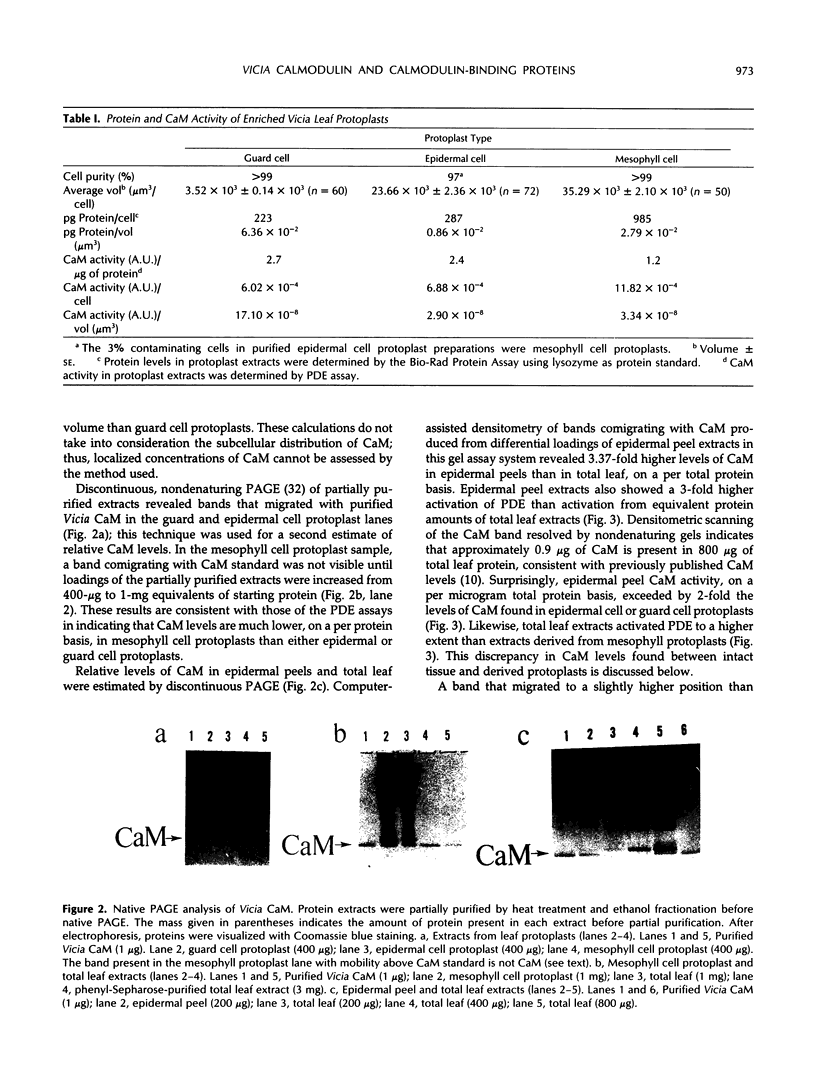
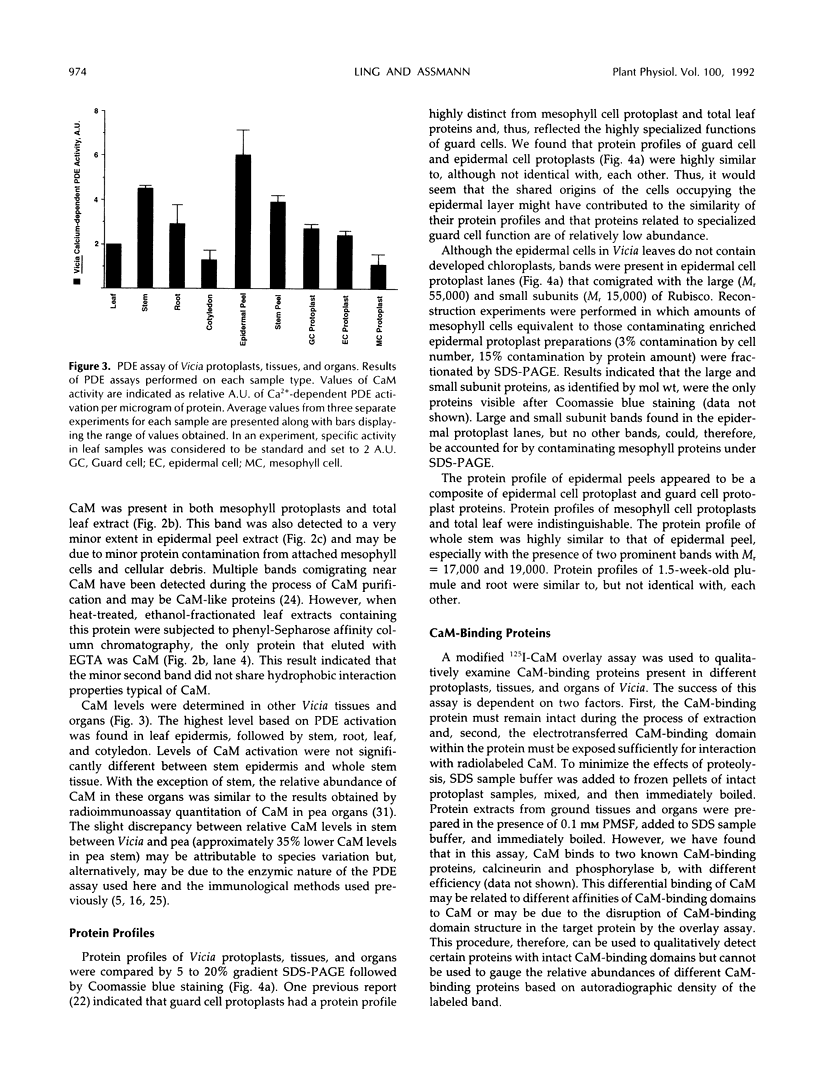
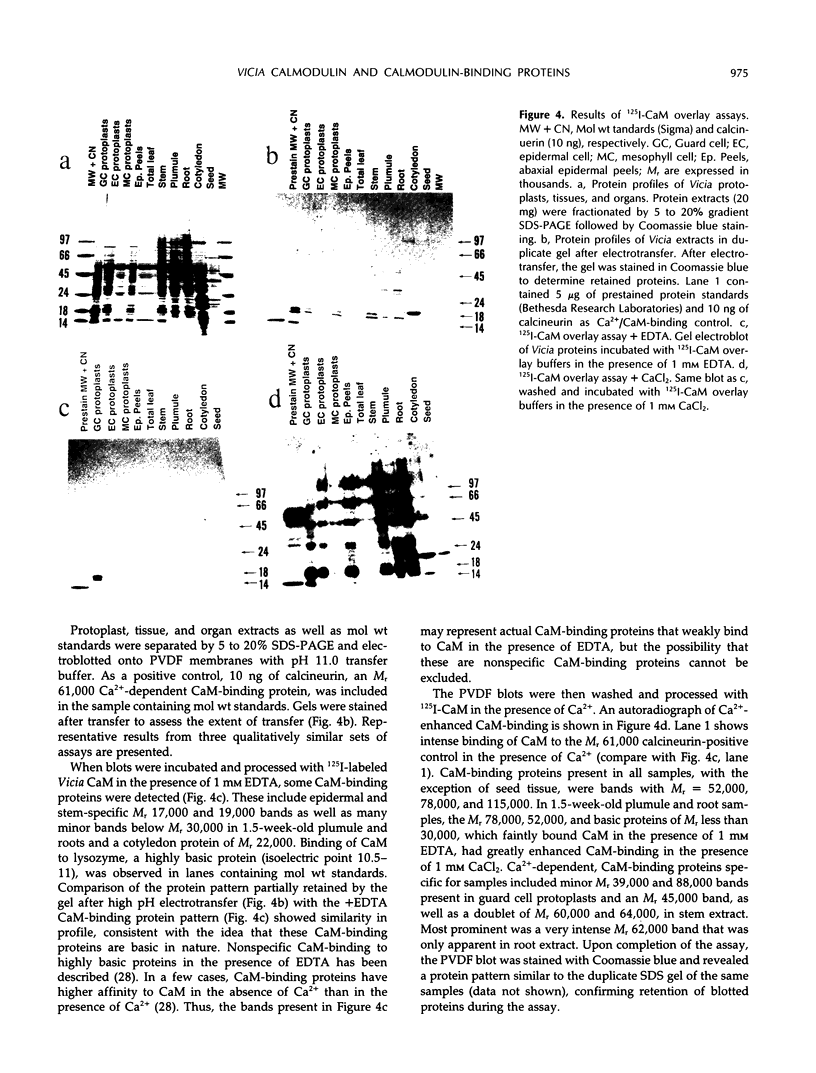
Abstract
The distribution of calmodulin (CaM) and CaM-binding proteins within Vicia faba was investigated. Both CaM and CaM-binding proteins were found to be differentially distributed among organs, tissues, and protoplast types. CaM levels, on a per protein basis, were found to be the highest in leaf epidermis, containing 3-fold higher levels of CaM than in total leaf. Similarly, guard cell and epidermal cell protoplasts were also found to have higher levels of CaM than mesophyll cell protoplasts. 125I-CaM blot overlay assays were performed to qualitatively examine CaM-binding proteins in these protoplast types as well as in whole tissues and organs. CaM-binding proteins with Mr 52,000, 78,000, and 115,000 were common in all metabolically active plant parts. Unique CaM-binding protein bands were detected in guard cell protoplasts (Mr 39,000, 88,000), stems (Mr 45,000, 60,000, 64,000), and roots (Mr 62,000), suggesting the presence of specialized CaM-dependent processes in these cells and organs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. Purification of plant calmodulin. Methods Enzymol. 1983;102:9–17. doi: 10.1016/s0076-6879(83)02004-2. [DOI] [PubMed] [Google Scholar]
- Biro R. L., Daye S., Serlin B. S., Terry M. E., Datta N., Sopory S. K., Roux S. J. Characterization of oat calmodulin and radioimmunoassay of its subcellular distribution. Plant Physiol. 1984 Jun;75(2):382–386. doi: 10.1104/pp.75.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J., Davis R. W. