 81.5 | 91.9 | 32.9 | 53.9 | 27.6 | 46.2 |
| ∑ P,C | 81.5 | 91.9 | 76.5 | 89.7 | 81.7 | 91.8 |
| Ae | 9.3 | 4.1 | 11.6 | 5.1 | 9.4 | 4.2 |
| Ai | 9.2 | 4.0 | 12.0 | 5.2 | 8.9 | 4.0 |
| (∑) A | 18.5 | 8.1 | 23.5 | 10.3 | 18.3 | 8.2 |
| pmd | – | 29 | – | 38 | – | 30 |
Caloric proportions calculated with respect to modeled protein proportions and the composition and caloric values of pulses (P); cereals (C); animals extensive pastured (Ae); animals intensive pastured (Ai), and for pure meat days (pmd).
For Stolniceni, the peas made up 43.6% and the cereals 32.9% of the consumed proteins, for a total of 76.5%. Of the consumed calories, peas made up 35.8% and cereals 53.9%, for a total of some 90% of caloric intake from crops in Stolniceni, which matches that at Kosenivka. At Maidanetske, the crops played approximately the same role in terms of protein (81.7%) and calories (91.8%) as at Kosenivka and Stolniceni, but pulses dominated the protein input (54.1%) and contributed about the same proportion to the caloric intake as cereals. At all three sites, the total animal portions are almost equally derived from Ae and Ai and vary in total for the protein between 18.3 to 23.5% and only 8.1 to 10.3% for calories.
For the animal protein proportion, the results of the three Trypillia food webs are close to some calculations for the Neolithic to Bronze Age in southern Poland (66) and the Neolithic in Turkey (67), central Europe (28), and southwestern Germany (68). When we convert these protein proportions into calories, we see that at all three Trypillia settlements, animal products contributed only a small proportion, some 8 to 10%, of people’s caloric intake. Results from archaeobotanical, landscape, chemical element, and ethnographical analyses indicate a similarly low consumption of animal products in the Neolithic (23, 66, 69, 70). This stands in contrast to the concept of an intensive Trypillia meat production system (71).
As an essential ingredient of feasting, meat may have played a central role in social cohesion and stability (7, 20, 72). We hypothesize that there would have been days during which only meat was consumed (pure meat days, pmd). Calculations of how many pmd would be needed to accommodate the stable isotope results vary from 4 wk to more than 5 wk per year. This seems to us an improbably large number of feasting days and suggests there was some everyday meat consumption, preferably from small animals, as well. During episodes of meat overconsumption, such as feasts, the excess protein would be broken down and excreted without leaving a trace in the bone collagen, causing some underestimation of the contribution of meat to human diet in the model outputs.
Conclusions
The phenomenon of Trypillia mega-sites is strongly correlated with high δ15N values in humans, animals, and plants, resulting from intensive management of animals on fenced pastures to enable labor-intensive gardening of pulses. The main focus of intensive cattle management was the extraction of manure to enable the large harvests of protein-rich pulses that could be produced on highly fertile dung-soils fertilized with this manure.
The strong intensification in animal and crop production was linked to the mega-site phenomenon (4200/4100–3650 BCE) and therefore restricted to the region between the Dniester and Dnepr. Farther west, in the Prut–Dniester interfluve, mega-sites are not known and δ15N values are relatively low. Low δ15N values are also generally the norm for Trypillia societies before and after the agglomeration of the mega-sites, i.e., in the early and dispersal phases.
Thus, the agglomeration process in the forest–steppe ecotone initiated a profound but temporary transformation in agricultural practices, including intensive animal management, while on the loess area in central Germany, nearly no changes in animal husbandry occurred over the four millennia spanning the Neolithic and the Bronze Age (73).
At the mega-sites, most of the cattle were probably fenced in to concentrate the dung in a small and confined area for ease of collection and to protect adjacent garden plots and fields from animal depredation. Fences made it possible for pastures, pulse plots, and cereal fields to adjoin each other and therefore to minimize land consumption as well as walking distances for animal tending, dung transport, field tilling, sowing, weed and pest management, and harvesting. Due to the high animal density on the pastures, it seems likely that the animals were given additional feed in the form of grass or hay from the steppes, the straw from the pulses (plentiful because of the manuring), and perhaps cereal straw. The intensive pasturing and manuring increased the biological turnover, resulting in the observed high δ15N in plants, animals, and humans, but possibly also created water eutrophication. Cattle and sheep/goat kept in the wider catchment of the Trypillia mega-sites may have acted not only as a hedge against unforeseeable complications in the intensive production chain but also as a continuous input of nutrients into it.
