Abstract
The multiple-memory-systems framework—that distinct types of memory are supported by distinct brain systems—has guided learning and memory research for decades. However, recent work challenges the one-to-one mapping between brain structures and memory types central to this taxonomy, with key memory-related structures supporting multiple functions across substructures. Here we integrate cross-species findings in the hippocampus, striatum, and amygdala to propose an updated framework of multiple memory subsystems (MMSS). We provide evidence for two organizational principles of the MMSS theory: First, opposing memory representations are colocated in the same brain structures; second, parallel memory representations are supported by distinct structures. We discuss why this burgeoning framework has the potential to provide a useful revision of classic theories of long-term memory, what evidence is needed to further validate the framework, and how this novel perspective on memory organization may guide future research.
Keywords: hippocampus, striatum, amygdala, episodic memory, habits, conditioning
Any experience can be stored as multiple kinds of memories, varying in strength, richness, and imageability. Some memories are episodic in nature, involving vivid recollections of a specific event in space and time (e.g., your last birthday party). Other memories may be less detailed or tied to specific cues (e.g., anticipating a cake when you hear the song “Happy Birthday” or blowing out lit candles placed in front of you). These differences in content also influence the way people use memories to guide future behavior (Biderman et al., 2020). People can call on specific experiences to flexibly guide behavior based on their current state, or people can rely on more general, rigid memories to drive behavior automatically (Tolman, 1948).
These varieties of remembering led to attempts to systematize long-term memory into an ontology or taxonomy. The theory of multiple memory systems resulted from these efforts and has remained the dominant, guiding framework for memory research. This theory maps functionally distinct representations onto discrete brain structures (Henke, 2010; Squire, 1992). Research across species has identified key hubs for distinct types of associative memory, with the hippocampus involved in episodic memory (e.g., remembering your last birthday party), the striatum involved in stimulus-response learning (e.g., linking candles with blowing), and the amygdala involved in classical conditioning (e.g., linking the birthday song with cake).
However, recent work challenges this taxonomy by revealing that multiple—sometimes competing—memories can exist within each of these structures and that similar mnemonic computations are supported across multiple structures. Specifically, the hippocampus, striatum, and amygdala can each rapidly form separated representations and gradually form integrated representations. In this article, we synthesize these findings into a new theoretical framework of multiple memory subsystems (MMSS). After characterizing these subsystems, we discuss why it may be adaptive to house competing representations within the same brain system, highlight commonalities in subsystems across regions, and discuss factors that modulate the use of subsystems. Together, MMSS provides a more nuanced and precise understanding of the relationship between brain structures and memory functions, unveils novel adaptive memory features, and presents a guide for future research on how these canonical brain regions support people’s multifaceted memories.
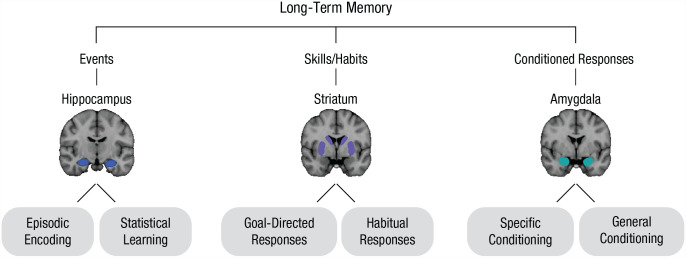
Traditional Taxonomy of Multiple Memory Systems: One Memory, One Brain Region
There is a rich history of characterizing how the human mind and brain support multiple types of memory. Early behavioral theories distinguished memories by process, for example, noting the distinction between stimulus-response and “cognitive map”-like episodic representations (Tolman, 1948). The majority of initial evidence for multiple memory systems came from lesion studies in human patients and rodents that localized different types of memories to particular brain regions (Fig. 1, top). By linking anatomical damage to mnemonic deficits, this work pushed against prior “equipotentiality” theories that memory is distributed across the brain (Lashley, 1950).
Fig. 1.
Multiple-memory-subsystems taxonomy. The traditional multiple-memory-systems taxonomy is depicted on the top. We propose the delineation of memory “subsystems” within each canonical memory system (bottom shaded regions).
Distinct memory representations in the hippocampus, amygdala, and striatum have been supported by double (Packard et al., 1989; Packard & McGaugh, 1996) and triple dissociations (McDonald & White, 1993) in rodents. For example, rodents with hippocampal damage had impaired episodic-like memory for places but intact stimulus-response memory for actions, whereas rodents with striatal damage showed the opposite (Packard et al., 1989; Packard & McGaugh, 1996). Such dissociations have been found in human lesion studies as well (Knowlton et al., 1996; LaBar et al., 1995; Milner, 1962).
The nature of multiple memory representations has been refined in important ways over time. Early theories emphasized the role of consciousness in delineating memory systems, with “declarative” forms of memory, such as episodic memory, relying on the hippocampus and “non-declarative” forms of memory, such as procedural memory and conditioned responses, relying on the striatum and amygdala, respectively (Squire, 1992).
Subsequent theories moved away from strict consciousness-based distinctions, proposing instead that the recruitment of a brain region for a memory process can be explained by computations facilitated by the brain region, with the hippocampus supporting the rapid encoding of flexible associations and the striatum supporting the slower encoding of rigid associations (Henke, 2010). Such an appeal to the computational properties of a brain region can also help explain the role of the hippocampus in nonmnemonic tasks (Shohamy & Turk-Browne, 2013). Although this perspective based on “processing modes” provides greater explanatory power and accounts for a wider range of contemporary findings, existing theories largely continue to treat these memory-related regions as homogeneous, ignoring the functional heterogeneity of subregions (but see White, 2009).
Beyond characterizing the relationship between brain structure and memory function, multiple-memory-systems theories delineate circumstances under which certain brain regions/memory systems are used preferentially. Segregating memory systems across structures allows for parallel representations to guide behavior through cooperation or compensation. For example, while you may be able to rely on “striatal” stimulus-response associations to get to work (e.g., turn left at the intersection), if there is a roadblock, more flexible “hippocampal” episodic memories could help efficiently circumvent the obstruction. Which memory system is used depends on factors including task demands. For example, reminders of past trials in a reinforcement-learning task can bias participants toward acting based on specific episodic memories rather than incrementally learned values (Bornstein et al., 2017; Duncan et al., 2019). Segregated memory systems can also compete. For example, rodents with hippocampal lesions can exhibit enhanced response-based striatal learning (Packard et al., 1989), which would have otherwise conflicted with place-based hippocampal learning (also Gardner et al., 2020). Likewise, in humans, hippocampal damage is associated with increased use of striatum-dependent strategies (Vikbladh et al., 2019).
This framework also promoted an appreciation of modulatory factors that bias the use of different memory systems. For example, across species, stress is associated with a bias toward striatal representations (stimulus-response associations) rather than hippocampal representations (episodic memories; Schwabe et al., 2022). Time also plays an important role in arbitrating between memory systems. For example, temporal gaps between conditioned and unconditioned stimuli in trace conditioning lead to a shift from the amygdala to the hippocampus (Clark & Squire, 1998), and delays between choice and feedback in reinforcement learning lead to a shift from the striatum to the hippocampus (Foerde et al., 2013).
Taxonomy of Memory Subsystems: Multiple Memories, One Brain Region
Although there is extensive evidence that different kinds of memory map onto different brain regions, recent work challenges this framework. Importantly, canonical memory-related brain regions are not functionally homogeneous. Instead, they contain subregions with different firing patterns and connectivity profiles, which may support distinct memory types. Here we review how subregions within the hippocampus, striatum, and amygdala support different, sometimes opposing memories. This led to our proposal of the MMSS theory, in which single brain regions can support multiple memory representations (Fig. 1, bottom).
Hippocampus
The hippocampus is traditionally considered the seat of episodic memory. Episodic memory reflects the rapid encoding of a discrete experience, enabling rich recollection while minimizing interference from related experiences. This is attributed to at least two functions of the hippocampus: relational binding, linking elements of an experience over space and time into a detailed and integrated episode (Henke, 2010), and pattern separation, orthogonalizing the representation of the episode with respect to related memories (Yassa & Stark, 2011).
However, the hippocampus has also been assigned other mnemonic functions, such as generalization (Zeithamova & Bowman, 2020) and statistical learning (Sherman et al., 2020). Specifically, the hippocampus extracts spatial and/or temporal regularities across experiences (Covington et al., 2018; Henin et al., 2021; Schapiro et al., 2012) and links across related associations (Schlichting et al., 2014, 2017; Wimmer & Shohamy, 2012). In stark contrast to the computational requirements of episodic memory (sparse, orthogonalized code to rapidly form unique memories), these processes require that memories get formed more gradually, using an overlapping neural code to reinforce common aspects of related memories over time without suffering catastrophic interference.
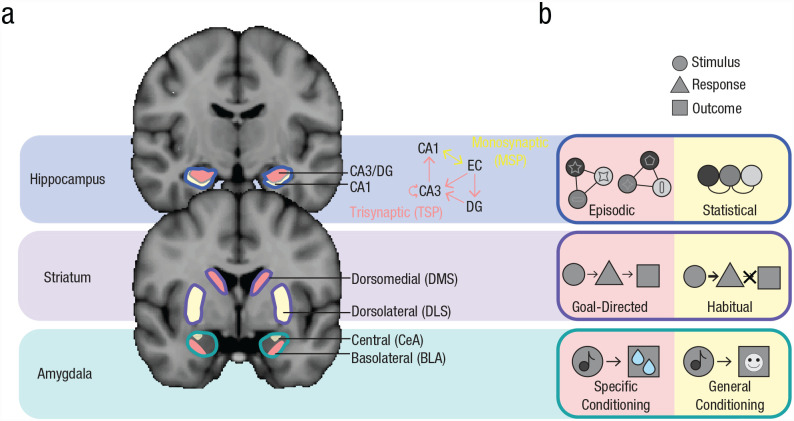
From a traditional multiple-memory-systems perspective, this is puzzling: How could the hippocampus support such different representations? A neural-network model of the hippocampus (Schapiro et al., 2017) suggests that its ability to perform these computationally opposing learning functions depends on two hippocampal pathways (Amaral, 1993; see Fig. 2, top). The trisynaptic pathway (TSP)—from entorhinal cortex (EC) to hippocampal subfield cornu ammonis 1 (CA1) via dentate gyrus (DG) and CA3—has high inhibition and a fast learning rate, ideally suited to support the rapid, pattern-separated codes of episodic memory (Bakker et al., 2008; Leutgeb et al., 2007). Pattern separation is often probed by examining hippocampal activity in response to two highly similar environments or images. For example, in monkeys, the firing rates across CA3/DG neurons discriminate between similar images (e.g., two images of the same breed of dog) such that the two similar images are represented by separate populations of neurons (Sakon & Suzuki, 2019). In addition, a recent human functional MRI (fMRI) study measuring multivoxel patterns in CA3/DG found that similar memories (e.g., highly similar object–scene pairs, such as Lighthouse 1–Guitar 1 and Lighthouse 2–Guitar 2) were represented in a nonoverlapping way such that they recruited distinct populations of voxels; such representational dissimilarity reduced interference and enhanced new behavioral learning (Wanjia et al., 2021). Beyond evidence of pattern separation in CA3/DG, TSP structural connectivity in humans has also been linked to behavioral evidence of mnemonic specificity in category learning (e.g., learning the exception to a category rule; Schlichting et al., 2021).
Fig. 2.
