Abstract
The alternative lengthening of telomeres (ALT) phenotype is characterized by ultra-bright telomeres on fluorescence in situ hybridization (FISH) and is a marker of a unique mechanism of telomere maintenance in tumors. ALT does not occur in normal tissues. ALT has been described in hepatocellular carcinoma (5–10%) and in primary hepatic angiosarcomas (75%). To study the frequency of ALT in other primary hepatic tumors, a wide range of primary hepatic neoplasms were retrieved. The tumors included the following: intrahepatic and hilar cholangiocarcinomas (N=110), hepatic adenomas (N=35), hepatocellular carcinomas (N=30), fibrolamellar carcinomas (n=11), combined cholangiocarcinoma-hepatocellular carcinomas (N=8), carcinosarcoma (N=10), hepatoblastomas (N=5), hemangiomas (N=4), angiosarcomas (N=8), epithelioid hemangioendotheliomas (N=10), calcified nested stromal epithelial tumor (N=2), embryonal sarcoma (N=2), rhabdoid tumor (N=1), bile duct adenoma (N=1), and angiomyolipoma (N=1). For epithelial tumors, ALT-FISH was positive in one carcinosarcoma (10% of cases,), one cholangiocarcinoma (1% of cases), and one combined hepatocellular carcinoma-cholangiocarcinoma (13% of cases). In the hepatocellular carcinoma component of both the carcinosarcoma and the combined hepatocellular carcinoma-cholangiocarcinoma, the tumor cells showed patchy marked nuclear pleomorphism akin to that described previously for chromophobe hepatocellular carcinoma, which are typically ALT FISH positive. The ALT-positive cholangiocarcinoma also showed patchy, striking nuclear pleomorphism. For soft tissue tumors, ALT was positive in two angiosarcomas (N=2; 25% of cases). In summary, this study shows that ALT-FISH is positive in rare carcinosarcomas, cholangiocarcinomas, and combined cholangiocarcinoma-hepatocellular carcinoma. ALT is not a significant mechanism of telomere maintenance in hepatocellular adenomas or fibrolamellar carcinomas and was negative in all other tested primary hepatic neoplasms. ALT-FISH is also positive in a subset of primary hepatic angiosarcomas.
Keywords: Hepatocellular carcinoma, fibrolamellar carcinoma, fibrolamellar hepatocellular carcinoma, angiosarcoma, hemangioendothelioma, cholangiocarcinoma, hepatoblastoma, alternative lenthening of telomeres, ALT, combined hepatocellular carcinoma-cholangiocarcinoma, carcinosarcoma
1. Introduction
Malignant cells need to maintain their telomere length in order to proliferate. There are two primary mechanisms for maintaining telomere lengths in malignant cells: (1) reactivation of the telomerase gene TERT through promoter mutations or other rearrangements of the TERT gene and (2) alternative lengthening of telomeres (ALT). A third, rare, proposed mechanism is called “ever-shorter telomeres” and was identified in neuroblastomas, where it is characterized by ultra-short telomeres without TERT mutations or ALT, with the molecular mechanism currently unknown [1]. Overall, telomerase activation is found in about 80% of all tumors and ALT is found in 10–20% of all human malignancies [2]. ALT can be detected by a variety of molecular methods, but the most commonly used technique is fluorescence in situ hybridization (FISH). ALT FISH, also called Telo-FISH, detects ultra-bright telomeres and is a robust marker of ALT [2]. ALT does not occur in normal tissues [3].
The frequency of ALT is highest in sarcomas such as osteosarcoma, chondrosarcoma, and leiomyosarcoma, where the frequency exceeds 50% [2]. In comparison, epithelial tumors have a lower frequency of ALT positivity, with the highest frequency found in neuroendocrine tumors (NET) of the pancreas (about 30%) [4]. A survey study of 6110 primary tumors found ALT positivity in 8/121 hepatocellular carcinomas (7%) and no ALT positive cases in 23 extrahepatic cholangiocarcinomas and 10 intrahepatic cholangiocarcinomas [3]. Subsequent studies found an ALT frequency of between 6–10% in hepatocellular carcinomas [5, 6]. ALT has also been reported in 8 of 12 primary angiosarcomas of the liver [7]. Despite these advances, the frequency of ALT positivity in primary tumors of the liver remains limited. The goal of this study was to perform a survey of primary liver neoplasms, extending ALT-FISH testing to a wider range of primary hepatic tumors.
ALT has been associated with a number of different mutations, including DAXX and ATRX mutations [8]. Loss of nuclear expression of ATRX and DAXX by immunohistochemistry has been shown to correlate with ALT in pancreatic neuroendocrine tumors [9], while loss of ATRX correlates with ALT in a subset of primary hepatic angiosarcomas [7]. For these reasons, ALT positive cases were also evaluated by ATRX immunostains.
2. Materials and Methods.
With IRB approval, cases were obtained from the pathology archives and consult service. H&E and relevant immunostains were reviewed to confirm the diagnosis.