Together with cereals, the manured pulses not only co-dominated the daily Trypillia food intake but also ensured a healthy diet, balanced in calories and IAA. Meat contributed no more than approximately 10% to the human diet overall but was important for vitamins, such as B12, and may have played an important role for social cohesion during feasts.
The proto-urban Trypillia mega-sites obviously developed a unique subsistence strategy for feeding large populations, based on intensification of farming and husbandry practices, which is very distinct from the extensive, low-labor-input but land-consuming, agricultural strategies of early urbanism in Mesopotamia and the Aegean (3, 65). It seems plausible, that the high-intensity, labor-limited cooperative and therefore egalitarian farming system contributed to the social stability of the mega-sites, as it per se has a limited degree of inequality (74). The favorable climate, indicated by the δ13C of crops and animals (27), together with the loess as soil substrate (37), offered the best preconditions for agricultural development. Due to the development of a pasture- and pulses-based mega-economy, including the wise management of nutrients, such as nitrogen, the flourishing of the Trypillia mega-sites did not result in any overexploitation of natural resources (1, 25, 26). This makes it all the more likely that the demise of these proto-urban societies, and their inability to continue further to develop urbanism, was the result of intra-social tensions caused by politically induced social inequality and the lack of functioning communication abilities, ultimately culminating in population dispersal (4, 6, 7).
Materials and Methods
The dataset for this study consists of more than 480 isotope measurements from some 40 sites, which were obtained in recent years within the Subproject D1 “Population Agglomerations at Tripolye-Cucuteni Mega-sites” of the Kiel Collaborative Research Centre 1266 “Scales of Transformations–Human-Environmental Interaction in Prehistoric and Archaic Societies.” The field sampling included settlements of different periods, (sub-)phases, and size categories. It was based on the definition of a specific excavation strategy after the completion of the magnetic surveys for each settlement, to guarantee the representation of all contexts and phases of the sites (5, 75, 76). Systematic sampling of all excavated archaeological features for archaeozoological and archaeobotanical material on the large and the smaller sites provides the basis for a solid representativeness of the archaeological data, including the isotope dataset. In addition, isotope measurements were carried out on archived material from previous excavations, e.g., on charred storage finds (27). Further, the isotope record was supplemented by previously published isotope data (20, 77). All isotope data were subject to critical evaluation before food web reconstruction. For the bone material, collagen quality was evaluated. The collagen C/N ratio of all human bones falls into the range of 2.9 to 3.5 (78) (Dataset S3). From the over 340 animal bones, two (both 2.8) are close below that range and nine are above (Dataset S1). All their δ13C and δ15N values are completely inconspicuous and were not excluded from our statistical analyses, especially since higher ratios point to some intermixture of nitrogen-free C-source like carbohydrates or fat, that does not affect the δ15N values (78). Botanical remains were examined for contaminations and cleaned as needed (27). The total amount of human bones for Trypillia is minimal because the rite de passage is not associated with formal burials or bone deposition. Burial sites dating prior to about 3600 BC are nearly unknown (79). Human remains from seven individuals from Kosenivka and one individual from Stolniceni provided the opportunity to perform the needed consumer measurements for our food webs (80, 81). From the partly burned material of Kosenivka, only unburned cranial bones from three individuals were selected here for isotopic measurements to avoid effects due to contact with fire (Dataset S3).
Animal isotope values generated by us were measured on bone collagen extracted for radiocarbon dating following the Poznań Radiocarbon Laboratory protocol and isotopic measurements performed in the Frankfurt Isotope and Element Research Center, at Goethe Universität (Germany), with an element analyzer (ThermoFisher, Flash 1112) connected to a continuous-flow inlet of a gas source mass spectrometer (ThermoFisher, MAT 253) with usually less than ±0.2‰ for C and ±0.3‰ for N isotopes, using the standards USGS 24, IAEA-CH-7, IAEA-N1, and IAEA-N2.
Despite the large number of datapoints, a very detailed spatial–temporal analyses had to be renounced. The data points are spread between several taxonomical groups over a large geographic area and a long time span. There is not enough representation of any single taxon through all regions and phases for profound detailed statistical analyses. For our spatial–temporal analyses, we simplified the chronological framework to the three most prominent developmental steps of the Trypillia phenomenon, the early phase (colonization), middle phase (agglomeration), and late phase (dispersal), and we subsumed the few datapoints from the preceding LBK of the multi-phase settlement of Nicolaevca into the Trypillia early phase. With three phases and three regions, each taxonomic group becomes divided into up to nine subsets. As demonstrated by ref. 82, at least eight values per taxonomic group, or subset, per site are needed for the data for statistical validity. Datasets with high value ranges, such as the ones presented here, require even larger numbers per subset (83). Assigned to the subset sizes, subsets with at least 14 isotope values are here considered statistically meaningful.