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990 Feb 9;60(3):357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- Brawley S. H., Roberts D. M. Calmodulin-binding proteins are developmentally regulated in gametes and embryos of fucoid algae. Dev Biol. 1989 Feb;131(2):313–320. doi: 10.1016/s0012-1606(89)80004-1. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Chen Y. R., Datta N., Roux S. J. Purification and partial characterization of a calmodulin-stimulated nucleoside triphosphatase from pea nuclei. J Biol Chem. 1987 Aug 5;262(22):10689–10694. [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Evidence for and properties of a protein activator. J Biol Chem. 1971 May 10;246(9):2859–2869. [PubMed] [Google Scholar]
- Dauwalder M., Roux S. J., Hardison L. Distribution of calmodulin in pea seedlings: immunocytochemical localization in plumules and root apices. Planta. 1986;168:461–470. [PubMed] [Google Scholar]
- Flanagan S. D., Yost B. Calmodulin-binding proteins: visualization by 125I-calmodulin overlay on blots quenched with Tween 20 or bovine serum albumin and poly(ethylene oxide). Anal Biochem. 1984 Aug 1;140(2):510–519. doi: 10.1016/0003-2697(84)90202-1. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Sussman M. R., Schaller G. E., Putnam-Evans C., Charbonneau H., Harmon A. C. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991 May 17;252(5008):951–954. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- Ido M., Lagacé L., Chafouleas J. G. Increased sensitivity to Vinca alkaloids in cells overexpressing calmodulin by gene transfection. Cancer Res. 1990 Oct 15;50(20):6554–6558. [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Kruse T., Tallman G., Zeiger E. Isolation of Guard Cell Protoplasts from Mechanically Prepared Epidermis of Vicia faba Leaves. Plant Physiol. 