2.1. Tissues studied
The first set of cholangiocarcinomas consisted of 37 intrahepatic or hilar cholangiocarcinomas; no extrahepatic cholangiocarcinomas were examined. Fifteen of the cholangiocarcinomas were from explanted livers, the remainder were resection specimens. Thirteen cases (35%) were classified as large duct cholangiocarcinomas based on morphology, the remainder as small duct cholangiocarcinomas (65%). After an ALT positive cholangiocarcinoma was identified, an additional TMA of 67 cholangiocarcinomas were studied. The TMA consists of two cores of tumor and non-tumor per patient, with 1 mm cores. On the stained TMA, 3 cases of tumor were entirely missing and 1 failed to hybridize on both cores. Four cases had a single core that was present and hybridized. Thus, the final number of analyzable cases on the TMA was 63. All cases were intrahepatic cholangiocarcinomas, with 38 (57%) having a small duct growth pattern based on morphology.
The frequency of ALT positivity in hepatocellular carcinomas is between 6–10% and has been linked to a distinctive morphology [5, 6]; this morphology consists of (1) tumor cells with moderately abundant pale to eosinophilic cytoplasm, (2) a background of low-to-moderate nuclear cytology, but admixed with scattered foci of striking nuclear atypia, and (3) often the presence of small pseudocysts. For the purposes of this study, cases were selected to extend the understanding of ALT frequency in hepatocellular carcinomas that lack this morphology, so all 30 hepatocellular carcinomas included in the study lacked the morphological findings that have been linked to ALT positive hepatocellular carcinomas. Twenty-eight cases were resection specimens and two were biopsy specimens. Tumors were graded according to the current WHO grading method: well differentiated (N=15), moderately differentiated (N=9), and poorly differentiated, (N=6). The morphological subtypes within the hepatocellular carcinoma were classified as follows [10]: not otherwise specified (N= 12), beta-catenin type morphology (N=5) [11], clear cell (N=4), scirrhous (N=4), steatohepatitic (N=3), lymphocyte rich (N=1) and myxoid hepatic adenoma [12] that had transformed to hepatocellular carcinoma (N=1). All fibrolamellar carcinomas had the classic morphology and were FISH positive for the chromosomal 19 deletion that is typical of fibrolamellar carcinoma [13, 14]. The combined hepatocellular carcinoma-cholangiocarcinomas (N=8) and carcinosarcomas (N=10) were all diagnosed based on standard morphology and immunostain methods [15, 14]; the carcinoma component of the carcinosarcomas was hepatocellular carcinoma in each case.
The 35 hepatic adenomas were diagnosed in the usual fashion using morphology supplemented with stains for reticulin, Ki-67 [16], and glypican 3 stain [17]. After a diagnosis of hepatic adenoma was made, the adenomas were subtyped using immunostains for LFABP, CRP, SAA, beta-catenin, and glutamine synthetase. Beta-catenin activation was defined as any nuclear positivity on beta catenin stain, or diffuse glutamine synthetase staining (>50%) with either a diffuse or mosaic staining pattern. If these stains had not been performed for clinical care, they were performed when tissue was available.
All 10 epithelioid hemangioendotheliomas were primary to the liver. All 8 angiosarcomas were primary to the liver [18]. All other tumors examined showed typical morphological features.
2.2. ALT FISH and ATRX immunostains
Five micron thick sections were cut for ALT-FISH. Unstained slides were pretreated following a reduced pepsin FISH protocol. The Telomere PNA FISH Kit/Cy3 (Dako, Denmark) probe was applied, hybridized and washed according to the Dako protocol. This is a qualitative assay ultra-bright, large FISH signals were scored as positive.
ATRX immunostains were performed on selected cases on 5 micron sections using the following antibody: ATRX (D-5): sc-55584, Santa Cruz.
3. Results
ALT-FISH was performed successfully on a total of 230 of 237 cases (97%); one hepatocellular carcinoma, one cholangiocarcinoma, 4 fibrolamellar carcinomas, and one angiosarcoma failed to hybridize and were excluded from the study. Benign hepatocellular lesions were consistently ALT-FISH negative (Table 1). All of the 35 hepatic adenomas were ALT-FISH negative, including 15 HNF1A inactivated hepatic adenomas, 13 inflammatory adenomas, 3 unclassified adenomas, and 4 adenomas where subtype information was not available. Beta-catenin activation was seen in 2 of the inflammatory adenomas and 2 of the unclassified adenomas. Of the 4 hemangiomas, 3 were cavernous hemangiomas and 1 was an anastomosing hemangioma; all were negative for ALT FISH.
Table 1.