MMSS circuitry and subsystem representations. (a) Anatomical visualization of different memory-related regions, segmented into subregions supporting different memory subsystems (MSP = monosynaptic pathway; TSP = trisynaptic pathway; DMS = dorsomedial striatum; DLS = dorsolateral striatum; CeA = central amygdala; BLA = basolateral amygdala). (b) Schematic of representations supported by memory subsystems associated with brain subregions in Figure 2a. Subsystems indicated in pink tend to support specific, rapidly formed, separated memory representations, whereas subsystems indicated in yellow tend to support general, gradually formed, integrated memory representations. (Top) The hippocampus forms associations among stimuli (circles). Episodic associations (left) bind together trial-unique stimuli (colored circles with unique symbols) into discrete episodes. Statistically learned associations (right) reflect the extraction of commonalities across experiences (e.g., the co-occurrence of the colored circles, abstracted away from idiosyncratic details). (Middle) The striatum forms associations between stimuli (circles), responses (triangles), and outcomes (squares). Goal-directed associations (left) are sensitive to outcomes, whereas habits (right) reflect continuing to make responses when the stimulus is presented, even in the absence of a (desirable) outcome. (Bottom) The amygdala forms associations between stimuli (circles) and outcomes (squares). Specific conditioning (left) reflects an association between a cue and an outcome (e.g., juice reward). General conditioning (right) reflects an association between a cue and a general motivational state (e.g., positive affect).
The monosynaptic pathway (MSP)—a direct recurrent EC/CA1 connection—has lower inhibition and a slower learning rate, leading to distributed and overlapping representations of related inputs in the service of an integrated code for statistical learning. Consistent with this model, human (Molitor et al., 2021; Schlichting et al., 2014) and rodent (McKenzie et al., 2013; Wood et al., 2000) studies have found that subfield CA1 supports pattern integration (such that related experiences are represented in a more overlapping manner). For example, a rodent study found that common populations of CA1 neurons fired at both old and new goal locations in a familiar environment, indicating that CA1 may represent the shared content of similar memories across events (McKenzie et al., 2013). In a human study of associative inference, CA1 reinstated patterns of encoding activity for overlapping memories during retrieval, providing a mechanism to link distinct encoding experiences in the service of inference behavior (Schlichting et al., 2014).
Further supporting the distinct roles of CA3/DG (TSP) and CA1 (MSP) in differentiation and integration, respectively, rodent studies have found that CA3 can support the rapid acquisition of memories, whereas CA1 is required for integrating memories more gradually over time (Lee et al., 2004; Lee & Kesner, 2002; Nakashiba et al., 2008). Importantly, although CA1 is part of both the MSP and TSP circuits, we refer to it as an “MSP” region throughout the article because it contains the necessary computational properties (i.e., pattern integration) to support MSP-dependent statistical learning, even in the absence of CA3/DG (Schapiro et al., 2017; Sucevic & Schapiro, 2022).
Striatum
The striatum has traditionally been associated with stimulus-response, or habit, memory (Knowlton et al., 1996). However, there is evidence that the striatum can also support flexible, goal-directed behavior (Balleine, 2019).
Both habit and goal-directed associations have three parts: A “stimulus” (e.g., birthday candles) indicates that an agent should make a “response” (e.g., blowing them out) in order to receive a desirable “outcome” (e.g., cake). Habit memory reflects rigid stimulus-response associations, marked by persistent stimulus-evoked responses even without a rewarding outcome. In contrast, goal-directed associations involve continued sensitivity to outcome value and the ability to flexibly update behavior based on current environment or goal states (Grospe et al., 2018; Lhost et al., 2021). Habit memories form slowly, as a result of extensive training, whereas goal-directed associations are formed rapidly (Balleine & O’Doherty, 2010).
As with the hippocampus, the existence of opposing associations within the striatum challenges the traditional multiple-memory-systems taxonomy. And again, the solution may come from considering functional heterogeneity within the striatum (Fig. 2, middle). Different subregions support distinct computations: The dorsolateral striatum (DLS; primate putamen, part of sensorimotor striatum) facilitates rigid, habitual behavior, whereas the dorsomedial striatum (DMS; primate caudate nucleus, part of associative striatum) supports flexible, goal-directed behavior (Turner et al., 2022; White, 2009). Although the majority of this evidence comes from rodents, human neuroimaging has provided converging support for this dissociation (McNamee et al., 2015; Patterson & Knowlton, 2018).
The DLS and DMS exhibit distinct firing patterns that may support these different associations. For example, Thorn et al. (2010) designed a task in which rodents were placed in a T-maze. As the animal moved along the linear track (before the choice point), a cue indicated which of the two goal arms would be rewarded. DMS and DLS were active at distinct time points within a trial: DMS activity peaked during the middle portion of the trial, when the animal had to select a behavior based on the cue, whereas DLS primarily fired at the beginning and end of the trial, marking the “action boundaries.” This “task-bracketing” of DLS (Jog et al., 1999; Smith & Graybiel, 2013; Thorn et al., 2010) emphasizes boundaries between learned sequences, perhaps chunking them into unified habitual associations. Indeed, DLS bracketing activity is both insensitive to reward devaluation and linked to automaticity (Smith & Graybiel, 2013). In contrast, ramping DMS activity tracks the progression of a sequence, suggesting sensitivity to current states (Vandaele et al., 2021).
Amygdala
The amygdala has been linked to remembering salient associations with fear and reward, particularly in the context of classical conditioning. However, stimuli and outcomes can be associated at different levels of granularity, which can motivate different behaviors. Specifically, there is burgeoning evidence that the basolateral amygdala (BLA) supports specific associations between a stimulus and a particular outcome, whereas the central amygdala (CeA) supports general associations between a stimulus and an ensuing affective state or behavioral output (Fig. 2, bottom).
Although there is less work on subsystem dissociations in the amygdala than in the hippocampal and striatal subsystems described above, there is compelling evidence for a BLA/CeA difference from the Pavlovian-instrumental-transfer paradigm. Pavlovian-instrumental transfer is an experimental paradigm in which classically conditioned cues promote instrumentally learned actions. It can be general (presence of a conditioned cue results in a general motivational increase or response regardless of the anticipated outcome) or specific (conditioned cue elicits a response associated with the outcome that the cue predicts). As an intuitive example, after a bout of food poisoning, you may show specific transfer by linking the aversive outcome with the dish you ate and thus avoid ordering that meal in the future. In the case of general transfer, you may form a broader representation of that experience, resulting in a general motivation/response change, thus becoming wary of ordering takeout entirely (see Cartoni et al., 2016). Human and rodent studies have linked specific transfer to BLA and general transfer to CeA (Cartoni et al., 2016; Corbit & Balleine, 2005; Prévost et al., 2012). In one such experiment (Corbit & Balleine, 2005), a rodent formed Pavlovian tone/reward associations and instrumental lever press/reward associations. Transfer was measured when the tone was presented while the lever was available. The tone selectively increased lever pressing if the tone and lever were paired with the same reward (specific transfer) but broadly increased lever pressing if they were associated with distinct rewards (general transfer). These findings provide empirical support for theorized BLA/CeA distinctions in classic models of amygdala function (Balleine & Killcross, 2006; Holland & Gallagher, 1999).
This mapping also extends beyond Pavlovian-instrumental transfer, with BLA supporting specific responses (Wassum & Izquierdo, 2015) and CeA supporting general responses (Fadok et al., 2018) in rodents. The BLA represents sensory-specific stimulus/outcome associations, supporting prediction (Lichtenberg et al., 2017) and detailed memory formation (Sias et al., 2021). In contrast, the CeA has been linked more broadly to motivation and reward-seeking behavior (Warlow & Berridge, 2021) and to more generalized forms of fear and anxiety (Ciocchi et al., 2010; Gilpin et al., 2015). Although studied less in humans, recent patient (Rosenberger et al., 2019; van Honk et al., 2013) and high-resolution fMRI (Sladky et al., 2021) findings have provided converging evidence, with BLA (but not CeA) supporting specific stimulus/outcome representations in a trust game.
The computational and connectivity profiles of these subregions help them represent specific sensory information versus general motivational information, respectively. The BLA primarily receives sensory input and has been described as “cortex-like” (Wassum & Izquierdo, 2015). BLA neurons fire strongly to stimuli and outcomes during learning (Sias et al., 2021), and glutamatergic activity in BLA tracks learning (Malvaez et al., 2019). This may potentiate specific stimulus-outcome links (Kyriazi et al., 2018). In contrast, the CeA is directly connected to the hypothalamus and brainstem, facilitating motor and autonomic responses (LeDoux et al., 1988). Stimulating CeA neurons or projections broadly elicits motivational responses such as freezing (Fadok et al., 2017; Li et al., 2013) or feeding (Douglass et al., 2017; W. Han et al., 2017), even without a stimulus-outcome association. Although these motivation-related responses may support generalized memory behaviors (i.e., a link between cue and motivation), such findings also suggest that the CeA may play a broader role as an output structure supporting defensive or appetitive behaviors (Fadok et al., 2018; Moscarello & Penzo, 2022) beyond memory. Furthermore, in contrast to the hippocampus and striatum, little work in the amygdala has simultaneously measured BLA and CeA computations in the same learning task in order to delineate their roles.
Interactions Between Subsystems Within a Region
Above we highlighted cross-species evidence that the hippocampus, striatum, and amygdala each contain at least two subsystems supporting distinct memories. These findings present a more precise brain/memory mapping and provide a framework for understanding behavioral consequences of colocated memory representations.
Nature of interactions
Just as having multiple memory systems spread across the brain can promote cooperation or competition, housing memory subsystems within the same brain region provides opportunities for interactions. Colocating may lead to competitive interactions because of shared anatomy and common input/output structures or to parallel processing, akin to the division of labor between the dorsal and ventral visual streams (Nassi & Callaway, 2009). Another related possibility is that the two subsystems could regulate each other such that one subsystem controls the expression of the subsystem. Here, we explore these possibilities and illustrate support for interacting and/or parallel representations across subsystems. We also consider why such colocation may be adaptive for facilitating behavior.
Hippocampus
By being colocated in the hippocampus, episodic memory and statistical learning can function in parallel to support differentiated or integrated memory representations, respectively. For example, CA1 (MSP) shows increased pattern similarity for related items (evidence of integration), while CA2/3/DG (TSP) simultaneously exhibits pattern differentiation of related items (Dimsdale-Zucker et al., 2018; Molitor et al., 2021).
That said, colocation of these opposing representations could foment competition, especially because the hippocampus ultimately sends a single output representation to cortex (Schapiro et al., 2017). For example, a recent neural-network model of category learning found a trade-off between MSP and TSP: Lesioning the TSP (such that only MSP was intact) led to enhanced categorization and generalization relative to if both systems were intact (Sucevic & Schapiro, 2022), suggesting that the TSP (which represented specific exemplars) impeded the ability to optimally learn category structure. Empirically, in a recent study, Sherman and Turk-Browne (2020) measured competition in a task that allowed for simultaneous encoding of statistical regularities and episodic memories. They found that statistical learning interfered with episodic memory: Statistically predictive images were remembered worse than nonpredictive images. Providing initial evidence that the hippocampus was the locus of this trade-off, patterns of hippocampal activity measured with fMRI represented statistical predictions, and the stronger the hippocampal prediction, the worse the average memory for predictive images across participants.
The presence of dual-coding strategies within the hippocampus, with CA1 (MSP) supporting integration and CA2/3/DG (TSP) supporting separation (Molitor et al., 2021; Schapiro et al., 2017), may facilitate adjusting behavior based on task demands. For example, when both integrated and separated encoding strategies are available (as in Sherman & Turk-Browne, 2020), it may be more efficient to focus on integration, thus enabling generalization and prediction, even at the cost of remembering specifics. Indeed, a recent neural-network model demonstrated that forgetting is necessary to efficiently learn statistics because encoding each individual experience may lead to overfitting and learning of unreliable statistical structures (Endress & Johnson, 2021). However, when the task is set up to clearly promote either a separation-based episodic strategy (Chanales et al., 2017) or an integration-based inference strategy (Molitor et al., 2021), then colocation may enable these subsystems to adaptively inhibit each other to support the optimal behavior.