For the spatial–temporal analysis, the zoological subsets are presented as boxplots (84) visualizing the changes in the regions through the three phases (Fig. 2). Small subsets are discussed with respect to their statistical uncertainty, and single finds are plotted for the sake of completeness. The software PAST v. 4.1 (85) was used to calculate k-means to indicate value limits between animal subgroups, calculate the equation and regression line of q3 (Fig. 3), and conduct Shapiro–Wilk tests.
The taxonomic classification of the animal bones varies in the original sources. Initially, only those bones originally classified as cattle, Bos, cf. Bos taurus, or Bos taurus were grouped under cattle. In this group, isotope values are not normally distributed (Shapiro–Wilk, p < 0.001). Since it is probable that bones classified as large ruminants and large mammals also come from cattle, we performed a statistical test to find out whether we could justify adding them to the cattle group. There is no significant difference between the groups (Kruskal-Wallis test), and we therefore subsumed them under cattle.
Initially, only those bones originally classified as Capra, goat, Ovis, Ovis/Capra, sheep, and sheep/goat are grouped under sheep/goat. Isotope values within this group are not normally distributed (Shapiro–Wilk, p < 0.002). Since it is possible that bones classified as small ruminants or mid-size mammals represent sheep or goats, we performed statistical tests. There are differences in the sample means (Kruskal-Wallis test), and these come from differences between sheep/goat and small ruminants (Mann–Whitney post hoc test). This is apparently caused by the high δ15N values of small ruminant bones from the biggest of the mega-sites, Nebelivka. Within Nebelivka, there is no significant difference between the means of sheep/goat and small ruminants (Kruskal-Wallis test, P = 0.133). Therefore, all small ruminant as well as medium mammal bones are included here in the sheep/goat group.
Crop isotope values were generated from charred remains identified to the species or variety level, mostly from pits of ephemeral, single-phased Trypillia settlements (27). They are used here especially for the food webs of the sites. A total of 99 measurements (n) of δ13C and δ15N have been performed for Maidanetske, Stolniceni, Chechelnyk, Bilyi Kamin, and Kosenivka (Dataset S4), mostly on single cereal grains (77 n) and single peas (19 n) and also on three assemblages of 10 glume bases of hulled wheats each. The δ15N in the text and figures was adjusted by −0.5‰ for charring, whereas the δ13C remains unchanged (27). Fourier-transform infrared (FTIR) spectroscopy pre-treatment of selected finds revealed no trace of contamination in the peas but did reveal carbonate contamination for the cereal grains, which were therefore processed following (86) before isotope measurement at the Department of Earth and Environmental Sciences of Katholieke Universiteit Leuven (Belgium). δ13C and δ15N were measured through combustion (elemental analyzer, ThermoFisher, Flash HT/EA or EA 1110) coupled via a continuous-flow interface (ThermoFisher, Conglo IV) to a mass spectrometer system (ThermoFisher, Delta V Advantage) with standards IAEA600 (−27.77‰ δ13C; 1‰ δ15N), Leucine (−13.73‰, 1.07‰), and Tuna (−18.72‰, 13.77‰). SDs of isotope results are <0.1‰. δ13C is reported relative to the international Vienna PeeDee Belemnite, δ15N relative to AIR.