ALT Telo-FISH results
| Tumor | Number studied | Number positive (percent) |
|---|---|---|
| Hepatic adenoma | 35 | 0 |
| Hepatocellular carcinoma | 30 | 0 |
| Fibrolamellar carcinoma | 11 | 0 |
| Combined hepatocellular carcinoma-cholangiocarcinoma | 8 | 1 (13%) |
| Carcinosarcoma | 10 | 1 (10%) |
| Cholangiocarcinoma, resection | 37 | 1 (3%) |
| Cholangiocarcinoma, TMA | 63 | 0 |
| hepatoblastoma | 5 | 0 |
| Calcified nested stromal epithelial tumor | 2 | 0 |
| Rhabdoid tumor | 1 | 0 |
| Hemangioma | 4 | 0 |
| Angiosarcoma | 8 | 2 (20%) |
| Epithelioid hemangioendothelioma | 10 | 0 |
| Embryonal sarcoma | 2 | 0 |
| Angiomyolipoma / FNH / VMC / Bile duct adenoma | 1 / 1/ 1 /1 | 0 / 0 / 0 /0 |
| Total Total benign Total malignant |
230 43 187 |
5 (2%) 0 5 (3%) |
The first set of cholangiocarcinomas were full resection specimens; 1 of 37 cholangiocarcinomas (3%) was ALT positive (Figure 1). The tumor had been previously treated with chemoradiation and the patient underwent liver transplantation, showing an ill-defined 2.5 cm hilar mass with 50% viable residual cholangiocarcinoma. The cholangiocarcinoma showed a large duct morphology with more striking nuclear pleomorphism than usually present in cholangiocarcinomas (Table 2). There was no angiolymphatic or perineural invasion and hilar lymph nodes were negative for carcinoma. ATRX immunohistochemistry showed retained staining (Table 2). There was no known underlying liver disease and the background liver showed mild nonspecific portal inflammation with Batts-Ludwig stage 2 fibrosis. Twenty months later, recurrent disease was detected in the abdominal wall. The abdominal wall recurrence was not available for FISH testing. Follow-up ALT-FISH on a cholangiocarcinoma TMA containing 63 intrahepatic cholangiocarcinomas showed was negative in all cases.
Figure 1. ALT positive cholangiocarcinoma.
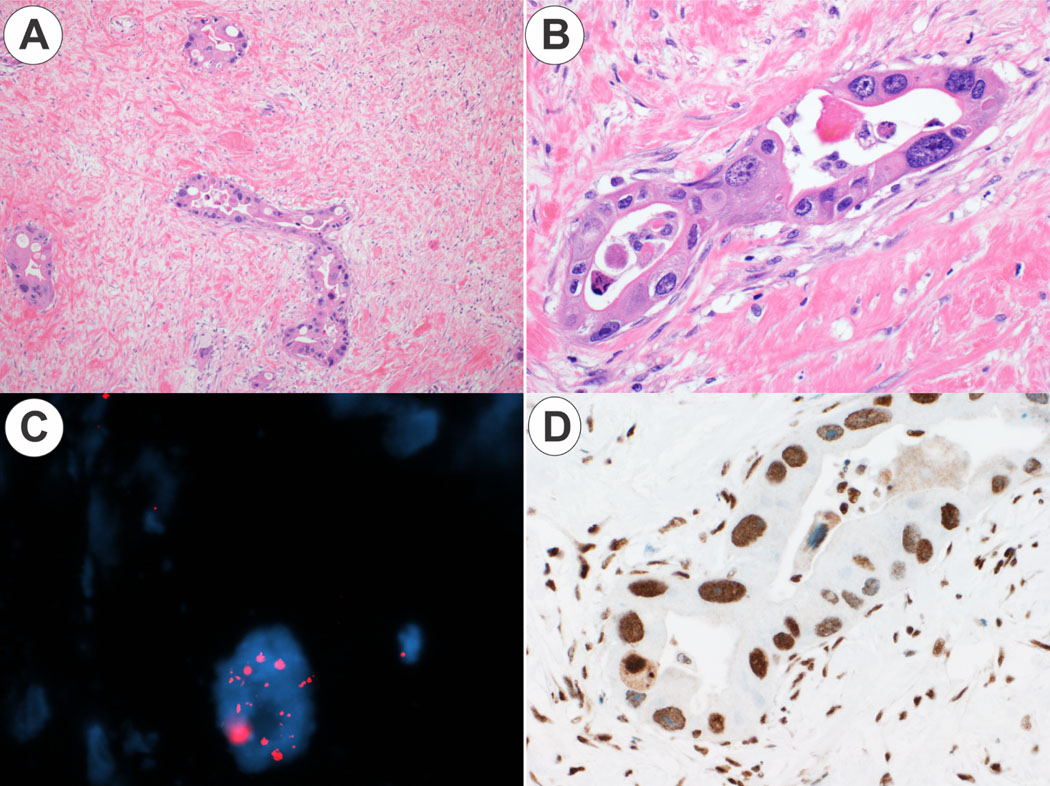
Panel 1A. At low power, malignant glands are seen in dense fibrosis (original magnification 5X). Panel 1B. At higher power, there is heterogenous but striking nuclear atypia (original magnification 20X). Panel 1C. The malignant glands are ALT positive, showing scattered, large ultra-bright foci on telomere FISH. Panel 1D. An immunostain for ATRX shows strong nuclear staining (original magnification 20X).
Table 2.