Striatum
A similar push-and-pull relationship exists in the striatum for habits and goal-directed associations, with evidence that these two subsystems can directly regulate one another (rather than merely compete under certain circumstances, as in the hippocampus). In animals, habits are thought to emerge from extended practice such that over time, responses persist even if the outcome is devalued (Dickinson, 1985; described in greater detail below). However, we note that it remains unclear whether such an “over-training” mechanism exists in humans (de Wit et al., 2018; Pool et al., 2022). Nevertheless, recent theories posit that habits and goals are intricately linked, with habits and goal-directed associations stored in parallel, working in concert to guide action (Balleine & Dezfouli, 2019; Bouton, 2021; Cushman & Morris, 2015). For example, a recent behavioral study of human motor sequencing by Hardwick et al. (2019) highlighted the importance of time in toggling between these representations: With time limits, habits automatically drove behavior, but given additional time, goal-directed associations replaced these responses. Attentional demands can also modulate the use of habits versus goal-directed associations: If the link between a stimulus and reward is uncertain, goal-directed behavior is maintained over habits, presumably because more attention must be allocated to the outcome (Thrailkill et al., 2018).
After a habit is established, inactivation of the DLS renews expression of goal-directed associations (Corbit et al., 2012; Zapata et al., 2010), suggesting an inhibitory relationship between DLS and DMS. However, a key challenge in detecting competition is that many tasks rely on insensitivity to outcome devaluation. Outcome devaluation is an experimental manipulation in which a rewarding outcome is made less desirable (e.g., through satiation or pairing the outcome with something aversive). Persistence in performing the response that produces the outcome reflects insensitivity to devaluation, often interpreted as evidence for habitual representations. However, despite the fact that such devaluation is interpreted as evidence of habits, outcome devaluation could in fact emerge from impaired goal-directed associations and/or enhanced habit formation (de Wit et al., 2018). Critically, Turner et al. (2022) developed a novel action-sequence task for rodents in which optimal performance could be achieved through habitual behavior. By incentivizing the use of habits, their task provided a measure of habitual behavior without relying on outcome devaluation. In this paradigm, they also observed a double dissociation between DLS/DMS and habit/goal behavior, supporting the notion that these representations map onto striatal subregions and do indeed compete.
The colocation of habits and goals in the striatum might allow them to regulate each other based on task demands. For example, under time pressure (Hardwick et al., 2019) or cognitive load (Haith & Krakauer, 2018), the striatum can default to habitual behavior (via DLS). However, colocated circuitry (DMS) promoting goal-directed behavior may override habits in favor of specific task- or context-relevant behaviors.
Amygdala
The separable amygdala subregions supporting specific versus general processing may also interact. Time may play a role in arbitrating between BLA and CeA representations. Early theories posited that these amygdala subregions operate “serially” such that information is first learned in BLA and then transferred to CeA, which controls the expression of learning (LeDoux, 2000). Indeed, BLA tends to be important for acquiring stimulus-outcome relations, whereas over time, CeA becomes responsible for expression of the learned association (Lázaro-Muñoz et al., 2010; Murray et al., 2015). However, these two systems can also operate in parallel (Balleine & Killcross, 2006). This parallel-processing theory is supported by double dissociations whereby lesions to the BLA result in decreased sensitivity to specific outcomes but lesions to the CeA result in general decreases in motivation or reward seeking (Corbit & Balleine, 2005; see also Everitt et al., 2003). Such dissociations suggest that the two subregions can operate independently rather than trading off in time. Human behavioral work has also provided some indirect evidence for such parallel processing, with demonstrations of dissociable, complementary components of classical conditioning, a form of learning that critically depends on the amygdala (Pool et al., 2019; S. Zhang et al., 2016). Pool et al. (2019) measured eye movements and pupil size during classical conditioning of a stimulus-outcome association. After the outcome was devalued, the pupil no longer dilated to the conditioned stimulus (indicating specificity and updated outcome value). Surprisingly, participants continued to gaze toward the expected location of the outcome. These findings suggest two parallel Pavlovian conditioning systems: one supporting “consummatory” responses by linking specific properties of a stimulus and salient outcome and one supporting more general “preparatory” or motivational orienting responses, which persist regardless of whether the outcome is desired. Although this behavioral evidence of parallel conditioning processes is intriguing, it is unclear whether or how these processes are instantiated in amygdala subregions. A possibility suggested by the MMSS framework is that these consummatory and preparatory forms of classical conditioning map onto the BLA and CeA, respectively.
There are also circumstances under which amygdala subsystems compete. A recent study examined the effect of BLA lesions in both humans and rodents on a threat-avoidance task (Terburg et al., 2018). BLA lesions decreased selection of a “rapid escape” behavior and facilitated autonomic reactions (human startle potential and rodent freezing) associated with CeA (Fadok et al., 2017; Li et al., 2013). Indeed, BLA acts on CeA in an inhibitory manner: During active escape, BLA inhibits CeA (thus reducing freezing), but when BLA is damaged or inactivated, the CeA representation dominates (see also Rosenkranz et al., 2006; Tye et al., 2011).
The presence of general and specific associations in the amygdala may enable flexibility. In real-world settings, outcome values are likely to fluctuate (e.g., shifting satiety levels change the desirability of food rewards). Thus, tracking stimulus-outcome contingencies through parallel systems (i.e., Pool et al., 2019) would enable flexible and efficient adaptation across states without having to reencode values as they subjectively fluctuate. However, in cases in which only one representation should ultimately serve to guide behavior (as in the case of freezing or avoiding; Terburg et al., 2018), these representations can inhibit one another to guide behavior.
Factors modulating use of subsystems
Above we provided examples of how colocating memory subsystems within a canonical memory-related brain region can promote adaptive interactions or parallel functions to support behavior. What are the circumstances under which a subsystem is preferentially used? A number of contextual factors may contribute, many of which overlap with the modulatory factors that have been considered in the traditional multiple-memory-systems framework (see “Traditional Taxonomy of Multiple Memory Systems: One Memory, One Brain Region”). For example, one modulatory factor may be time: In the hippocampus, episodic memories are formed after a single experience, but statistical regularities require integration across multiple experiences (e.g., Schapiro et al., 2017); in the striatum, goal-directed learning can occur quickly, but habits are formed gradually (e.g., Balleine & O’Doherty, 2010); and in the amygdala, memory for specific stimulus-outcome associations may drive behavior early in learning, but general approach behaviors come to be expressed over time (e.g., Murray et al., 2015). Another modulatory factor may be stress, which we consider in greater depth later (see “Implications of Multiple Memory Subsystems: Stress as a Case Study”). Here we consider neurobiological forms of modulation, that is, how prefrontal cortex and neurochemical signaling may control the expression of different memory subsystems.
Prefrontal cortical modulation
Hippocampus
Medial prefrontal cortex (mPFC) is a key candidate for arbitrating between hippocampal subsystems. Hippocampal–mPFC interactions have been shown to support both gradual, integrated memory representations in humans (Schlichting & Preston, 2015) and rapid, separated memory representations in rodents (Eichenbaum, 2017). Such interactions may play a specialized role during memory consolidation, which has been associated with the gradual transformation of episodic memories into more generalized, statistical, and semantic memories (McClelland et al., 1995) in both humans (Graves et al., 2022; Tompary & Davachi, 2017) and rodents (Richards et al., 2014).
Striatum
mPFC is also a candidate for modulating striatal subsystems, with rodent studies showing that separable mPFC subregions support the gradual formation of habits (Smith & Graybiel, 2013) versus the rapid learning of goal-directed associations (Hart et al., 2018; Killcross & Coutureau, 2003). Orbitofrontal cortex (OFC) also plays an important role in shifting between habits and goals (Turner et al., 2022), with OFC activation promoting DMS-dependent goal-directed behavior (Gremel & Costa, 2013).
Amygdala
Similar prefrontal regions (OFC and mPFC) have extensive connectivity with BLA: BLA–OFC interactions support stimulus-specific reward memories (Lichtenberg et al., 2017; Malvaez et al., 2019; Sias et al., 2021), and mPFC–BLA interactions regulate fear memory expression (Marek et al., 2013). However, mPFC does not seem to be directly connected to CeA (Arruda-Carvalho & Clem, 2015); instead, BLA–CeA circuits may themselves provide a mechanism for flexibly switching between general and specific representations based on task demands (J. Kim et al., 2017).
Neuromodulatory influences
Hippocampus
Acetylcholine may play an important role in arbitrating between hippocampal subsystems. High levels of acetylcholine are associated with facilitated inputs from EC into the hippocampus and reduced influence of CA3 on CA1 (for a review, see Hasselmo, 2006), suggesting that acetylcholine could bias the hippocampus toward MSP-dependent statistical learning. There is also indirect evidence for the role of acetylcholine in toggling between memory states in humans from studies that have capitalized on the fact that novelty is associated with acetylcholine release in the hippocampus (Giovannini et al., 2001). That is, participants are biased toward encoding when the preceding trial contains a novel item and toward retrieval when the preceding trial contains a familiar item (Duncan et al., 2012). Novelty also biases decisions away from the use of individual episodes and toward the use of incrementally learned associations (Duncan et al., 2019), consistent with our proposal that acetylcholine may promote statistical learning.
Striatum
Multiple neuromodulatory systems may mediate the switching between habits and goals. Dopamine transmission plays a role in the transition from goal-directed to habitual behavior, with dopamine down-regulation associated with reduced habit formation and dopamine up-regulation associated with faster habit formation (for a review, see Costa, 2007; Wickens et al., 2007). Such an effect may result from opposing effects of dopamine in DLS and DMS: Systemic exposure to a dopamine agonist (methamphetamine) promotes dendritic spine growth in DLS but reduces spines in DMS (Jedynak et al., 2007). The endocannabinoid system has also been implicated in the shift from goal-directed to habit behavior: Blocking endocannabinoid signaling (via mutations to endocannabinoid receptors, both systemically and locally in OFC-striatal circuits) results in reduced habit formation and a bias toward goal-directed behavior (Gremel et al., 2016; Hilário et al., 2007).
Amygdala
There are several neuromodulatory candidates for mediating the switching between specific and general representations in the amygdala. For example, dopamine modulates fear generalization within CeA, with higher dopamine levels associated with greater discriminability (less generalization; De Bundel et al., 2016; Jo et al., 2018). Noradrenaline is also associated with increased precision of memories in BLA (Bahtiyar et al., 2020), which may promote specific representations. However, although much work has examined the effect of neuromodulators within BLA or CeA independently, the MMSS framework highlights the need to investigate neuromodulatory mechanisms that mediate switching between these two subsystems and their representations.
Why colocate opposing subsystems?
Throughout this section, we have explored modulatory factors and behavioral consequences arising from colocating opposing subsystems. We propose that housing opposing memory representations within the same region may promote adaptive behavior. This is perhaps most evident in the hippocampus, in which the output subregion of the hippocampus to cortex (CA1) is part of both the MSP and TSP. CA1 may therefore serve as a computationally efficient “bottleneck,” ensuring that only one memory representation (episodic or statistical) is propagated forward to drive behavior rather than relying on a separate brain region to arbitrate between the two competing memory representations. Similar principles may also be at play in the striatum, with DMS and DLS inhibiting one another to regulate the expression of habits versus goals, and in the amygdala, with BLA–CeA circuits arbitrating between specific and general responses (perhaps because of CeA being the output region of the amygdala). Future work aimed at precisely targeting the nature of these interactions at both the behavioral and neural levels will be important for supporting these hypotheses.
