Abstract
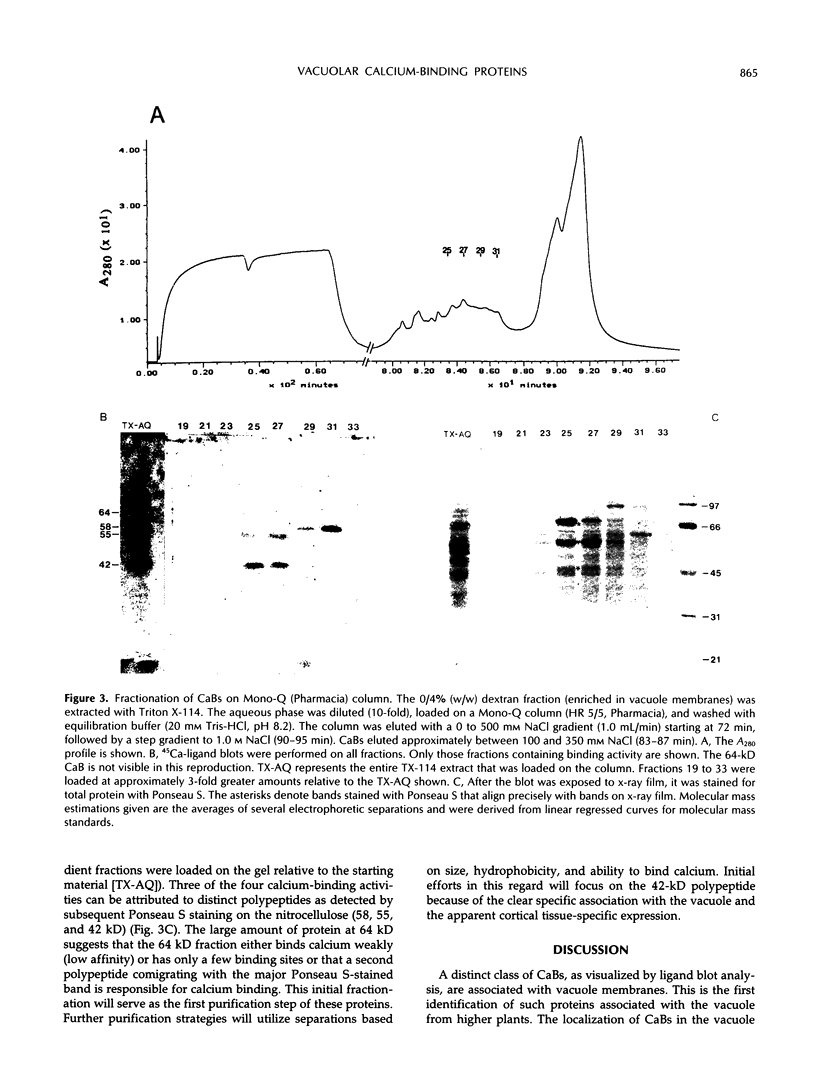
The vacuole plays a major structural and biochemical role in the higher plant cell. Among the most studied properties of the vacuole have been transport activities. One important aspect of vacuolar function is its participation in the regulation of cytosolic calcium levels. To identify the molecular entities involved in calcium regulation, a study of vacuole-associated, calcium-binding proteins (CaBs) was initiated. A competition assay was used, and it was observed that the majority of the total cellular membrane-associated, calcium-binding activity resided in low-density fractions enriched in vacuole membranes. Much of that calcium-binding activity was inactivated by a 0.5 m KI wash, and of the remaining activity, 77% was estimated to be peripherally associated with vacuolar membranes, whereas 23% was integrally associated with the vacuolar membrane. Calcium-ligand blots were used, and four major CaBs, with apparent molecular masses of 64, 58, 55, and 42 kD, were detected in purified vacuole membrane fractions. Two of these, the 58- and the 55-kD polypeptide, also appear to be present in significant amounts in endoplasmic reticulum-enriched fractions. However, the 64- and the 42-kD polypeptide are found primarily in vacuolar fractions. It is interesting that expression of the 42-kD polypeptide appears to be restricted to the heavily vacuolated cortical tissues (i.e. it is not found in vascular tissues). The localization of CaBs in the vacuole is consistent with the presence of calcium uptake (H+/Ca2+ antiport) and release mechanisms (inositol trisphosphate sensitive) on vacuolar membranes. These vacuole-associated CaBs, which may play a role in calcium buffering, together with the calcium transport systems, could mediate the vacuolar component of cellular calcium homeostasis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Cormier M. J. Calcium-dependent regulation of NAD kinase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):595–602. doi: 10.1016/0006-291x(78)90747-7. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Kinetics of Ca/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris L. Plant Physiol. 1986 Mar;80(3):727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bush D. R., Sze H. Calcium transport in tonoplast and endoplasmic reticulum vesicles isolated from cultured carrot cells. Plant Physiol. 1986 Feb;80(2):549–555. doi: 10.1104/pp.80.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou M., Krause K. H., Campbell K. P., Jensen K. G., Sjolund R. D. Antibodies against the Calcium-Binding Protein: Calsequestrin from Streptanthus tortuosus (Brassicaceae). Plant Physiol. 1989 Dec;91(4):1259–1261. doi: 10.1104/pp.91.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. B., Dauwalder M., Roux S. J. Purification and immunolocalization of an annexin-like protein in pea seedlings. Planta. 