ALT positive cases
| Tumor type | ALT-FISH | ATRX immunostain | Key Morphology findings |
|---|---|---|---|
| Cholangiocarcinoma | Positive | Retained nuclear staining | Large duct pattern with striking nuclear anaplasia |
| Combined hepatocellular and cholangiocarcinoma | Positive in hepatocellular component and in cholangiocarcinoma component | Not tested | Hepatocellular carcinoma: moderately abundant eosinophilic to focally clear cytoplasm; patchy striking nuclear atypia Cholangiocarcinoma: small duct pattern; nuclear cytology homogenous without striking nuclear atypia |
| Carcinosarcoma | Positive in hepatocellular carcinoma component Equivocal positive in sarcoma component |
Retained nuclear staining | Hepatocellular carcinoma: moderately abundant eosinophilic to focally clear cytoplasm; patchy striking nuclear atypia Sarcoma: Undifferentiated sarcoma |
| Angiosarcoma Case 1 | Positive | Retained nuclear staining | Epithelioid, multinucleated tumor giant cells |
| Angiosarcoma Case 2 | Positive | Retained nuclear staining | Vasoformative |
One of 8 cases of combined hepatocellular carcinoma-cholangiocarcinoma (13%) was ALT-FISH positive. The tumor was a single 2 cm mass in the liver of a 57-year-old woman with a history of chronic hepatitis B who was HBsAg positive and HBeAg negative. The liver also showed mild macrovesicular steatosis, but there was no fibrosis. No follow-up was available. Sections of the tumor showed a moderately differentiated hepatocellular carcinoma with a solid and macrotrabecular growth pattern, patchy chromophobe cytoplasm and focal areas of striking nuclear atypia (Figure 2). The cholangiocarcinoma component showed a small duct morphology but did not have the patchy, distinctive nuclear anaplasia that was seen in the hepatocellular carcinoma component. ALT-FISH was positive in both components.
Figure 2. ALT positive combined hepatocellular carcinoma-cholangiocarcinoma.
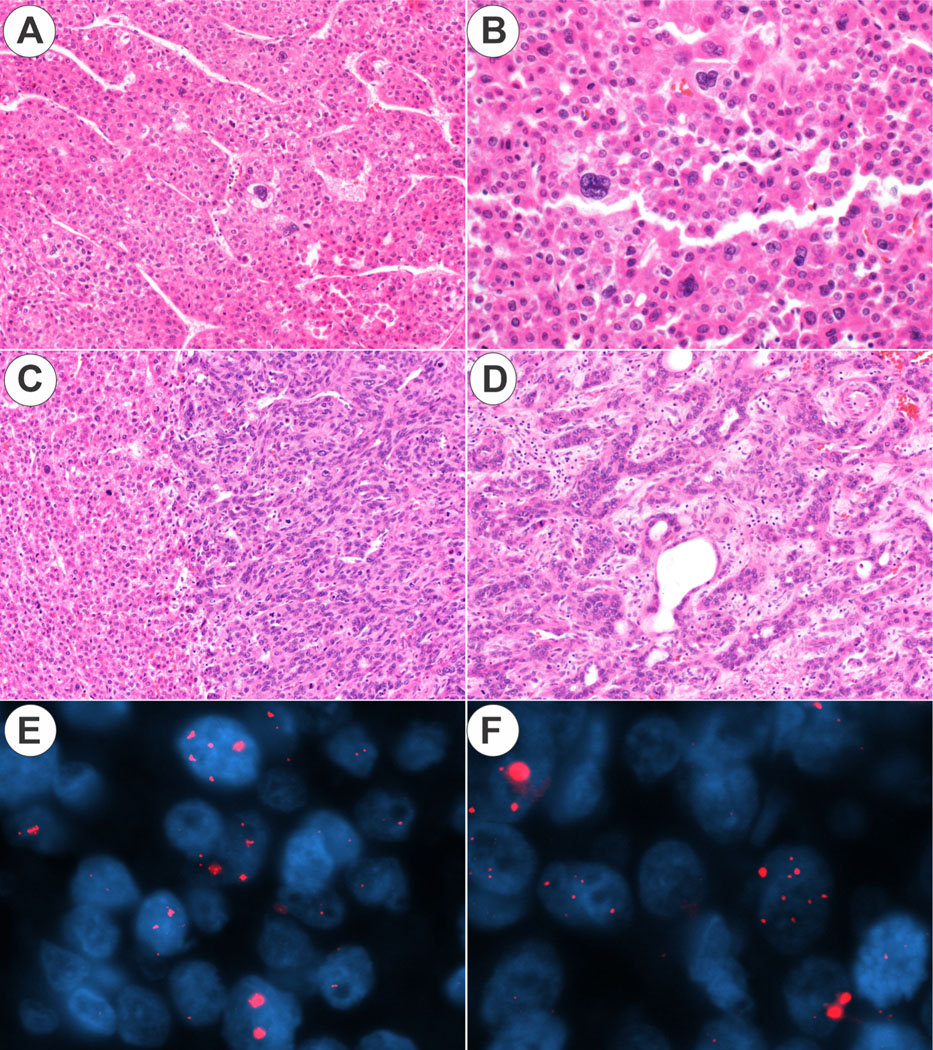
Panel 2A. At low power, the tumor has a macrotrabecular growth pattern (original magnification 10X). Patchy, striking anaplasia is evident. Panel 2B. The hepatocellular carcinoma component shows patchy nuclear atypia (original magnification 20X). Figure 2C. The interface is shown between the hepatocellular carcinoma component (left side of image) and cholangiocarcinoma component (right side of image) (original magnification 10X). Panel 2D. The cholangiocarcinoma component is shown (original magnification 10X). Panel 3E. The hepatocellular carcinoma component is ALT positive. Panel 3F. The cholangiocarcinoma component is ALT positive.