Interactions Between Subsystems Across Regions
Thus far, we have outlined how the hippocampus, striatum, and amygdala each contain two subsystems supported by local circuitry and promoting distinct memories. Carefully examining these memory subsystems illuminates mnemonic features that are consistent across these three regions.
For example, episodic memories (TSP of hippocampus), goal-directed associations (DMS of striatum), and specific cue-outcome associations (BLA of amygdala) represent more specific associations. Episodic memories reflect the encoding of a single, unique experience; goal-directed associations reflect a link between a context- or goal-specific stimulus and an associated response/outcome; and specific conditioned associations reflect the link between a certain sensory cue and a given outcome. In this way, these representations may all be considered “separated” in that they are acquired rapidly and held distinct from other, related memories in order to avoid interference (hippocampus: Wanjia et al., 2021; DMS: Bradfield et al., 2013; amygdala: Sias et al., 2021). This separation enables the flexible use of such memories; for example, storing multiple, separated goal-directed associations allows for the appropriate selection of an action based on a given context or goal.
In contrast, statistical learning (MSP of the hippocampus), habits (DLS of the striatum), and general cue-outcome associations (CeA of amygdala) represent more general associations that require the gradual integration of related experiences across time. Statistical learning reflects the abstraction over many idiosyncratic instances in order to uncover generalizable patterns, habits reflect persistent expression of stimulus-evoked responses acquired over many repetitions, and general conditioned associations reflect the link between a stimulus and a broad motivational outcome (rather than a stimulus-specific response). Such integrated learning may give rise to more rigid representations that guide behavior automatically or in a context-insensitive manner.
Distinguishing along these axes highlights shared computations across subsystems from different brain regions. By labeling these commonalities, we aim to highlight representational similarities that can clarify existing findings and generate novel hypotheses rather than define a strict taxonomy. These also suggest key organizational principles, indicating broader categories of memory representations that may coordinate or be similarly modulated. In this section, we explore examples of how subsystems across regions coincide in the learning computations they recruit and the behaviors they support, and we discuss predictions arising from these commonalities.
Nature of interactions
Consistent with our proposal of similar computations across memory-related brain regions, there is evidence for overlap in the kinds of memory representations supported by subsystems housed in the hippocampus, striatum, and amygdala. Here we consider how similarities in mnemonic processes may give rise to shared function and facilitation across subsystems.
Hippocampus and striatum
Coordinated representations of flexible, specific, and distinct memories have been studied most thoroughly for the hippocampus and DMS. These are anatomically connected (Groenewegen et al., 1991) and jointly involved in spatial learning and memory (Goodroe et al., 2018; White, 2009), although they may play distinct roles (van der Meer et al., 2010). Hippocampal–DMS connectivity has been related to memory success (Delcasso et al., 2014; Ness et al., 2022; Nyberg et al., 2016) and spatial-navigation ability (Brown et al., 2012; DeCoteau et al., 2007) across species. One prediction arising from the MMSS framework is that TSP (DG and CA3) would be most similar to and interactive with DMS, supporting rapidly acquired, separated representations. Future work examining functional connectivity among subregions of the hippocampus and striatum could directly test these hypotheses. For example, when goal-directed or single-shot learning is required, this may promote coupling between DMS and hippocampal area CA3/DG. The MMSS framework also raises hypotheses about the other subsystems within these regions: For example, there may be more coupling between DLS and CA1 when expressing more rigid, habit-like memories.
Hippocampus and amygdala
Interactions between the hippocampus and the BLA subsystem in the amygdala also facilitate episodic (Inman et al., 2018) or episodic-like memory (Bass et al., 2014). Again, the MMSS framework predicts that the TSP hippocampal subsystem in particular would be most closely tied to BLA. Furthermore, given the overlap between the CeA and MSP in supporting generalized forms of memory, we hypothesize that these regions facilitate each other. This would be consistent with behavioral work that demonstrated that general (category-level) classical conditioning facilitates episodic memory generalization (Starita et al., 2019). After undergoing a threat-conditioning paradigm in which shocks were paired with exemplars of one category (e.g., animals) but not another (e.g., tools), participants showed greater generalization of episodic memories from the threat-associated category (i.e., memories for animal exemplars were less precise). This work suggests that the general association between shock and a semantic category (perhaps supported by CeA) may bias the hippocampus toward a more generalized memory-encoding strategy (perhaps supported by MSP).
Striatum and amygdala
DMS and BLA both support learning specific stimulus-response-outcome associations (Corbit et al., 2013; Fisher et al., 2020) and updating temporal expectations during classical conditioning (Dallérac et al., 2017). This pairing is consistent with our MMSS framework in that both DMS and BLA are argued to support rapidly acquired, separated representations. Coordination between the striatum and amygdala may extend to general, integrated subsystems as well, given that DLS and CeA work together to support habit formation (Lingawi & Balleine, 2012; Murray et al., 2015) and classical conditioning (J. S. Han et al., 1997).
Competitive interactions
Just as housing multiple memory subsystems within a given brain region can give rise to competition, similarities in mnemonic processes (combined with subtle computational differences) across regions may promote antagonistic interactions. For example, despite playing cooperative roles as described above, lesions to hippocampus and DMS can have opposite effects in reversal-learning paradigms. In a typical reversal-learning paradigm, rodents learn cue/reward associations; eventually, these are “reversed,” requiring the rodent to relearn which cue predicts reward. Note that performance is worse if reversal occurs in the same context as learning (compared with in a new environment). Lesions to the hippocampus block this context sensitivity (i.e., rodents perform equally well on the reversal task regardless of context), whereas DMS lesions exaggerate context sensitivity (even worse performance when reversal was encountered in the same environment as initial learning; McDonald et al., 2008), suggesting that these regions may support parallel representations when both are intact. However, evidence of direct competition is lacking, and further work is needed to assess how subsystems supporting similar mnemonic representations across regions might compete.
Factors modulating use of subsystems
A central tenet of MMSS is the observation that similar mnemonic processes can be housed in different structures. Although above we discussed many examples in which related subsystems may support similar behaviors, this raises questions about how and why they may support distinct aspects of behavior. Here, we consider how connectivity patterns and neuromodulatory influences may control the use (and facilitation) of computationally similar subsystems.
The connectivity patterns (and associated modality preferences) of a given brain region may bias the use of memory subsystems. For example, both the MSP (hippocampus) and DLS (striatum) support gradual learning of temporally predictable sequences. However, the MSP has been implicated in the passive extraction of stimulus regularities (Schapiro et al., 2016), whereas DLS has been implicated in the learning of motor sequences (Yin, 2010). The hippocampus receives input from many regions that process different sensory modalities (Turk-Browne, 2019) and thus may be well suited to represent visual or auditory sequences, whereas the striatum is highly connected to the motor system (Hintiryan et al., 2016) and thus may be well suited to represent action sequences. This raises the intriguing possibility that DLS and MSP may jointly enable the learning of visuomotor information, which may perhaps shed light on why the hippocampus is required for the consolidation of motor-sequence memories (Schapiro et al., 2019).
Neuromodulators may also play a role in broadly promoting particular mnemonic processes, facilitating cooperation among related subsystems. For example, the noradrenergic system is thought to promote the formation of specific, vivid memories (particularly under arousal), whereas the dopaminergic system is thought to promote more integrated memory representations (particularly when engaging in exploration or novelty seeking; Clewett & Murty, 2019). Although this model of neuromodulator function has largely been considered in the context of emotional memory, the MMSS framework presented here—which proposes the existence of similar mnemonic processes in different memory-related brain regions—makes novel and broader predictions about the roles of these neuromodulators in the formation of memory. We anticipate that the noradrenergic system will broadly facilitate subsystems across brain regions that are involved in rapidly acquired, specific associations (including episodic, goal-directed, and specific conditioning representations; see also Atucha et al., 2017; Bahtiyar et al., 2020). In contrast, we predict that the dopaminergic system will broadly facilitate subsystems involved in more integrated, generalized associations (statistical learning, habits, and general conditioning; see also Shohamy & Wagner, 2008).
Other neuromodulators may also arbitrate between the use of similar subsystems across regions. Although existing work has so far focused on memory systems rather than subsystems, this literature suggests intriguing neuromodulator candidates. For example, acetylcholine modulates switching between hippocampal place and striatal response strategies (Chang & Gold, 2003; Pych et al., 2005), suggesting a role in modulating competition between memory systems (see Gold, 2003). Drawing closer to the MMSS model, acetylcholine may modulate related memory subsystems housed in the amygdala and hippocampus. Specifically, hippocampal acetylcholine levels modulate the acquisition of hippocampal-dependent context conditioning versus amygdala-dependent tone conditioning in a dose-dependent manner (Calandreau et al., 2006). This suggests that acetylcholine may modulate competition between specific representations across the hippocampus and amygdala (i.e., specific context shock vs. specific tone shock). To the extent that acetylcholine facilitates the preferential expression of related memories supported by different brain regions, it may also arbitrate between memory subsystems housed in these distinct structures.
Why distribute related memory processes across brain regions?
In “Why Colocate Opposing Subsystems?” we considered why it may be adaptive to house opposing memory representations within the same brain region. Here, we have highlighted evidence for similar mnemonic processes across different brain regions. Rather than being fully redundant, we propose that similar computations across memory regions may function adaptively by supporting distinct aspects of behavior. For example, perhaps the hippocampus and DMS are both useful for spatial navigation because the hippocampus supports memory for the environment (e.g., a cognitive map) and DMS promotes the use of a specific goal-directed motor plan within that environment. On the other hand, overlapping computations across brain regions provide redundancy that may allow for compensation in the case of brain damage. Future work employing precise lesion techniques (i.e., to knock out specific subregions/circuits of these regions) is needed to adjudicate these hypotheses.
Implications of Multiple Memory Subsystems: Stress as a Case Study
In proposing the MMSS framework, our goal is to not only capture contemporary findings of memory organization in the brain but also to drive future research and help to account for some findings that traditional multiple-memory-systems theory fails to explain. Here, as a case study, we explore how the MMSS framework may guide future work on how stress affects different forms of memory.
The multiple-memory-systems framework has been highly influential in stress research. Rather than unilaterally enhancing or impairing all memories, stress is thought to modulate the relative engagement of memory systems (e.g., shifting from hippocampal-dependent to striatal-dependent memories; Goldfarb & Phelps, 2017; Schwabe et al., 2022). The MMSS framework proposed herein suggests novel perspectives on how stress alters memory. Specifically, we consider how the traditional multiple-memory-systems framework does not account for some aspects of how stress influences memory, and we consider how the MMSS framework may or may not account for such findings.
Hippocampus
Consistent with traditional multiple-memory-systems proposals that stress disrupts hippocampal-based memory processing (Goldfarb & Phelps, 2017), stress negatively affects the TSP subsystem in rodents (C. C. Chen et al., 2010; Y. Chen et al., 2010) and episodic-memory retrieval in humans (Gagnon & Wagner, 2016). However, our framework raises the possibility that the other subsystem (MSP) may not be affected in the same way. Indeed, rodent CA1 (MSP) is largely spared from negative effects of stress (Yamada et al., 2003) and may even be enhanced by stress (Karst & Joëls, 2005; Vandael et al., 2021). In humans, stress promotes “gist”-like memories with less specificity (e.g., Qin et al., 2012) and integration across related episodes (Bierbrauer et al., 2021) as well as detection of probability-based regularities associated with statistical learning (Tóth-Fáber et al., 2021). The MMSS framework both helps to reconcile these apparently contradictory stress-induced enhancements and impairments of hippocampal function and raises the novel possibility that other “general,” MSP-dependent hippocampal memory functions may be enhanced under stress.