The food web analyses are based on isotopes of cereal grains, peas, and animals as sources for the values in the human bones. Values of the animal sources are divided according to our identifications of pasturing practices into <9.8‰ (extensively pastured) and >9.8‰ (intensively pastured). The food webs were modeled with the Bayesian software FRUITS v. 3.1 (SI Appendix, Table S6), with a SD for the mean values of the sources and the offsets of ±0.5 (28, 29). The modeled proportions of the sources primarily reflect the proteins, because some 75% of the collagen carbon and most of the nitrogen derives from food proteins (87). Compared with meat, cereals are quite low in proteins; they mostly consist of nitrogen-free hydrocarbons, such as starch. Legumes also consist of a certain proportion of hydrocarbons. The overall caloric role of crops in human nutrition is greater than the mainly protein-based calculations suggest and was therefore calculated separately, from the reconstructed protein proportions.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Acknowledgments
We thank Svetlana Ibens for help in the field; Tanja Reiser for support during the macrobotanical analyses, and Dragana Filipović for cross-checking identifications; students Pia Bodden, Ricarda Liedtke (Kiel), and Nikita Kuzemko (Uman), as well as other students and colleagues, for sample collection and flotation support; Steven Bouillon and Yannick Stroobandt (Leuven) for processing the botanical stable isotopes; I.G. Emelyanov (Kyiv) for archiving charred plant remains; and Cheryl Makarewicz and Rebecca Eckelmann for identification of animal bones. We thank Suzanne Needs-Howarth for English-language and text editing and Carsten Reckweg for graphic editing. Research and open access to this paper was made possible within the scope of the Sonderforschungsbereich (Collaborative Research Centre) 1266 “Scales of Transformation. - Human-Environmental Interaction in Prehistoric and Archaic Societies” at Kiel University, funded by the Deutsche Forschungsgemeinschaft (German Research Foundation) (project number 290391021).
Author contributions
R.H., J.M., and W.K. designed research; R.H., M.d.C., G.P., S.D., M.S., A.T., K.F., M.V., V.R., J.M., and W.K. performed research; F.S. and R.H. analyzed data; F.S. analyzed and interpreted isotope data, drafted figures; R.H. operated isotope and archaeological database, analyzed and interpreted archaeological data, drafted figures; M.d.C., S.D., J.M., and W.K. provided interpretation; and F.S. and R.H. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.Diachenko A., “Demography reloaded” in Trypillia Mega-sites and European Prehistory 4100-3400 BCE, Müller J., Rassmann K., Videiko M., Eds. (Routledge, 2016), pp. 181–193. [Google Scholar]
- 2.Müller J., Pollock S., “Trypillia and Uruk” in Trypillia mega-sites and European prehistory 4100-3400 BCE, Müller J., Rassmann K., Videiko M., Eds. (Routledge, 2016), pp. 281–287. [Google Scholar]
- 3.Gaydarska B., Nebbia M., Chapman J., Trypillia megasites in context: Independent urban development in Chalcolithic Eastern Europe. Cam. Arch. Jnl. 30, 97–121 (2020). [Google Scholar]
- 4.Hofmann R., Shatilo L., Tripolye population aggregation and dispersal in light of regional settlement trajectories. Universitätsforschungen zur prähistorischen Archäologie (UPA) 376, 197–212 (2022). [Google Scholar]
- 5.Müller J., Hofmann R., Brandtstätter L., Ohlrau R., Videiko M., “Chronology and demography: How many people lived in a mega-site?” in Trypillia Mega-sites and European Prehistory 4100–3400 BCE, Müller J., Rassmann K., Videiko M., Eds. (Routledge, 2016), pp. 133–170. [Google Scholar]
- 6.Müller J., Hofmann R., Shatilo M., Tripolye mega-sites: “Collective computational abilities” of prehistoric proto-urban societies? J. Soc. Comput. 3, 75–90 (2022). [Google Scholar]
- 7.Hofmann R., et al. , Governing Tripolye: Integrative architecture in Tripolye settlements. PLoS One 14, e0222243 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman J., Videiko M. Y., Gaydarska B., Burdo N., Hale D., Architectural differentiation on a Trypillia mega-site: Preliminary report on the excavation of a mega-structure at Nebelivka, Ukraine: 2014a. J. Neolithic Archaeol. 16, 135–157 (2014). [Google Scholar]
- 9.Ohlrau R., Maidanets’ke: Development and Decline of a Trypillia Mega-Site in Central Ukraine (Sidestone Press, 2020). [Google Scholar]