One of 10 cases of carcinosarcoma was ALT-FISH positive (10%). The tumor was 12 cm and occurred in a non-cirrhotic liver in a 61-year-old man. No underlying disease was evident. Sections of the primary tumor showed a moderately-differentiated hepatocellular carcinoma. The cytoplasm was >90% eosinophilic but there were focal areas of chromophobe type cytoplasm. No pseudocysts were seen. Patchy, striking nuclear atypia was prominent. The sarcomatous component was negative for keratins (pankeratin, CAM5.2) and for hepatocellular markers. The sarcoma component did not show morphological or immunostain evidence of a specific type of sarcoma. ALT was positive in the hepatocellular carcinoma component and showed equivocal positivity in the sarcoma component (Figure 3). ATRX was retained in both components. Metastatic disease to the lungs and pleura was discovered 11 months after partial hepatectomy. The metastatic disease had a sarcomatoid morphology.
Figure 3. ALT positive carcinosarcoma.
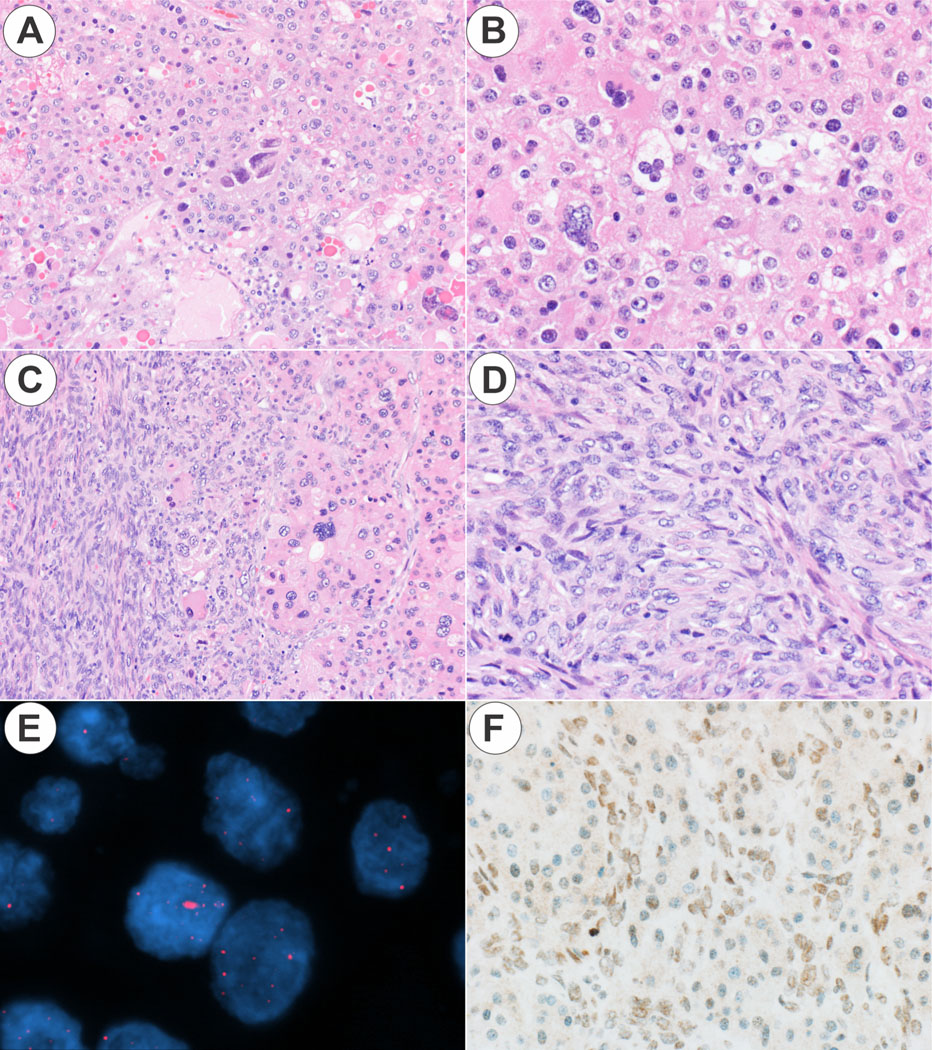
Panel 3A. The hepatocellular carcinoma component shows pale to eosinophilic cytoplasm, a small pseudocyst, and patchy nuclear anaplasia (original magnification 10X). Panel 3B. The hepatocellular carcinoma component is shown at higher magnification (original magnification 20X). Panel 3C. The interface between the sarcoma and the hepatocellular carcinoma component is shown (original magnification 10X). Panel 3D. The sarcoma component is undifferentiated (original magnification 20X). Panel 3E. The hepatocellular carcinoma component is ALT positive, while the sarcoma showed equivocal ALT staining; the hepatocellular carcinoma component is shown. Panel 3F. ATRX protein expression was retained in both components. The hepatocellular carcinoma component is shown (original magnification 20X).