Striatum
In contrast to opposing effects across hippocampal subsystems, emerging evidence indicates that stress may enhance both striatal subsystems. In rodents, administration of the stress-related glucocorticoid hormone into DLS (Siller-Pérez et al., 2017) enhances stimulus-response memory, consistent with human findings that stress facilitates a shift toward stimulus-response memory (Schwabe et al., 2022) and habit formation (i.e., higher affective stress associated with early adoption of habitual behavior; Pool et al., 2022). Intriguingly, there is preliminary evidence in rodents that administration of glucocorticoids into DMS also enhances memory associated with this subsystem (Lozano et al., 2013) and in humans that glucocorticoid responses are associated with enhanced DMS-related updating in a cognitive-flexibility task (Goldfarb et al., 2017). However, other recent work found that when pitting stimulus-response and goal-directed strategies against one another in the same task, stress reduced reliance on goal-directed behavior (Meier et al., 2022). The MMSS framework underscores the need to consider the differential effects of stress on behaviors associated with subsystems, calling for more work in this area.
Amygdala
It remains unclear how stress affects amygdala subsystems. Behaviorally, stress appears to promote general rather than specific representations. In rodents, systemic glucocorticoid agonists disrupt specific Pavlovian-instrumental transfer (Zorawski & Killcross, 2003). Systemic glucocorticoids and acute stressors also impair flexible updating of conditioned associations in humans (Merz et al., 2014; Raio et al., 2017) while promoting general transfer, or increased “wanting” (i.e., greater desire/increased effort to obtain a reward despite no increases in the hedonic value of the reward; Pool et al., 2015). Work across species demonstrates that stress and glucocorticoids promote overgeneralization of fear memories (dos Santos Corrêa et al., 2019; Dunsmoor et al., 2017). However, this behavioral evidence that stress biases the amygdala toward general representations needs neurobiological validation. Rodent research has yielded mixed findings, with evidence that stress both facilitates BLA (Roozendaal et al., 2009) and impairs BLA structure and function, with effects varying according to stressor type and BLA neuron-connectivity profiles (W.-H. Zhang et al., 2021). Furthermore, effects of stress on CeA are understudied (Roozendaal & McGaugh, 1997). Although there is some evidence that glucocorticoid administration in CeA leads to the enhanced retention of fear memories (B. L. Thompson et al., 2004), whether these memories are general or specific remains unclear. Finally, it is unclear how stress-induced BLA enhancements map onto behavioral expressions of memory. Here, too, the MMSS framework indicates novel testable hypotheses regarding stress effects on these representations.
Summary
Together, the MMSS framework may help to account for distinct effects of stress across memory subsystems within a canonical memory-related brain region. Indeed, claiming that stress impairs an entire brain region (e.g., “hippocampal memory”) may be misleading. The MMSS theory suggests the intriguing possibility that stress enhances general representations across brain structures (a proposal that would not have arisen from the traditional multiple-memory-systems framework), although more work is needed to determine whether this occurs in the striatum (for which there is some evidence indicating that both subsystems are enhanced) and how the neural correlates of stress actions on the amygdala map onto behavioral observations of more generalized conditioned associations. This framework also provides a roadmap for future work to gain a comprehensive understanding of how stress adaptively alters memory. For example, the majority of studies examining the effects of stress on multiple memory systems have designed tasks to pit episodic/cognitive map-dependent behavior (hippocampus) and stimulus-response-dependent behavior (striatum) against one another. However, to understand how stress influences memory subsystems, it will be important for future work to design tasks that pit the dual mnemonic functions of a given brain region against each other (i.e., examining whether stress promotes the learning of statistical regularities over episodic memories or the formation of general over specific conditioned associations).
Avenues Forward and Concluding Remarks
In this review, we present comprehensive behavioral and biological evidence for a novel framework of multiple memory subsystems, or MMSS. We identified that three traditional memory-related structures in the brain—the hippocampus, striatum, and amygdala—all support at least two distinct and seemingly incompatible mnemonic representations. Our goal in putting forth this framework is to (a) account for existing findings that cannot be accounted for by traditional multiple-memory-systems theories and (b) present novel avenues for future research. Here, we discuss several additional areas in which we think the MMSS framework holds promise while identifying gaps to be filled by future research.
Assessing the strengths and weaknesses of evidence
In developing this framework, we integrated findings across nonhuman animal, human, and computational research (Table 1). Although synthesizing across multiple lines of evidence helped to build a comprehensive theory, we also identified gaps for future research across species. For example, whereas animal work allows for high resolution to measure and manipulate subsystems, human work is better suited to probe behavioral correlates of different subsystems. To test the predictions of the MMSS framework, there is a need to combine neural precision with sophisticated task design and behavioral phenotyping. For example, in nonhuman-animal models, the quality of memory representations supported by distinct hippocampal subsystems are either understudied (e.g., statistical learning) or difficult to compare with human processes (e.g., recollection of episodic memories). In humans, on the other hand, many neuroimaging studies do not have the (anatomical and/or functional) resolution to distinguish hippocampal subfields and thus may be insensitive to subsystem representations. That said, differences in representation along the longitudinal axis of the hippocampus may be informative (Poppenk et al., 2013). Specifically, the distribution of hippocampal subfields differs across the longitudinal axis such that MSP is overrepresented in anterior hippocampus and the TSP is overrepresented in posterior hippocampus (Malykhin et al., 2010; Schapiro et al., 2017). This proposed distinction converges with findings that anterior hippocampal activation relates to statistical learning (Ellis et al., 2021; Turk-Browne et al., 2010) and that representational differentiation in posterior hippocampus predicts episodic memory (Callaghan et al., 2021).
Table 1.
Key Evidence (Across Species) for the Primary Claims of the MMSS Framework
| Claim | Region | Evidence strength | Primary evidence | Key citations |
|---|---|---|---|---|
| The same memory structure can support opposing computations. | Hippocampus | *** | CA3 (TSP) supports a pattern-separated code. CA1 (MSP) supports an integrated code. |
Lee et al. (2004)
a
Molitor et al. (2021) b |
| Striatum | *** | DLS activity marks “boundaries” between action sequences. DMS activity ramps up until decision is executed. |
Thorn et al. (2010)
a
Vandaele et al. (2021) a |
|
| Amygdala | ** | BLA facilitates learning of stimulus-outcome links. CeA facilitates motivational responses/behavioral output. |
Sias et al. (2021)
a
Fadok et al. (2017) a |
|
| The same memory structure can support both “specific” and “general” memory representations. | Hippocampus | *** | TSP can support episodic memory (specific). MSP can support statistical learning (general). |
Schlichting et al. (2014)
b
Schapiro et al. (2017) c Schlichting et al. (2021) b |
| Striatum | ** | DMS can support goal-directed behavior (specific). DLS can support habits (general). |
McNamee et al. (2015)
b
Turner et al. (2022) a |
|
| Amygdala | * | BLA supports specific Pavlovian-instrumental transfer. CeA supports general Pavlovian-instrumental transfer. |
Corbit & Balleine (2005)
a
Prévost et al. (2012) b |
|
| Similar mnemonic computations are supported by distinct structures. | Hippocampus & Striatum | *** | Hippocampus (subregion unspecified) and DMS are jointly involved in learning specific associations in context of spatial learning. |
DeCoteau et al. (2007)
a
Brown et al. (2012) b |
| Hippocampus & Amygdala | *** | Hippocampus (subregion unspecified) and BLA facilitate specific associations in context of episodic/episodic-like memory. |
Bass et al. (2014)
a
Inman et al. (2018) b |
|
| Striatum & Amygdala | *** | BLA and DMS support learning specific stimulus-response-outcome associations. CeA and DLS support formation of general habit memories. |
Corbit et al. (2013)
a
Lingawi & Balleine (2012) a |
Note: CA3 = cornu ammonis 3; TSP = trisynaptic pathway; CA1 = cornu ammonis 1; MSP = monosynaptic pathway; DLS = dorsolateral striatum; DMS = dorsomedial striatum; BLA = basolateral amygdala; CeA = central amygdala.
Evidence type is rodent.
Evidence type is human.
Evidence type is neural-network model.
Number of asterisks indicate relative strength of evidence (e.g., *** indicates strong evidence).
Combining across human and animal work is also necessary to derive a comprehensive understanding of how subsystems interact and ultimately give rise to behavior. Although animal work is well suited to deeply characterize individual subsystems or regions and neuromodulatory factors (perhaps enabling work on how subsystems within a region interact), human whole-brain imaging enables the interrogation of multiple subsystems at once (perhaps enabling work on how subsystems across regions interact). Neural-network models of memory regions that incorporate subsystem representations may also be critical for advancing the MMSS theory: Such models enable the selective lesioning of one subsystem (as has been done in the hippocampus; Schapiro et al., 2017; Sucevic & Schapiro, 2022), which may help delineate the circumstances under which a given subsystem representation will dominate and yield precise, testable, empirical hypotheses.
Fortifying the MMSS framework
The MMSS framework was inspired by key observations that the hippocampus (e.g., Schapiro et al., 2017), striatum (e.g., Balleine & O’Doherty, 2010), and amygdala (e.g., Balleine & Killcross, 2006) each have opposing memory representations across subregions/circuits within these brain regions (see also Table 1). However, relative to the vast literature on these memory-related regions, there is relatively little work on their subsystems, requiring integration across disparate studies and fields designed to study a given subregion in isolation. A goal of our framework is to inspire future, targeted investigations of subsystems that will not only provide important tests of our framework but also have the potential to uncover novel, unexpected empirical findings.
There are open questions about the neurobiological and contextual tenets that govern the use of memory subsystems (also see “Implications of Multiple Memory Subsystems: Stress as a Case Study”). Such questions will benefit from more carefully considering the behavior—rather than merely the brain region—that a particular task solicits and designing tasks suited to isolate a given subsystem(s). Further studies investigating multiple subsystems in tandem (as in Sherman & Turk-Browne, 2020) will be especially important for validating the MMSS model, particularly in cases in which the evidence is limited, such as the amygdala.
Finally, although we have focused on the hippocampus, striatum, and amygdala, it is possible that the principles identified here may apply to other memory-related regions. For example, the cerebellum has been implicated in some forms of conditioning, such as eyeblink conditioning, in which an auditory or visual cue becomes associated with a puff of air to the eye, thus leading to a conditioned eyeblink response. Although more work is needed to understand whether the cerebellum supports multiple distinct types of conditioning, there are indications of functional dissociations within the cerebellum supporting distinct aspects of conditioning behavior (Medina et al., 2002; R. Thompson & Steinmetz, 2009). For example, separable subregions (cerebellar cortex vs. deep cerebellar nuclei) may support the initial learning versus storage of conditioned responses (Medina et al., 2002). Intriguingly, there is evidence that cerebellar nuclei continue to represent conditioned associations even after extinction (Medina et al., 2001), perhaps paralleling behavioral evidence for outcome-insensitive conditioned responses (Pool et al., 2019).
Promise of MMSS as a unifying theory of memory organization
In further validating MMSS, it is possible that the framework will provide a means of clarifying what might seem to be inconsistent findings in the literature. For example, some studies have found that greater hippocampal pattern similarity relates to better episodic memory (see Brunec et al., 2020), contrary to the view that pattern separation supports episodic memory. However, many of these tasks probed only episodic memory; it is possible that increased pattern similarity reflects increased memory integration or statistical learning, processes that were not directly measured in behavior. Future work more carefully tailored to isolate the behaviors associated with given subsystems will clarify the link between neural representations and behavioral outcomes.