1992;187:1–9. [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Joyce D. C., Cramer G. R., Reid M. S., Bennett A. B. Transport Properties of the Tomato Fruit Tonoplast : III. Temperature Dependence of Calcium Transport. Plant Physiol. 1988 Dec;88(4):1097–1103. doi: 10.1104/pp.88.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Nonomura Y. High molecular weight calcium binding protein in the microsome of scallop striated muscle. J Biochem. 1984 Sep;96(3):859–870. doi: 10.1093/oxfordjournals.jbchem.a134904. [DOI] [PubMed] [Google Scholar]
- Miller A. J., Vogg G., Sanders D. Cytosolic calcium homeostasis in fungi: roles of plasma membrane transport and intracellular sequestration of calcium. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9348–9352. doi: 10.1073/pnas.87.23.9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam S. K., Towers T. Subcellular distribution of calcium-binding proteins and a calcium-ATPase in canine pancreas. J Cell Biol. 1990 Jul;111(1):197–200. doi: 10.1083/jcb.111.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostwald T. J., MacLennan D. H. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J Biol Chem. 1974 Feb 10;249(3):974–979. [PubMed] [Google Scholar]
- Pietrobon D., Di Virgilio F., Pozzan T. Structural and functional aspects of calcium homeostasis in eukaryotic cells. Eur J Biochem. 1990 Nov 13;193(3):599–622. doi: 10.1111/j.1432-1033.1990.tb19378.x. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Burns A. L., Rojas E. Synexin, a new member of the annexin gene family, is a calcium channel and membrane fusion protein. Prog Clin Biol Res. 1990;349:159–172. [PubMed] [Google Scholar]
- Randall S. K., Sze H. Probing the catalytic subunit of the tonoplast H+-ATPase from oat roots. Binding of 7-chloro-4-nitrobenzo-2-oxa-1,3,-diazole to the 72-kilodalton polypeptide. J Biol Chem. 1987 May 25;262(15):7135–7141. [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. A Ca/H Antiport System Driven by the Proton Electrochemical Gradient of a Tonoplast H-ATPase from Oat Roots. Plant Physiol. 1985 Dec;79(4):1111–1117. doi: 10.1104/pp.79.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J Biol Chem. 1986 Sep 15;261(26):12172–12178. [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of oat roots. J Biol Chem. 1987 Mar 25;262(9):3944–3946. [PubMed] [Google Scholar]
- Waisman D. M., Rasmussen H. A reexamination of the chelex competitive calcium binding assay. Cell Calcium. 1983 Apr;4(2):89–105. doi: 10.1016/0143-4160(83)90038-6. [DOI] [PubMed] [Google Scholar]
- Waisman D. M., Salimath B. P., Anderson M. J. Isolation and characterization of CAB-63, a novel calcium-binding protein. J Biol Chem. 1985 Feb 10;260(3):1652–1660. [PubMed] [Google Scholar]