Two of 8 primary hepatic angiosarcomas were ALT-FISH positive (25%) (Figure 4), both were in men with an average age of 81 years at diagnosis. One of the angiosarcomas showed an epithelioid growth pattern with scattered bizarre multinucleated giant cells (Figure 4), while the other showed a vasoformative growth pattern. Sequencing (Tempus xT) was performed on the vasoformative case and showed mutations in TP53 and ATRX (c.5566+2T>G, affection splicing, predicted to lead to loss of function), but there was retained expression of ATRX protein expression (Table 2).
Figure 4. ALT positive angiosarcoma.
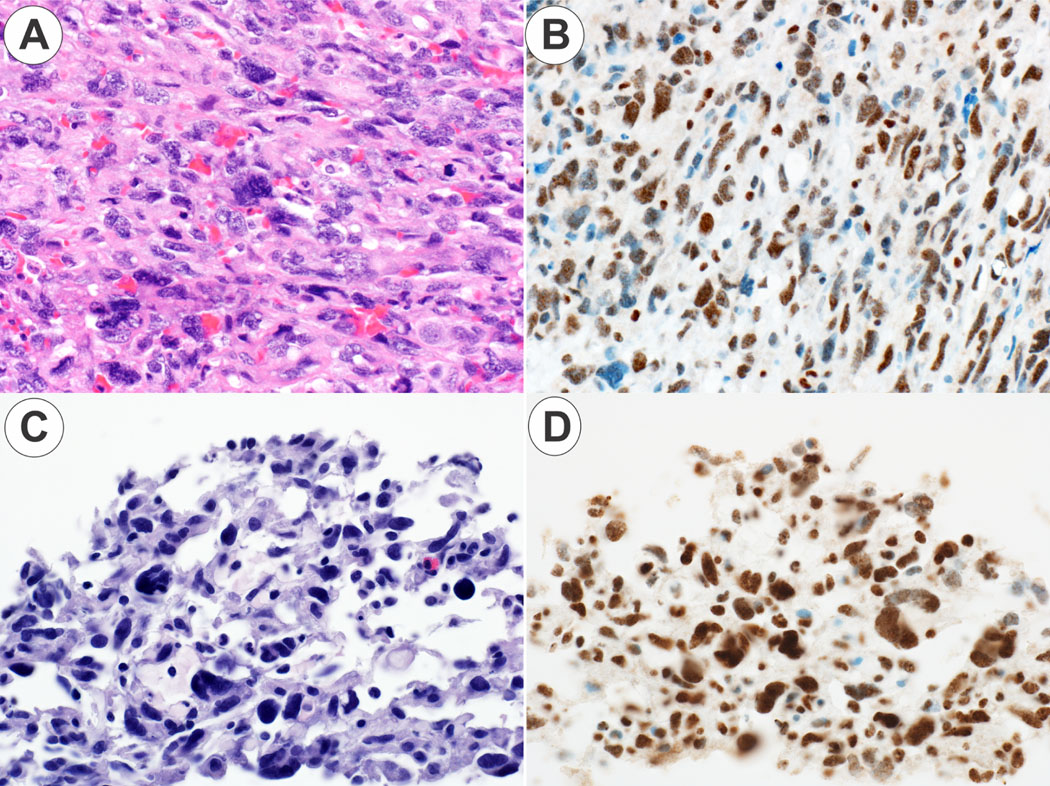
Panel 4A. The angiosarcoma case 1 shows a solid, epithelioid growth pattern (original magnification 20X). Panel 4B. The angiosarcoma case 1 is ATRX positive (original magnification 20X). Panel 4C. The angiosarcoma case 2 shows a vasoformative growth pattern (original magnification 30X). Panel 4DB. The angiosarcoma case 2 is ATRX positive (original magnification 30X).
4. Discussion
ALT positivity in hepatocellular carcinoma was first reported in a large survey study of tumors throughout the body, identifying a frequency of 7% [3]. A subsequent study on morphological findings in hepatocellular carcinoma identified a subset of hepatocellular carcinoma with distinctive morphology that included a triad of features: (1) pale to sometimes eosinophilic cytoplasm, (2) patchy areas of striking nuclear atypia, (3) and microscopic pseudocysts. This subtype was called chromophobe hepatocellular carcinoma and the findings were subsequently mapped back to the ALT-FISH results and found to largely identify the same group of tumors [5]. A subsequent study from South Korea confirmed the core findings for chromophobe hepatocellular carcinoma, including both the morphology and ALT-FISH positivity [6].