- 10.Müller J., Hofmann R., Ohlrau R., Shatilo L., “The social constitution and political organsiation of Tripolye mega-sites: hierarchy and balance” in Überschuss ohne Staat–politische Formen in der Vorgeschichte: 10. Mitteldeutscher Archäologentag vom 19. bis 21. Oktober 2017 in Halle (Saale) = Surplus without the state - political forms in Prehistory: 10th Archaeological Conference of Central Germany October 19-21, 2017 in Halle (Saale), Meller H., Gronenborn D., Risch R., Eds. (Landesamt für Denkmalpflege und Archäologie Sachsen-Anhalt Landesmuseum für Vorgeschichte, 2018), pp. 247–260. [Google Scholar]
- 11.Hofmann R., Müller-Scheeßel N., Müller J., Trypillia-Mega-sites: A social levelling concept? Antiquity, in press. [Google Scholar]
- 12.Budd C., Lillie M. C., “The prehistoric populations of Ukraine: Stable isotope studies of fisher-hunter-forager and pastoralist-incipient farmer dietary pathways: 2020a” in Prehistoric Ukraine: From the First Hunters to the First Farmers, Lillie M. C., Potehina I. D., Budd C. E., Eds. (Oxbow Books, 2020), pp. 283–307. [Google Scholar]
- 13.Budd C., Potekhina I., Lillie M., Continuation of fishing subsistence in the Ukrainian Neolithic: Diet isotope studies at Yasinovatka, Dnieper Rapids. Archaeol. Anthropol. Sci. 12, 64 (2020). [Google Scholar]
- 14.Lillie M. C., Budd C. E., Potehina I. D., Stable isotope analysis of prehistoric populations from the cemeteries of the Middle and Lower Dnieper Basin, Ukraine. J. Archaeol. Sci. 38, 57–68 (2011). [Google Scholar]
- 15.Lillie M. C., et al. , First isotope analysis and new radiocarbon dating of Trypillia (Tripolye) farmers from Verteba Cave, Bilche Zolote, Ukraine. Documenta Praehistorica. Poročilo o raziskovanju paleolitika, neolotika in eneolitika v Sloveniji. Neolitske študije = Neolithic studies 44, 306–325 (2017). [Google Scholar]
- 16.Lillie M. C., Budd C. E., Diet isotope analysis and related studies in prehistoric Ukraine: Fact, fiction and fantasy. Archaeol. Early History Ukraine 37, 251–267 (2020). [Google Scholar]
- 17.Ledogar S. H., et al. , New AMS dates for Verteba cave and stable isotope evidence of human diet in the Holocene Forest-Steppe, Ukraine. Radiocarbon 61, 141–158 (2019). [Google Scholar]
- 18.Nikitin A. G., et al. , Radiocarbon dating and stable isotope analysis of human remains from the Usatovo Culture Site of Mayaki in Ukraine. SSRN J. (2022). 10.2139/ssrn.4236123. Accessed 7 February 2023. [DOI] [Google Scholar]
- 19.Nikitin A. G., Videiko M., Patterson N., Renson V., Reich D., Interactions between Trypillian farmers and North Pontic forager-pastoralists in Eneolithic central Ukraine. PLoS One 18, e0285449 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makarewicz C. A., Hofmann R., Videiko M. Y., Müller J., Community negotiation and pasture partitioning at the Trypillia settlement of Maidanetske. Antiquity 96, 831–847 (2022). [Google Scholar]
- 21.Kriszan M., et al. , Revealing N management intensity on grassland farms based on natural δ15N abundance. Agric. Ecosyst. Environ. 184, 158–167 (2014). [Google Scholar]
- 22.Schwertl M., Auerswald K., Schäufele R., Schnyder H., Carbon and nitrogen stable isotope composition of cattle hair: Ecological fingerprints of production systems? Agric. Ecosyst. Environ. 109, 153–165 (2005). [Google Scholar]
- 23.Fischer E., et al. , “Landnutzung im Umkreis der Zentralorte Hohenasperg, Heuneburg und Ipf. Archäobotanische und archäozoologische Untersuchungen und Modellberechnungen zum Ertragspotential von Ackerbau und Viehhaltung” in "Fürstensitze" und Zentralorte der frühen Kelten: Abschlusskolloquium des DFG-Schwerpunktprogramms 1171 in Stuttgart, 12–15 October 2009, Krausse D., Beilharz D., Eds. (Theiss, 2010), pp. 195–265. [Google Scholar]
- 24.Dreibrodt S., et al. , Earthworms, Darwin and prehistoric agriculture-Chernozem genesis reconsidered. Geoderma 409, 115607 (2022). [Google Scholar]
- 25.Ohlrau R., Dal Corso M., Kirleis W., Müller J., “Living on the edge? Carrying capacities of Trypillian settlements in the Buh-Dnipro interfluve” in Trypillia Mega-Sites and European Prehistory 4100–3400 BCE, Müller J., Rassmann K., Videiko M., Eds. (Routledge, 2016), pp. 207–220. [Google Scholar]
- 26.Dal Corso M., et al. , Modelling landscape transformation at the Chalcolithic Tripolye mega-site of Maidanetske (Ukraine): Wood demand and availability. Holocene 29, 1622–1636 (2019). [Google Scholar]
- 27.Kirleis W., et al. , A complex subsistence regime revealed for Cucuteni-Trypillia sites in Chalcolithic eastern Europe based on new and old macrobotanical data. Veget Hist Archaeobot. (2023), 10.1007/s00334-023-00936-y. Accessed 19 July 2023. [DOI] [Google Scholar]