1989 Aug;90(4):1382–1386. doi: 10.1104/pp.90.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mac Neil S., Walker S. W., Senior H. J., Bleehen S. S., Tomlinson S. Effects of extracellular calmodulin and calmodulin antagonists on B16 melanoma cell growth. J Invest Dermatol. 1984 Jul;83(1):15–19. doi: 10.1111/1523-1747.ep12261637. [DOI] [PubMed] [Google Scholar]
- Outlaw W. H., Mayne B. C., Zenger V. E., Manchester J. Presence of Both Photosystems in Guard Cells of Vicia faba L: IMPLICATIONS FOR ENVIRONMENTAL SIGNAL PROCESSING. Plant Physiol. 1981 Jan;67(1):12–16. doi: 10.1104/pp.67.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter G. R., Means A. R. Use of the 125I-labeled protein gel overlay technique to study calmodulin-binding proteins. Methods Enzymol. 1987;139:433–444. doi: 10.1016/0076-6879(87)39104-9. [DOI] [PubMed] [Google Scholar]
- Spalding E. P., Cosgrove D. J. Large plasma-membrane depolarization precedes rapid blue-light-induced growth inhibition in cucumber. Planta. 1989;178:407–410. [PubMed] [Google Scholar]
- Stinemetz C. L., Kuzmanoff K. M., Evans M. L., Jarrett H. W. Correlation between calmodulin activity and gravitropic sensitivity in primary roots of maize. Plant Physiol. 1987;84:1337–1342. doi: 10.1104/pp.84.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A., Gilroy S. Signal transduction in plant cells. Trends Genet. 1991 Nov-Dec;7(11-12):356–361. doi: 10.1016/0168-9525(91)90255-o. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Yang R. L., Evans M. L., Moore R. Microsurgical removal of epidermal and cortical cells: evidence that the gravitropic signal moves through the outer cell layers in primary roots of maize. Planta. 1990;180:530–536. [PubMed] [Google Scholar]