By observing that there may be common computations across subsystems housed in different regions (see “Interactions Between Subsystems Across Regions”), our framework also promotes the discovery of interventions that act similarly across these regions. As one example, prediction errors (or encountering something that deviates from what you expected) appear to act on these computations in a way that extends across brain regions. Prediction error may support both specific and general representations. On the one hand, prediction error can support separation and maintenance of specific representations, in part by shielding memories from interference. In the TSP of the hippocampus, prediction errors promote differentiation of memories (G. Kim et al., 2017); in the DMS of the striatum, prediction errors shield previously learned information from updated knowledge (Bradfield et al., 2013); and in classical conditioning (presumably supported by the amygdala), prediction error is thought to separate memories for conditioning versus extinction (thus making fear memories return even after extinction; Gershman et al., 2013). On the other hand, prediction error can also support the gradual learning of integrated representations via the incremental updating of predictions. This process may contribute to statistical learning (Sherman & Turk-Browne, 2020; Turk-Browne et al., 2010), habit memory (Drummond & Niv, 2020), and general cue–outcome associations (Rescorla, 1972).
In other words, prediction error may play distinct roles in facilitating computations underlying separated versus integrated representations and appears to do so in a way that transcends canonical memory-related brain regions. This example holds promise not only for understanding shared computations across similar subsystems housed in different regions but also for understanding potential mechanisms that govern the use of opposing subsystems within a region. Although untested, one possibility is that when the errors are large and deviate from existing knowledge, a separated representation is rapidly created; in contrast, smaller errors may trigger incremental updating of gradually formed, integrated representations.
In addition to accounting for empirical data and generating novel theoretical hypotheses, the MMSS framework also highlights two notable yet underexplored patterns in the organization of memory in the brain: The colocation of competing memory representations within a given brain region and the overlap in mnemonic computations supported by subsystems housed in different brain regions. Given that the traditional multiple-memory-systems framework highlights the adaptive role of having discrete, anatomically distinct memory systems, the evidence for colocated competition and distal overlap presented here may be puzzling. We argue that both of these organizational principles are adaptive: First, housing competing memory representations within a brain region allows for self-regulation of memory-guided behavior, for example, allowing the goal-directed subsystem to suppress habit memory under disadvantageous circumstances. Second, overlapping computations across canonical memory regions may serve complementary functions. With specific connectivity patterns and computational properties, certain regions may be better suited to support distinct dimensions of memory-guided behavior, and some degree of redundancy across brain regions may be a critical mechanism for shielding complex memories.
Footnotes
ORCID iDs: Brynn E. Sherman  https://orcid.org/0000-0002-5071-7101
https://orcid.org/0000-0002-5071-7101
Nicholas B. Turk-Browne  https://orcid.org/0000-0001-7519-3001
https://orcid.org/0000-0001-7519-3001
Transparency
Action Editor: Tim Pleskac
Editor: Interim Editorial Panel
The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This article was supported by a National Science Foundation Graduate Research Fellowship (B. E. Sherman), the Canadian Institute for Advanced Research (N. B. Turk-Browne), and National Institutes of Health Grants R01 MH069456 (N. B. Turk-Browne), K01 AA027832 (E. V. Goldfarb), and R21 MH128740 (E. V. Goldfarb).
References
- Amaral D. G. (1993). Emerging principles of intrinsic hippocampal organization. Current Opinion in Neurobiology, 3(2), 225–229. [DOI] [PubMed] [Google Scholar]
- Arruda-Carvalho M., Clem R. L. (2015). Prefrontal-amygdala fear networks come into focus. Frontiers in Systems Neuroscience, 9, Article 145. 10.3389/fnsys.2015.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atucha E., Vukojevic V., Fornari R. V., Ronzoni G., Demougin P., Peter F., Atsak P., Coolen M. W., Papassotiropoulos A., McGaugh J. L., de Quervain D. J., Roozendaal B. (2017). Noradrenergic activation of the basolateral amygdala maintains hippocampus-dependent accuracy of remote memory. Proceedings of the National Academy of Sciences, USA, 114(34), 9176–9181. 10.1073/pnas.1710819114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahtiyar S., Karaca K. G., Henckens M. J., Roozendaal B. (2020). Norepinephrine and glucocorticoid effects on the brain mechanisms underlying memory accuracy and generalization. Molecular and Cellular Neuroscience, 108, Article 103537. 10.1016/j.mcn.2020.103537 [DOI] [PubMed] [Google Scholar]
- Bakker A., Kirwan C. B., Miller M., Stark C. E. (2008). Pattern separation in the human hippocampal CA3 and dentate gyrus. Science, 319(5870), 1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B. W. (2019). The meaning of behavior: Discriminating reflex and volition in the brain. Neuron, 104(1), 47–62. [DOI] [PubMed] [Google Scholar]
- Balleine B. W., Dezfouli A. (2019). Hierarchical action control: Adaptive collaboration between actions and habits. Frontiers in Psychology, 10, Article 2735. 10.3389/fpsyg.2019.02735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B. W., Killcross S. (2006). Parallel incentive processing: An integrated view of amygdala function. Trends in Neurosciences, 29(5), 272–279. [DOI] [PubMed] [Google Scholar]
- Balleine B. W., O’Doherty J. P. (2010). Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology, 35(1), 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. I., Nizam Z. G., Partain K. N., Wang A., Manns J. R. (2014). Amygdala-mediated enhancement of memory for specific events depends on the hippocampus. Neurobiology of Learning and Memory, 107, 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biderman N., Bakkour A., Shohamy D. (2020). What are memories for? The hippocampus bridges past experience with future decisions. Trends in Cognitive Sciences, 24(7), 542–556. [DOI] [PubMed] [Google Scholar]
- Bierbrauer A., Fellner M.-C., Heinen R., Wolf O. T., Axmacher N. (2021). The memory trace of a stressful episode. Current Biology, 31(23), 5204–5213. [DOI] [PubMed] [Google Scholar]
- Bornstein A. M., Khaw M. W., Shohamy D., Daw N. D. (2017). Reminders of past choices bias decisions for reward in humans. Nature Communications, 8(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton M. E. (2021). Context, attention, and the switch between habit and goal-direction in behavior. Learning & Behavior, 49(4), 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield L. A., Bertran-Gonzalez J., Chieng B., Balleine B. W. (2013). The thalamostriatal pathway and cholinergic control of goal-directed action: Interlacing new with existing learning in the striatum. Neuron, 79(1), 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. I., Ross R. S., Tobyne S. M., Stern C. E. (2012). Cooperative interactions between hippocampal and striatal systems support flexible navigation. NeuroImage, 60(2), 1316–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunec I. K., Robin J., Olsen R. K., Moscovitch M., Barense M. D. (2020). Integration and differentiation of hippocampal memory traces. Neuroscience & Biobehavioral Reviews, 118, 196–208. [DOI] [PubMed] [Google Scholar]
- Calandreau L., Trifilieff P., Mons N., Costes L., Marien M., Marighetto A., Micheau J., Jaffard R., Desmedt A. (2006). Extracellular hippocampal acetylcholine level controls amygdala function and promotes adaptive conditioned emotional response. The Journal of Neuroscience, 26(52), 13556–13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B., Gasser C., Silvers J. A., VanTieghem M., Choy T., O’Sullivan K., Tompary A., Davachi L., Tottenham N. (2021). Age-related increases in posterior hippocampal granularity are associated with remote detailed episodic memory in development. The Journal of Neuroscience, 41(8), 1738–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni E., Balleine B., Baldassarre G. (2016). Appetitive Pavlovian-instrumental transfer: A review. Neuroscience & Biobehavioral Reviews, 71, 829–848. [DOI] [PubMed] [Google Scholar]
- Chanales A. J., Oza A., Favila S. E., Kuhl B. A. (2017). Overlap among spatial memories triggers repulsion of hippocampal representations. Current Biology, 27(15), 2307–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Gold P. E. (2003). Switching memory systems during learning: Changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. The Journal of Neuroscience, 23(7), 3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-C., Yang C.-H., Huang C.-C., Hsu K.-S. (2010). Acute stress impairs hippocampal mossy fiber-CA3 long-term potentiation by enhancing cAMP-specific phosphodiesterase 4 activity. Neuropsychopharmacology, 35(7), 1605–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Rex C. S., Rice C. J., Dubé C. M., Gall C. M., Lynch G., Baram T. Z. (2010). Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proceedings of the National Academy of Sciences, USA, 107(29), 13123–13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S., Herry C., Grenier F., Wolff S. B., Letzkus J. J., Vlachos I., Ehrlich I., Sprengel R., Deisseroth K., Stadler M. B., Müller C., Lüthi A. (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature, 468(7321), 277–282. 10.1038/nature09559 [DOI] [PubMed] [Google Scholar]
- Clark R. E., Squire L. R. (1998). Classical conditioning and brain systems: The role of awareness. Science, 280(5360), 77–81. [DOI] [PubMed] [Google Scholar]
- Clewett D., Murty V. P. (2019). Echoes of emotions past: How neuromodulators determine what we recollect. eNeuro, 6(2), Article ENEURO.0108-18.2019. 10.1523/ENEURO.0108-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit L. H., Balleine B. W. (2005). Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. The Journal of Neuroscience, 25(4), 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit L. H., Leung B. K., Balleine B. W. (2013). The role of the amygdala-striatal pathway in the acquisition and performance of goal-directed instrumental actions. The Journal of Neuroscience, 33(45), 17682–17690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit L. H., Nie H., Janak P. H. (2012). Habitual alcohol seeking: Time course and the contribution of subregions of the dorsal striatum. Biological Psychiatry, 72(5), 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. M. (2007). Plastic corticostriatal circuits for action learning: What’s dopamine got to do with it? Annals of the New York Academy of Sciences, 1104, 172–191. 10.1196/annals.1390.015 [DOI] [PubMed] [Google Scholar]
- Covington N. V., Brown-Schmidt S., Duff M. C. (2018). The necessity of the hippocampus for statistical learning. Journal of Cognitive Neuroscience, 30(5), 680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman F., Morris A. (2015). Habitual control of goal selection in humans. Proceedings of the National Academy of Sciences, USA, 112(45), 13817–13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallérac G., Graupner M., Knippenberg J., Martinez R. C. R., Tavares T. F., Tallot L., El Massioui N., Verschueren A., Höhn S., Bertolus J. B., Reyes A., LeDoux J. E., Schafe G. E., Diaz-Mataix L., Doyère V. (2017). Updating temporal expectancy of an aversive event engages striatal plasticity under amygdala control. Nature Communications, 8, Article 13920. 10.1038/ncomms13920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bundel D., Zussy C., Espallergues J., Gerfen C. R., Girault J.-A., Valjent E. (2016). Dopamine D2 receptors gate generalization of conditioned threat responses through mtorc1 signaling in the extended amygdala. Molecular Psychiatry, 21(11), 1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoteau W. E., Thorn C., Gibson D. J., Courtemanche R., Mitra P., Kubota Y., Graybiel A. M. (2007). Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proceedings of the National Academy of Sciences, USA, 104(13), 5644–5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcasso S., Huh N., Byeon J. S., Lee J., Jung M. W., Lee I. (2014). Functional relationships between the hippocampus and dorsomedial striatum in learning a visual scene-based memory task in rats. The Journal of Neuroscience, 34(47), 15534–15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit S., Kindt M., Knot S. L., Verhoeven A. A., Robbins T. W., Gasull-Camos J., Evans M., Mirza H., Gillan C. M. (2018). Shifting the balance between goals and habits: Five failures in experimental habit induction. Journal of Experimental Psychology: General, 147(7), 1043–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. (1985). Actions and habits: The development of behavioural autonomy. Philosophical Transactions of the Royal Society B: Biological Sciences, 308(1135), 67–78. [Google Scholar]