- 28.Bickle P., Stable isotopes and dynamic diets: The Mesolithic-Neolithic dietary transition in terrestrial central Europe. J. Archaeol. Sci. Rep. 22, 444–451 (2018). [Google Scholar]
- 29.Fernandes R., A simple(R) model to predict the source of dietary carbon in individual consumers. Archaeometry 58, 500–512 (2016). [Google Scholar]
- 30.Nitsch E., et al. , A bottom-up view of food surplus: Using stable carbon and nitrogen isotope analysis to investigate agricultural strategies and diet at Bronze Age Archontiko and Thessaloniki Toumba, northern Greece. World Archaeol. 49, 105–137 (2016). [Google Scholar]
- 31.Fraser R. A., et al. , Manuring and stable nitrogen isotope ratios in cereals and pulses: Towards a new archaeobotanical approach to the inference of land use and dietary practices. J. Archaeol. Sci. 38, 2790–2804 (2011). [Google Scholar]
- 32.Treasure E. R., Church M. J., Gröcke D. R., The influence of manuring on stable isotopes (δ13C and δ15N) in Celtic bean (Vicia faba L.): Archaeobotanical and palaeodietary implications. Archaeol. Anthropol. Sci. 8, 555–562 (2016). [Google Scholar]
- 33.Glaser B., Prehistorically modified soils of central Amazonia: A model for sustainable agriculture in the twenty-first century. Philos. Transact. R. Soc. Lond. B Biol. Sci. 362, 187–196 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogaard A., et al. , Crop manuring and intensive land management by Europe’s first farmers. Proc. Natl. Acad. Sci. U.S.A. 110, 12589–12594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sykut M., et al. , Environmental factors shaping stable isotope signatures of modern red deer (Cervus elaphus) inhabiting various habitats. PLoS One 16, e0255398 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheng W., et al. , Regional patterns of 15N natural abundance in forest ecosystems along a large transect in eastern China. Sci. Rep. 4, 4249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreibrodt S., et al. , Holocene soil erosion in Eastern Europe-land use and/or climate controlled? The example of a catchment at the Giant Chalcolithic settlement at Maidanetske, central Ukraine. Geomorphology 367, 107302 (2020). [Google Scholar]
- 38.Blanz M., et al. , Seaweed fertilisation impacts the chemical and isotopic composition of barley: Implications for analyses of archaeological skeletal remains. J. Archaeol. Sci. 104, 34–44 (2019). [Google Scholar]
- 39.Bedard-Haughn A., van Groenigen J. W., van Kessel C., Tracing 15N through landscapes: Potential uses and precautions. J. Hydrol. 272, 175–190 (2003). [Google Scholar]
- 40.Post D. M., Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83, 703–718 (2002). [Google Scholar]
- 41.Kolbe G., Stumpe H., Neunzig Jahre “Ewiger Roggenbau”. Arch. Agron. Soil Sci. 13, 933–949 (1969). [Google Scholar]
- 42.Ferrio J. P., Araus J. L., Buxó R., Voltas J., Bort J., Water management practices and climate in ancient agriculture: Inferences from the stable isotope composition of archaeobotanical remains. Veget. Hist. Archaeobot. 14, 510–517 (2005). [Google Scholar]
- 43.Kanstrup M., Thomsen I. K., Andersen A. J., Bogaard A., Christensen B. T., Abundance of 13C and 15N in emmer, spelt and naked barley grown on differently manured soils: Towards a method for identifying past manuring practice. Rapid Commun. Mass Spectrom. 25, 2879–2887 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Schlipf J. A., Populäres Handbuch der Landwirthschaft: in besonderer Beziehung für den würdigen Bauernstand im Großherzogthum Baden, Hessen, Königreich Würtemberg und deren benachbarten Ländern nach dem gegenwärtigen Standpunkte der Fortschritte im Acker-, Wiesen- und Weinbau, in der Obstbaumzucht, der Rindvieh-, Pferde-, Schweine- und Bienenzucht (Joh. Conr, Mäcken, 1841). [Google Scholar]
- 45.Wallace M., et al. , Stable carbon isotope analysis as a direct means of inferring crop water status and water management practices. World Archaeol. 45, 388–409 (2013). [Google Scholar]
- 46.Gillis R. E., et al. , Stable isotopic insights into crop cultivation, animal husbandry, and land use at the Linearbandkeramik site of Vráble-Veľké Lehemby (Slovakia). Archaeol. Anthropol. Sci. 12, 1–15 (2020). [Google Scholar]
- 47.Bogaard A., Heaton T. H. E., Poulton P., Merbach I., The impact of manuring on nitrogen isotope ratios in cereals: Archaeological implications for reconstruction of diet and crop management practices. J. Archaeol. Sci. 34, 335–343 (2007). [Google Scholar]
