Abstract
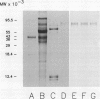
Highly purified mitochondria isolated from 14-day-old pea (Pisum sativum L., cv Little Marvel) seedlings contain a homolog of the 70,000 molecular weight heat-shock protein. The amount of this heat-shock cognate (Hsc70) was not reduced by limited proteolysis of intact mitochondria or by preparation of mitoplasts, indicating that the protein is located within the matrix compartment. Pea mitochondrial Hsc70 binds to immobilized ATP and reacts on western blots with anti-tomato Hsc70 antiserum. When a mitochondrial matrix fraction was incubated with [γ-32P]ATP, there was phosphorylation of Hsc70. The extent of phosphorylation was increased by including calcium chloride in the reactions. Phospho amino acid analysis of purified mitochondrial Hsc70, phosphorylated in the calcium-stimulated reaction, revealed only phosphothreonine. Pea mitochondrial Hsc70, purified by a combination of ATP-agarose affinity chromatography and gel permeation chromatography, was labeled when incubated with ATP plus calcium, suggesting autophosphorylation rather than phosphorylation by an associated kinase. In analogy to mammalian cells and yeast, it is likely that mitochondrial Hsc70 acts as a molecular chaperone, and it is possible that phosphorylation plays a role in chaperone function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir-Shapira D., Leustek T., Dalie B., Weissbach H., Brot N. Hsp70 proteins, similar to Escherichia coli DnaK, in chloroplasts and mitochondria of Euglena gracilis. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1749–1752. doi: 10.1073/pnas.87.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang D., Liberek K., Skowyra D., Zylicz M., Georgopoulos C. Biological role and regulation of the universally conserved heat shock proteins. J Biol Chem. 1991 Dec 25;266(36):24233–24236. [PubMed] [Google Scholar]
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- Budde R. J., Fang T. K., Randall D. D. Regulation of the phosphorylation of mitochondrial pyruvate dehydrogenase complex in situ: effects of respiratory substrates and calcium. Plant Physiol. 1988 Dec;88(4):1031–1036. doi: 10.1104/pp.88.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Kramer J., Shilling J., Werner-Washburne M., Holmes S., Kosic-Smithers J., Nicolet C. M. SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol Cell Biol. 1989 Jul;9(7):3000–3008. doi: 10.1128/mcb.9.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., Hemmingsen S. M. Molecular chaperones: proteins essential for the biogenesis of some macromolecular structures. Trends Biochem Sci. 1989 Aug;14(8):339–342. doi: 10.1016/0968-0004(89)90168-0. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M., Hemmingsen S. M. The molecular chaperone concept. Biochem Soc Symp. 1989;55:145–153. [PubMed] [Google Scholar]
- Gatenby A. A., Ellis R. J. Chaperone function: the assembly of ribulose bisphosphate carboxylase-oxygenase. Annu Rev Cell Biol. 1990;6:125–149. doi: 10.1146/annurev.cb.06.110190.001013. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Leustek T., Dalie B., Amir-Shapira D., Brot N., Weissbach H. A member of the Hsp70 family is localized in mitochondria and resembles Escherichia coli DnaK. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7805–7808. doi: 10.1073/pnas.86.20.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. Y., Duck N. B., Winter J., Folk W. R. Sequences of two hsc 70 cDNAs from Lycopersicon esculentum. Plant Mol Biol. 1991 Mar;16(3):475–478. doi: 10.1007/BF00023998. [DOI] [PubMed] [Google Scholar]
- McCarty J. S., Walker G. C. DnaK as a thermometer: threonine-199 is site of autophosphorylation and is critical for ATPase activity. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9513–9517. doi: 10.1073/pnas.88.21.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk J. A., Dennis D. T. Mitochondrial, plastid, and cytosolic isozymes of hexokinase from developing endosperm of Ricinus communis. Arch Biochem Biophys. 1983 Oct 15;226(2):458–468. doi: 10.1016/0003-9861(83)90315-6. [DOI] [PubMed] [Google Scholar]
- Miernyk J. A., Duck N. B., Shatters R. G., Jr, Folk W. R. The 70-Kilodalton Heat Shock Cognate Can Act as a Molecular Chaperone during the Membrane Translocation of a Plant Secretory Protein Precursor. Plant Cell. 1992 Jul;4(7):821–829. doi: 10.1105/tpc.4.7.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk J. A., Fang T. K., Randall D. D. Calmodulin antagonists inhibit the mitochondrial pyruvate dehydrogenase complex. J Biol Chem. 1987 Nov 15;262(32):15338–15340. [PubMed] [Google Scholar]
- Miernyk J. A., Randall D. D. A synthetic peptide-directed antibody as a probe of the phosphorylation site of pyruvate dehydrogenase. J Biol Chem. 1989 Jun 5;264(16):9141–9144. [PubMed] [Google Scholar]
- Pelham H. R. Heat shock and the sorting of luminal ER proteins. EMBO J. 1989 Nov;8(11):3171–3176. doi: 10.1002/j.1460-2075.1989.tb08475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Scherer P. E., Krieg U. C., Hwang S. T., Vestweber D., Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990 Dec;9(13):4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. A., Calderwood S. K. Members of the 70-kilodalton heat shock protein family contain a highly conserved calmodulin-binding domain. Mol Cell Biol. 1990 Mar;10(3):1234–1238. doi: 10.1128/mcb.10.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts F. Z., Walters A. J., Moore A. L. Characterisation of PHSP1, a cDNA encoding a mitochondrial HSP70 from Pisum sativum. Plant Mol Biol. 1992 Jan;18(1):23–32. doi: 10.1007/BF00018453. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooden S. K., Li L. J., Navarro D., Qadri I., Pereira L., Lee A. S. Transactivation of the grp78 promoter by malfolded proteins, glycosylation block, and calcium ionophore is mediated through a proximal region containing a CCAAT motif which interacts with CTF/NF-I. Mol Cell Biol. 1991 Nov;11(11):5612–5623. doi: 10.1128/mcb.11.11.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]