- Dimsdale-Zucker H. R., Ritchey M., Ekstrom A. D., Yonelinas A. P., Ranganath C. (2018). CA1 and CA3 differentially support spontaneous retrieval of episodic contexts within human hippocampal subfields. Nature Communications, 9(1), Article 294. 10.1038/s41467-017-02752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos Corrêa M., dos Santos Vaz B., Grisanti G. D. V., de Paiva J. P. Q., Tiba P. A., Fornari R. V. (2019). Relationship between footshock intensity, post-training corticosterone release and contextual fear memory specificity over time. Psychoneuroendocrinology, 110, Article 104447. 10.1016/j.psyneuen.2019.104447 [DOI] [PubMed] [Google Scholar]
- Douglass A. M., Kucukdereli H., Ponserre M., Markovic M., Gründemann J., Strobel C., Morales P. L. A., Conzelmann K.-K., Lüthi A., Klein R. (2017). Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nature Neuroscience, 20(10), 1384–1394. [DOI] [PubMed] [Google Scholar]
- Drummond N., Niv Y. (2020). Model-based decision making and model-free learning. Current Biology, 30(15), R860–R865. [DOI] [PubMed] [Google Scholar]
- Duncan K., Sadanand A., Davachi L. (2012). Memory’s penumbra: Episodic memory decisions induce lingering mnemonic biases. Science, 337(6093), 485–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K., Semmler A., Shohamy D. (2019). Modulating the use of multiple memory systems in value-based decisions with contextual novelty. Journal of Cognitive Neuroscience, 31(10), 1455–1467. [DOI] [PubMed] [Google Scholar]
- Dunsmoor J. E., Otto A. R., Phelps E. A. (2017). Stress promotes generalization of older but not recent threat memories. Proceedings of the National Academy of Sciences, USA, 114(34), 9218–9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. (2017). Prefrontal–hippocampal interactions in episodic memory. Nature Reviews Neuroscience, 18(9), 547–558. [DOI] [PubMed] [Google Scholar]
- Ellis C. T., Skalaban L. J., Yates T. S., Bejjanki V. R., Córdova N. I., Turk-Browne N. B. (2021). Evidence of hippocampal learning in human infants. Current Biology, 31(15), 3358–3364. [DOI] [PubMed] [Google Scholar]
- Endress A. D., Johnson S. P. (2021). When forgetting fosters learning: A neural network model for statistical learning. Cognition, 213, Article 104621. 10.1016/j.cognition.2021.104621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B. J., Cardinal R. N., Parkinson J. A., Robbins T. W. (2003). Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Annals of the New York Academy of Sciences, 985(1), 233–250. [PubMed] [Google Scholar]
- Fadok J. P., Krabbe S., Markovic M., Courtin J., Xu C., Massi L., Botta P., Bylund K., Müller C., Kovacevic A., Tovote P., Lüthi A. (2017). A competitive inhibitory circuit for selection of active and passive fear responses. Nature, 542(7639), 96–100. 10.1038/nature21047 [DOI] [PubMed] [Google Scholar]
- Fadok J. P., Markovic M., Tovote P., Lüthi A. (2018). New perspectives on central amygdala function. Current Opinion in Neurobiology, 49, 141–147. [DOI] [PubMed] [Google Scholar]
- Fisher S. D., Ferguson L. A., Bertran-Gonzalez J., Balleine B. W. (2020). Amygdala-cortical control of striatal plasticity drives the acquisition of goal-directed action. Current Biology, 30(22), 4541–4546. [DOI] [PubMed] [Google Scholar]
- Foerde K., Race E., Verfaellie M., Shohamy D. (2013). A role for the medial temporal lobe in feedback-driven learning: Evidence from amnesia. The Journal of Neuroscience, 33(13), 5698–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon S. A., Wagner A. D. (2016). Acute stress and episodic memory retrieval: Neurobiological mechanisms and behavioral consequences. Annals of the New York Academy of Sciences, 1369(1), 55–75. [DOI] [PubMed] [Google Scholar]
- Gardner R. S., Gold P. E., Korol D. L. (2020). Inactivation of the striatum in aged rats rescues their ability to learn a hippocampus-sensitive spatial navigation task. Neurobiology of Learning and Memory, 172, Article 107231. 10.1016/j.nlm.2020.107231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman S. J., Jones C. E., Norman K. A., Monfils M.-H., Niv Y. (2013). Gradual extinction prevents the return of fear: Implications for the discovery of state. Frontiers in Behavioral Neuroscience, 7, Article 164. 10.3389/fnbeh.2013.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin N. W., Herman M. A., Roberto M. (2015). The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biological Psychiatry, 77(10), 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini M., Rakovska A., Benton R., Pazzagli M., Bianchi L., Pepeu G. (2001). Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience, 106(1), 43–53. [DOI] [PubMed] [Google Scholar]
- Gold P. E. (2003). Acetylcholine modulation of neural systems involved in learning and memory. Neurobiology of Learning and Memory, 80(3), 194–210. [DOI] [PubMed] [Google Scholar]
- Goldfarb E. V., Froböse M. I., Cools R., Phelps E. A. (2017). Stress and cognitive flexibility: Cortisol increases are associated with enhanced updating but impaired switching. Journal of Cognitive Neuroscience, 29(1), 14–24. [DOI] [PubMed] [Google Scholar]
- Goldfarb E. V., Phelps E. A. (2017). Stress and the trade-off between hippocampal and striatal memory. Current Opinion in Behavioral Sciences, 14, 47–53. [Google Scholar]
- Goodroe S. C., Starnes J., Brown T. I. (2018). The complex nature of hippocampal-striatal interactions in spatial navigation. Frontiers in Human Neuroscience, 12, Article 250. 10.3389/fnhum.2018.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves K. N., Sherman B. E., Huberdeau D., Damisah E., Quraishi I. H., Turk-Browne N. B. (2022). Remembering the pattern: A longitudinal case study on statistical learning in spatial navigation and memory consolidation. Neuropsychologia, 174, Article 108341. 10.1016/j.neuropsychologia.2022.108341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel C. M., Chancey J. H., Atwood B. K., Luo G., Neve R., Ramakrishnan C., Deisseroth K., Lovinger D. M., Costa R. M. (2016). Endocannabinoid modulation of orbitostriatal circuits gates habit formation. Neuron, 90(6), 1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel C. M., Costa R. M. (2013). Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nature Communications, 4, Article 2264. 10.1038/ncomms3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen H. J., Berendse H. W., Wolters J. G., Lohman A. H. (1991). The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: Evidence for a parallel organization. Progress in Brain Research, 85, 95–118. [DOI] [PubMed] [Google Scholar]
- Grospe G. M., Baker P. M., Ragozzino M. E. (2018). Cognitive flexibility deficits following 6-OHDA lesions of the rat dorsomedial striatum. Neuroscience, 374, 80–90. [DOI] [PubMed] [Google Scholar]
- Haith A. M., Krakauer J. W. (2018). The multiple effects of practice: Skill, habit and reduced cognitive load. Current Opinion in Behavioral Sciences, 20, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.-S., McMahan R. W., Holland P., Gallagher M. (1997). The role of an amygdalo-nigrostriatal pathway in associative learning. The Journal of Neuroscience, 17(10), 3913–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W., Tellez L. A., Rangel M. J., Jr., Motta S. C., Zhang X., Perez I. O., Canteras N. S., Shammah-Lagnado S. J., van den Pol A. N., de Araujo I. E. (2017). Integrated control of predatory hunting by the central nucleus of the amygdala. Cell, 168(1–2), 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick R. M., Forrence A. D., Krakauer J. W., Haith A. M. (2019). Time-dependent competition between goal-directed and habitual response preparation. Nature Human Behaviour, 3(12), 1252–1262. [DOI] [PubMed] [Google Scholar]
- Hart G., Bradfield L. A., Fok S. Y., Chieng B., Balleine B. W. (2018). The bilateral prefronto-striatal pathway is necessary for learning new goal-directed actions. Current Biology, 28(14), 2218–2229. [DOI] [PubMed] [Google Scholar]
- Hasselmo M. E. (2006). The role of acetylcholine in learning and memory. Current Opinion in Neurobiology, 16(6), 710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henin S., Turk-Browne N. B., Friedman D., Liu A., Dugan P., Flinker A., Doyle W., Devinsky O., Melloni L. (2021). Learning hierarchical sequence representations across human cortex and hippocampus. Science Advances, 7(8), Article eabe4530. 10.1126/sciadv.abc4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K. (2010). A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience, 11(7), 523–532. [DOI] [PubMed] [Google Scholar]
- Hilário M. R., Clouse E., Yin H. H., Costa R. M. (2007). Endocannabinoid signaling is critical for habit formation. Frontiers in Integrative Neuroscience, 1, Article 6. 10.3389/neuro.07.006.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintiryan H., Foster N. N., Bowman I., Bay M., Song M. Y., Gou L., Yamashita S., Bienkowski M. S., Zingg B., Zhu M., Yang X. W., Shih J. C., Toga A. W., Dong H. W. (2016). The mouse cortico-striatal projectome. Nature Neuroscience, 19(8), 1100–1114. 10.1038/nn.4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. C., Gallagher M. (1999). Amygdala circuitry in attentional and representational processes. Trends in Cognitive Sciences, 3(2), 65–73. [DOI] [PubMed] [Google Scholar]
- Inman C. S., Manns J. R., Bijanki K. R., Bass D. I., Hamann S., Drane D. L., Fasano R. E., Kovach C. K., Gross R. E., Willie J. T. (2018). Direct electrical stimulation of the amygdala enhances declarative memory in humans. Proceedings of the National Academy of Sciences, USA, 115(1), 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak J. P., Uslaner J. M., Esteban J. A., Robinson T. E. (2007). Methamphetamine-induced structural plasticity in the dorsal striatum. European Journal of Neuroscience, 25(3), 847–853. [DOI] [PubMed] [Google Scholar]
- Jo Y. S., Heymann G., Zweifel L. S. (2018). Dopamine neurons reflect the uncertainty in fear generalization. Neuron, 100(4), 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog M. S., Kubota Y., Connolly C. I., Hillegaart V., Graybiel A. M. (1999). Building neural representations of habits. Science, 286(5445), 1745–1749. [DOI] [PubMed] [Google Scholar]
- Karst H., Joëls M. (2005). Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. Journal of Neurophysiology, 94(5), 3479–3486. [DOI] [PubMed] [Google Scholar]
- Killcross S., Coutureau E. (2003). Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral Cortex, 13(4), 400–408. [DOI] [PubMed] [Google Scholar]
- Kim G., Norman K. A., Turk-Browne N. B. (2017). Neural differentiation of incorrectly predicted memories. The Journal of Neuroscience, 37(8), 2022–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Zhang X., Muralidhar S., LeBlanc S. A., Tonegawa S. (2017). Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron, 93(6), 1464–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton B. J., Mangels J. A., Squire L. R. (1996). A neostriatal habit learning system in humans. Science, 273(5280), 1399–1402. [DOI] [PubMed] [Google Scholar]
- Kyriazi P., Headley D. B., Pare D. (2018). Multi-dimensional coding by basolateral amygdala neurons. Neuron, 99(6), 1315–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K. S., LeDoux J. E., Spencer D. D., Phelps E. A. (1995). Impaired fear conditioning following unilateral temporal lobectomy in humans. The Journal of Neuroscience, 15(10), 6846–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley K. (1950). In search of the engram. In Society of Experimental Biology Symposium, No. 4: Psychological Mechanisms in Animal Behavior (pp. 454–455, 468–473, 477–480). Cambridge University Press. [Google Scholar]
- Lázaro-Muñoz G., LeDoux J. E., Cain C. K. (2010). Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated pavlovian processes. Biological Psychiatry, 67(12), 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. E. (2000). The amygdala and emotion: A view through fear. In Aggleton J. P. (Ed.), The amygdala: A functional analysis (pp. 289–310). Oxford University Press. [Google Scholar]