- 48.Zeven A. C., Landraces: A review of definitions and classifications. Euphytica 104, 127–139 (1998). [Google Scholar]
- 49.Pampana S., Masoni A., Mariotti M., Ercoli L., Arduini I., Nitrogen fixation of grain legumes differs in response to nitrogen fertilisation. Ex. Agric. 54, 66–82 (2018). [Google Scholar]
- 50.Krafft G., Die Pflanzenbaulehre (Parey, 1918). [Google Scholar]
- 51.Krünitz J. G., “1785 En -Ez” in Oekonomische Encyklopädie, oder allgemeines System der Staats- Stadt- Haus- und Landwirthschaft, Krünitz J. G., Ed. (Joachim Pauli, 1785), pp. 1–897. [Google Scholar]
- 52.Fraas C., Die Schule des Landbaues ( Literarisch-artistische Anstalt, München, 1852).
- 53.Ryder M. L., Sheep & Man (Duckworth, 2007). [Google Scholar]
- 54.Fraser R. A., Bogaard A., Schäfer M., Arbogast R., Heaton T. H. E., Integrating botanical, faunal and human stable carbon and nitrogen isotope values to reconstruct land use and palaeodiet at LBK Vaihingen an der Enz, Baden-Württemberg. World Archaeol. 45, 492–517 (2013). [Google Scholar]
- 55.Styring A. K., et al. , Centralisation and long-term change in farming regimes: Comparing agricultural practices in Neolithic and Iron Age south-west Germany. Proc. Prehist. Soc. 83, 357–381 (2017). [Google Scholar]
- 56.Vaiglova P., et al. , Further insight into Neolithic agricultural management at Kouphovouno, southern Greece: Expanding the isotopic approach. Archaeol. Anthropol. Sci. 12, 1–17 (2020). [Google Scholar]
- 57.Baum T., Nendel C., Jacomet S., Colobran M., Ebersbach R., “Slash and burn” or “weed and manure”? A modelling approach to explore hypotheses of late Neolithic crop cultivation in pre-alpine wetland sites: A modelling approach to explore hypotheses of late Neolithic crop cultivation in pre-alpine wetland sites. Veget. Hist. Archaeobot. 25, 611–627 (2016). [Google Scholar]
- 58.Matthews D. E., Review of lysine metabolism with a focus on humans. J. Nutr. 150, 2548S–2555S (2020). [DOI] [PubMed] [Google Scholar]
- 59.Tomé D., Bos C., Lysine requirement through the human life cycle. J. Nutr. 137, 1642S–1645S (2007). [DOI] [PubMed] [Google Scholar]
- 60.Erbersdobler H. F., Barth C. A., Jahreis G., Legumes in human nutrition: Nutrient content and protein quality of pulses. Ernaehrungs Umschau Int. 64, 134–144 (2017). [Google Scholar]
- 61.Leinonen I., et al. , Lysine supply is a critical factor in achieving sustainable global protein economy. Front. Sustain. Food Syst. 3, 27 (2019). [Google Scholar]
- 62.Layman D. K., Assessing the role of cattle in sustainable food systems. Nutr. Today 53, 160–165 (2018). [Google Scholar]
- 63.Pimentel D., Pimentel M., Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 78, 660S–663S (2003). [DOI] [PubMed] [Google Scholar]
- 64.La Poutré H. J. P., The contribution of legumes to the diet of English peasants and farm servants, c.1300. Agri. Hist. Rev. 63, 19–38 (2015). [Google Scholar]
- 65.Styring A. K., et al. , Urban form and scale shaped the agroecology of early “cities” in northern Mesopotamia, the Aegean and Central Europe. J. Agrar. Change 22, 831–854 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mnich B., et al. , Terrestrial diet in prehistoric human groups from southern Poland based on human, faunal and botanical stable isotope evidence. J. Archaeol. Sci. Rep. 32, 102382 (2020). [Google Scholar]
- 67.Sebald S. V., Papathanasiou A., Grupe G., Changing subsistence economies in the course of the Neolithic transition: Isotopic sourcing of collagen isotopic ratios in human skeletons from early Neolithic Anatolia and Greece. J. Archaeol. Sci. Rep. 43, 103450 (2022). [Google Scholar]
- 68.Denaire A., et al. , The Cultural Project: Formal chronological modelling of the Early and Middle Neolithic sequence in Lower Alsace. J. Archaeol. Method Theor. 24, 1072–1149 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaupová S. D., et al. , The diet of settled Neolithic farmers of east-central Europe: Isotopic and dental microwear evidence. Archaeol. Anthropol. Sci. 15, 1–29 (2023). [Google Scholar]
- 70.Szostek K., Grygiel R., Bogucki P., The diet and social paleostratigraphy of Neolithic agricultural population of the Lengyel culture from Osłonki (Poland). Anthropol. Rev. 68, 29–41 (2005). [Google Scholar]
- 71.Harper T. K., Sustaining Talianki: A model of Eneolithic subsistence economics at a giant-settlement of the Western Tripolye Culture, Ukraine (Unpublished M.A. thesis, Department of Anthropology, State University of New York at Buffalo, 2012).