This survey extends the understanding of ALT positivity in primary liver tumors, identifying cases of ALT positive cholangiocarcinoma, ALT positive combined hepatocellular carcinoma-cholangiocarcinoma, and ALT positive carcinosarcoma composed of hepatocellular carcinoma-undifferentiated sarcoma. From these findings, several observations follow. First, the frequency for combined hepatocellular carcinoma-cholangiocarcinoma and for carcinosarcoma was about 10%, similar to that seen for conventional hepatocellular carcinoma. Second, ALT does not appear to play a significant role in the biology of hepatoblastomas or fibrolamellar carcinomas. Third, all of the ALT positive cases in the liver continue to share the cytological feature of patchy but striking nuclear atypia. The hepatocellular carcinoma component, when present, also showed the pale cytoplasm first described in chromophobe hepatocellular carcinoma. Fourth, rare intrahepatic cholangiocarcinomas are ALT positive.
The clinical significance of ALT positivity in primary liver tumors is still under active investigation. Studies on chromophobe hepatocellular carcinoma have consistently found the frequency in men and women to be similar [5, 6], which is distinct from the male predominance found in conventional hepatocellular carcinomas. Most cases are single tumors [6] and the background liver can be cirrhotic or non-cirrhotic [5, 6]. There have been mixed findings in terms of potential association with underlying liver disease, with the first paper describing an enrichment for chronic HBV [5], an observation that was not confirmed in another study [6]. To date, no prognostic significance has been identified [5, 6]. Strategies are being developed to target the ALT mechanism for cancer treatment [19], but have not reached clinical care at this time.
In terms of the other epithelial tumors, ductal adenocarcinoma of the breast and neuroendocrine tumors of the pancreas have also been shown to be ALT positive. In breast ductal adenocarcinoma, the frequency is about 6% and is associated with HER2 overexpression [20]. ALT positivity is associated with a worse prognosis in both NETs [4, 21] and breast ducal adenocarcinomas [20].
At the molecular level, ALT is strongly associated with ATRX mutations in sarcomas. For example, if an osteosarcoma shows loss of ATRX protein expression, then the tumors are ALT positive nearly 100% of the time [2]. NETs also have frequent ATRX mutations, but also are enriched for DAXX mutations, with about 40% of all ALT positive NETs having DAXX mutations. In hepatocellular carcinomas, other genes must play a role as ATRX mutations were found in only 1 of 23 cases (4%) [6] and DAXX mutations have not been reported [5]. TP53 mutations have been associated with the ALT pathway in gliomas, but were not found in chromophobe hepatocellular carcinomas [6].
One survey study of vascular neoplasms from different organs found ALT to be positive in about 17/70 (24%) of all angiosarcomas, with a striking enrichment for hepatic angiosarcomas, where 8/12 were FISH positive [7]; ALT positivity was also strongly associated with loss of ATRX protein expression. In the current study, all hemangiomas were negative. Of the 8 angiosarcomas tested, two were ALT positive and one with available testing showed an ATRX mutation; but neither case showed loss of ATRX by immunohistochemistry. None of the tested epithelioid hemangioendotheliomas were ALT-FISH positive.
ALT-FISH was negative in all tested hepatoblastomas. Conventional hepatoblastomas also lack TERT promoter mutations [22], suggesting a third mechanism for maintaining telomeres. Likewise, only about 20% of FLC have TERT promoter mutations [23, 24], and none show ALT-FISH positivity.
One of the limits of this study is that we were not able to establish the molecular mechanism for most of the ALT-FISH positive cases. Loss of ATRX or DAXX protein expression by immunostains have been used as surrogates for their respective mutations, but none of the cases in this study showed ATRX loss; DAXX stains were unavailable to us. Of note, the angiosarcoma with an ATRX mutation in this study had a mutation predicted to lead to loss of function, but showed retained nuclear expression by immunostaining. Until the underlying genetic mechanism are better understood, ALT-FISH appears to be the most robust mechanism to identify ALT positive primary liver tumors.
In summary, this ALT survey extends the types of primary epithelial liver tumors that can be ALT positive to include cholangiocarcinomas, combined hepatocellular carcinoma-cholangiocarcinoma, and carcinosarcomas. Fibrolamellar carcinomas and hepatoblastomas are negative for ALT FISH. Of the tested primary hepatic soft tissue tumors, only angiosarcomas were found to be positive.
Highlights.
Alternative lengthening of telomeres (ALT) phenotype can be found in several primary livetumors including hepatocellular carcinoma, combined hepatocellular carcinoma-cholangiocarcinoma, carcinosarcoma, cholangiocarcinoma, and angiosarcoma.
Many ALT positive carcinomas show significant nuclear pleomorphism.