- 72.Gaydarska B., Millard A., Buchanan B., Chapman J., Place and time at Trypillia mega-sites: Towards a new synthesis of analyses and social theory. J. Urban Archaeol. 7, 115–145 (2023). [Google Scholar]
- 73.Münster A., et al. , 4000 years of human dietary evolution in central Germany, from the first farmers to the first elites. PLoS One 13, e0194862 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bogaard A., Fochesato M., Bowles S., The farming-inequality nexus: New insights from ancient Western Eurasia. Antiquity 93, 1129–1143 (2019). [Google Scholar]
- 75.Hofmann R., et al. , Tripolye–Strategy and results of an ongoing Ukrainian-European project. Vita Antiqua 10, 146–154 (2018). [Google Scholar]
- 76.Müller J., Videjko M. J., Eds., Maidanetske 2013: New excavations at a Trypillia mega-site = Majdanec'ke 2013: novi rozkopky velykoho Trypil's'koho poselennja (Dr. Rudolf Habelt GmbH, 2017). [Google Scholar]
- 77.Chapman J. C., et al. , GEOINFORM Ukrainii, Trypillia mega-sites of the Ukraine (2018). 10.5284/1047599. Accessed 18 September 2023. [DOI]
- 78.van Klinken G. J., Bone Collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 26, 687–695 (1999). [Google Scholar]
- 79.Bailey D. W., “On the absence of burial ritual in Cucuteni-Tripolie communities” in Scripta Praehistorica. Miscellanea in honorem nonagenarii magistri Mircea Petrescu-Dîmboviţa oblata, Spinei V., Lazarovici C.-M., Monah D., Eds. (Trinitas, 2005), pp. 319–340. [Google Scholar]
- 80.Kruts V. A., et al. , Issledovanie poselenij-gigantov tripol’skoj kul’tury v 2002-2004 gg (Institute of Archaeology, Kiev, 2005).
- 81.Ţerna S., et al. , Stolniceni–Excavation results from the 2017 campaign. J. Neolithic Archaeol. 21, 209–282 (2019). [Google Scholar]
- 82.Pearson J., Grove M., Counting sheep: Sample size and statistical inference in stable isotope analysis and palaeodietary reconstruction. World Archaeol. 45, 373–387 (2013). [Google Scholar]
- 83.Syväranta J., Lensu A., Marjomaki T. J., An empirical evaluation of the utility of convex hull and standard ellipse areas for assessing population niche widths from stable isotope data. PLoS One 8, e56094 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaiglova P., Lazar N. A., Stroud E. A., Loftus E., Makarewicz C. A., Best practices for selecting samples, analyzing data, and publishing results in isotope archaeology. Quat. Int. 650, 86–100 (2023). [Google Scholar]
- 85.Hammer Ø., Harper D. A. T., Ryan P. D., PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4, 1–9 (2001). [Google Scholar]
- 86.Vaiglova P., Snoeck C., Nitsch E., Bogaard A., Lee-Thorp J., Impact of contamination and pre-treatment on stable carbon and nitrogen isotopic composition of charred plant remains. Rapid Commun. Mass Spectrom. 28, 2497–2510 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernandes R., Nadeau M.-J., Grootes P. M., Macronutrient-based model for dietary carbon routing in bone collagen and bioapatite. Archaeol. Anthropol. Sci. 4, 291–301 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Data Availability Statement
All study data are included in the article and/or supporting information.