Loss of ATRX by immunostaining was not seen in ALT positive cases
Grant support:
P50 CA210964 (MST, SMS)
Footnotes
Conflicts of interest: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dagg RA, Pickett HA, Neumann AA, Napier CE, Henson JD, Teber ET, et al. Extensive Proliferation of Human Cancer Cells with Ever-Shorter Telomeres. Cell Rep 2017;19:2544–56. [DOI] [PubMed] [Google Scholar]
- 2.MacKenzie D Jr., Watters AK, To JT, Young MW, Muratori J, Wilkoff MH, et al. ALT Positivity in Human Cancers: Prevalence and Clinical Insights. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, Gabrielson E, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol 2011;179:1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhi AD, Liu TC, Roncaioli JL, Cao D, Zeh HJ, Zureikat AH, et al. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clin Cancer Res 2017;23:600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood LD, Heaphy CM, Daniel HD, Naini BV, Lassman CR, Arroyo MR, et al. Chromophobe hepatocellular carcinoma with abrupt anaplasia: a proposal for a new subtype of hepatocellular carcinoma with unique morphological and molecular features. Mod Pathol 2013;26:1586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang HJ, Oh JH, Kim YW, Kim W, An J, Sung CO, et al. Clinicopathological and molecular characterization of chromophobe hepatocellular carcinoma. Liver Int 2021;41:2499–510. [DOI] [PubMed] [Google Scholar]
- 7.Liau JY, Tsai JH, Yang CY, Lee JC, Liang CW, Hsu HH, et al. Alternative lengthening of telomeres phenotype in malignant vascular tumors is highly associated with loss of ATRX expression and is frequently observed in hepatic angiosarcomas. Hum Pathol 2015;46:1360–6. [DOI] [PubMed] [Google Scholar]
- 8.Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science 2011;333:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heaphy CM, Singhi AD. The diagnostic and prognostic utility of incorporating DAXX, ATRX, and alternative lengthening of telomeres to the evaluation of pancreatic neuroendocrine tumors. Hum Pathol 2022;129:11–20. [DOI] [PubMed] [Google Scholar]
- 10.Torbenson MS. Morphologic Subtypes of Hepatocellular Carcinoma. Gastroenterol Clin North Am 2017;46:365–91. [DOI] [PubMed] [Google Scholar]
- 11.Torbenson M, McCabe CE, O’Brien DR, Yin J, Bainter T, Tran NH, et al. Morphological heterogeneity in beta-catenin-mutated hepatocellular carcinomas: implications for tumor molecular classification. Hum Pathol 2022;119:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowan DJ, Yasir S, Chen ZE, Mounajjed T, Erdogan Damgard S, Cummins L, et al. Morphologic and Molecular Findings in Myxoid Hepatic Adenomas. Am J Surg Pathol 2021;45:1098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham RP, Yeh MM, Lam-Himlin D, Roberts LR, Terracciano L, Cruise MW, et al. Molecular testing for the clinical diagnosis of fibrolamellar carcinoma. Mod Pathol 2018;31:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torbenson MS. Hepatocellular Carcinoma: Making Sense of Morphological Heterogeneity, Growth Patterns, and Subtypes. Hum Pathol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Digestive Systems Tumors. 5th ed. International Agency fo Research on Cancer: Lyon, France, 2019. [Google Scholar]
- 16.Jones A, Kroneman TN, Blahnik AJ, Graham RP, Mounajjed T, Torbenson MS, et al. Ki-67 “hot spot” digital analysis is useful in the distinction of hepatic adenomas and well-differentiated hepatocellular carcinomas. Virchows Arch 2020. [DOI] [PubMed] [Google Scholar]
- 17.Torbenson M. Hepatic Adenomas: Classification, Controversies, and Consensus. Surg Pathol Clin 2018;11:351–66. [DOI] [PubMed] [Google Scholar]
- 18.Yasir S, Torbenson MS. Angiosarcoma of the Liver: Clinicopathologic Features and Morphologic Patterns. Am J Surg Pathol 2019;43:581–90. [DOI] [PubMed] [Google Scholar]
- 19.O’Rourke JJ, Bythell-Douglas R, Dunn EA, Deans AJ. ALT control, delete: FANCM as an anti-cancer target in Alternative Lengthening of Telomeres. Nucleus 2019;10:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subhawong AP, Heaphy CM, Argani P, Konishi Y, Kouprina N, Nassar H, et al. The alternative lengthening of telomeres phenotype in breast carcinoma is associated with HER-2 overexpression. Mod Pathol 2009;22:1423–31. [DOI] [PubMed] [Google Scholar]
- 21.Hackeng WM, Brosens LAA, Kim JY, O’Sullivan R, Sung YN, Liu TC, et al. Non-functional pancreatic neuroendocrine tumours: ATRX/DAXX and alternative lengthening of telomeres (ALT) are prognostically independent from ARX/PDX1 expression and tumour size. Gut 2022;71:96173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagae G, Yamamoto S, Fujita M, Fujita T, Nonaka A, Umeda T, et al. Genetic and epigenetic basis of hepatoblastoma diversity. Nature communications 2021;12:5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kastenhuber ER, Lalazar G, Houlihan SL, Tschaharganeh DF, Baslan T, Chen CC, et al. DNAJB1PRKACA fusion kinase interacts with beta-catenin and the liver regenerative response to drive fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci U S A 2017;114:13076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Vanderbilt CM, Lin YT, Benhamida JK, Jungbluth AA, Rana S, et al. A Pan-Cancer Study of Somatic TERT Promoter Mutations and Amplification in 30,773 Tumors Profiled by Clinical Genomic Sequencing. J Mol Diagn 2021;23:253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