- LeDoux J. E., Iwata J., Cicchetti P., Reis D. J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience, 8(7), 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., Kesner R. P. (2002). Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nature Neuroscience, 5(2), 162–168. [DOI] [PubMed] [Google Scholar]
- Lee I., Rao G., Knierim J. J. (2004). A double dissociation between hippocampal subfields: Differential time course of CA3 and CA1 place cells for processing changed environments. Neuron, 42(5), 803–815. [DOI] [PubMed] [Google Scholar]
- Leutgeb J. K., Leutgeb S., Moser M.-B., Moser E. I. (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315(5814), 961–966. [DOI] [PubMed] [Google Scholar]
- Lhost J., More S., Watabe I., Louber D., Ouagazzal A.-M., Liberge M., Amalric M. (2021). Interplay between inhibitory control and behavioural flexibility: Impact of dorsomedial striatal dopamine denervation in mice. Neuroscience, 477, 25–39. [DOI] [PubMed] [Google Scholar]
- Li H., Penzo M. A., Taniguchi H., Kopec C. D., Huang Z. J., Li B. (2013). Experience-dependent modification of a central amygdala fear circuit. Nature Neuroscience, 16(3), 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg N. T., Pennington Z. T., Holley S. M., Greenfield V. Y., Cepeda C., Levine M. S., Wassum K. M. (2017). Basolateral amygdala to orbitofrontal cortex projections enable cue-triggered reward expectations. The Journal of Neuroscience, 37(35), 8374–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingawi N. W., Balleine B. W. (2012). Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. The Journal of Neuroscience, 32(3), 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano Y. R., Serafín N., Prado-Alcalá R. A., Roozendaal B., Quirarte G. L. (2013). Glucocorticoids in the dorsomedial striatum modulate the consolidation of spatial but not procedural memory. Neurobiology of Learning and Memory, 101, 55–64. [DOI] [PubMed] [Google Scholar]
- Malvaez M., Shieh C., Murphy M. D., Greenfield V. Y., Wassum K. M. (2019). Distinct cortical–amygdala projections drive reward value encoding and retrieval. Nature Neuroscience, 22(5), 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin N. V., Lebel R. M., Coupland N. J., Wilman A. H., Carter R. (2010). In vivo quantification of hippocampal subfields using 4.7 T fast spin echo imaging. NeuroImage, 49(2), 1224–1230. [DOI] [PubMed] [Google Scholar]
- Marek R., Strobel C., Bredy T. W., Sah P. (2013). The amygdala and medial prefrontal cortex: Partners in the fear circuit. The Journal of Physiology, 591(10), 2381–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland J. L., McNaughton B. L., O’Reilly R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3), 419–457. [DOI] [PubMed] [Google Scholar]
- McDonald R. J., King A. L., Hong N. S. (2008). Neurotoxic damage to the dorsomedial striatum exaggerates the behavioral influence of a context-specific inhibitory association mediated by the ventral hippocampus. Behavioral Neuroscience, 122(1), 27–35. [DOI] [PubMed] [Google Scholar]
- McDonald R. J., White N. M. (1993). A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behavioral Neuroscience, 107(1), 3–22. [DOI] [PubMed] [Google Scholar]
- McKenzie S., Robinson N. T., Herrera L., Churchill J. C., Eichenbaum H. (2013). Learning causes reorganization of neuronal firing patterns to represent related experiences within a hippocampal schema. The Journal of Neuroscience, 33(25), 10243–10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee D., Liljeholm M., Zika O., O’Doherty J. P. (2015). Characterizing the associative content of brain structures involved in habitual and goal-directed actions in humans: A multivariate fMRI study. The Journal of Neuroscience, 35(9), 3764–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J. F., Christopher Repa J., Mauk M. D., LeDoux J. E. (2002). Parallels between cerebellum-and amygdala-dependent conditioning. Nature Reviews Neuroscience, 3(2), 122–131. [DOI] [PubMed] [Google Scholar]
- Medina J. F., Garcia K. S., Mauk M. D. (2001). A mechanism for savings in the cerebellum. The Journal of Neuroscience, 21(11), 4081–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. K., Staresina B. P., Schwabe L. (2022). Stress diminishes outcome but enhances response representations during instrumental learning. eLife, 11, Article e67517. 10.7554/eLife.67517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz C. J., Hermann A., Stark R., Wolf O. T. (2014). Cortisol modifies extinction learning of recently acquired fear in men. Social Cognitive and Affective Neuroscience, 9(9), 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. (1962). Les troubles de la memoire accompagnant des lesions hippocampiques bilaterales. In Milner B. M., Glickman S. (Eds.), Physiologie de l’hippocampe, 107, 257–272. (English Translation in Cognitive Process and the Brain (pp. 97–111). Princeton: Van Nostrand. 1965; ). [Google Scholar]
- Molitor R. J., Sherrill K. R., Morton N. W., Miller A. A., Preston A. R. (2021). Memory reactivation during learning simultaneously promotes dentate gyrus/CA2, 3 pattern differentiation and CA1 memory integration. The Journal of Neuroscience, 41(4), 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscarello J. M., Penzo M. A. (2022). The central nucleus of the amygdala and the construction of defensive modes across the threat-imminence continuum. Nature Neuroscience, 25, 999–1008. [DOI] [PubMed] [Google Scholar]
- Murray J. E., Belin-Rauscent A., Simon M., Giuliano C., Benoit-Marand M., Everitt B. J., Belin D. (2015). Basolateral and central amygdala differentially recruit and maintain dorsolateral striatum-dependent cocaine-seeking habits. Nature Communications, 6, Article 10088. 10.1038/ncomms10088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T., Young J. Z., McHugh T. J., Buhl D. L., Tonegawa S. (2008). Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science, 319(5867), 1260–1264. [DOI] [PubMed] [Google Scholar]
- Nassi J. J., Callaway E. M. (2009). Parallel processing strategies of the primate visual system. Nature Reviews Neuroscience, 10(5), 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness H. T., Folvik L., Sneve M. H., Vidal-Piñeiro D., Raud L., Geier O. M., Nyberg L., Walhovd K. B., Fjell A. M. (2022). Reduced hippocampal-striatal interactions during formation of durable episodic memories in aging. Cerebral Cortex, 32(11), 2358–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L., Karalija N., Salami A., Andersson M., Wåhlin A., Kaboovand N., Köhncke Y., Axelsson J., Rieckmann A., Papenberg G., Garrett D. D., Riklund K., Lövdén M., Lindenberger U., Bäckman L. (2016). Dopamine D2 receptor availability is linked to hippocampal–caudate functional connectivity and episodic memory. Proceedings of the National Academy of Sciences, USA, 113(28), 7918–7923. 10.1073/pnas.1606309113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M. G., Hirsh R., White N. M. (1989). Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: Evidence for multiple memory systems. The Journal of Neuroscience, 9(5), 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M. G., McGaugh J. L. (1996). Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory, 65(1), 65–72. [DOI] [PubMed] [Google Scholar]
- Patterson T. K., Knowlton B. J. (2018). Subregional specificity in human striatal habit learning: A meta-analytic review of the fMRI literature. Current Opinion in Behavioral Sciences, 20, 75–82. [Google Scholar]
- Pool E., Brosch T., Delplanque S., Sander D. (2015). Stress increases cue-triggered “wanting” for sweet reward in humans. Journal of Experimental Psychology: Animal Learning and Cognition, 41(2), 128–136. [DOI] [PubMed] [Google Scholar]
- Pool E. R., Gera R., Fransen A., Perez O. D., Cremer A., Aleksic M., Tanwisuth S., Quail S., Ceceli A. O., Manfredi D. A., Nave G., Tricomi E., Balleine B., Schonberg T., Schwabe L., O’Doherty J. P. (2022). Determining the effects of training duration on the behavioral expression of habitual control in humans: A multilaboratory investigation. Learning & Memory, 29(1), 16–28. 10.1101/lm.053413.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool E. R., Pauli W. M., Kress C. S., O’Doherty J. P. (2019). Behavioural evidence for parallel outcome-sensitive and outcome-insensitive Pavlovian learning systems in humans. Nature Human Behaviour, 3(3), 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J., Evensmoen H. R., Moscovitch M., Nadel L. (2013). Long-axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17(5), 230–240. [DOI] [PubMed] [Google Scholar]
- Prévost C., Liljeholm M., Tyszka J. M., O’Doherty J. P. (2012). Neural correlates of specific and general Pavlovian-to-instrumental transfer within human amygdalar subregions: A high-resolution fMRI study. The Journal of Neuroscience, 32(24), 8383–8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pych J. C., Chang Q., Colon-Rivera C., Haag R., Gold P. E. (2005). Acetylcholine release in the hippocampus and striatum during place and response training. Learning & Memory, 12(6), 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Hermans E. J., Van Marle H. J., Fernández G. (2012). Understanding low reliability of memories for neutral information encoded under stress: Alterations in memory-related activation in the hippocampus and midbrain. The Journal of Neuroscience, 32(12), 4032–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raio C. M., Hartley C. A., Orederu T. A., Li J., Phelps E. A. (2017). Stress attenuates the flexible updating of aversive value. Proceedings of the National Academy of Sciences, USA, 114(42), 11241–11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla R. A. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In Black A. H., Prokasy W. F. (Eds.), Classical conditioning II: Current research and theory (pp. 64–99). Appleton-Century-Crofts. [Google Scholar]
- Richards B. A., Xia F., Santoro A., Husse J., Woodin M. A., Josselyn S. A., Frankland P. W. (2014). Patterns across multiple memories are identified over time. Nature Neuroscience, 17(7), 981–986. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McEwen B. S., Chattarji S. (2009). Stress, memory and the amygdala. Nature Reviews Neuroscience, 10(6), 423–433. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McGaugh J. L. (1997). Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiology of Learning and Memory, 67(2), 176–179. [DOI] [PubMed] [Google Scholar]
- Rosenberger L. A., Eisenegger C., Naef M., Terburg D., Fourie J., Stein D. J., van Honk J. (2019). The human basolateral amygdala is indispensable for social experiential learning. Current Biology, 29(20), 3532–3537. [DOI] [PubMed] [Google Scholar]
- Rosenkranz J. A., Buffalari D. M., Grace A. A. (2006). Opposing influence of basolateral amygdala and footshock stimulation on neurons of the central amygdala. Biological Psychiatry, 59(9), 801–811. [DOI] [PubMed] [Google Scholar]
- Sakon J. J., Suzuki W. A. (2019). A neural signature of pattern separation in the monkey hippocampus. Proceedings of the National Academy of Sciences, USA, 116(19), 9634–9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro A. C., Kustner L. V., Turk-Browne N. B. (2012). Shaping of object representations in the human medial temporal lobe based on temporal regularities. Current Biology, 22(17), 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro A. C., Reid A. G., Morgan A., Manoach D. S., Verfaellie M., Stickgold R. (2019). The hippocampus is necessary for the consolidation of a task that does not require the hippocampus for initial learning. Hippocampus, 29(11), 1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro A. C., Turk-Browne N. B., Botvinick M. M., Norman K. A. (2017). Complementary learning systems within the hippocampus: A neural network modelling approach to reconciling episodic memory with statistical learning. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1711), Article 20160049. 10.1098/rstb.2016.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro A. C., Turk-Browne N. B., Norman K. A., Botvinick M. M. (2016). Statistical learning of temporal community structure in the hippocampus. Hippocampus, 26(1), 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting M. L., Guarino K. F., Schapiro A. C., Turk-Browne N. B., Preston A. R. (2017). Hippocampal structure predicts statistical learning and associative inference abilities during development. Journal of Cognitive Neuroscience, 29(1), 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting M. L., Gumus M., Zhu T., Mack M. L. (2021). The structure of hippocampal circuitry relates to rapid category learning in humans. Hippocampus, 31, 1179–1190. [DOI] [PubMed] [Google Scholar]
- Schlichting M. L., Preston A. R. (2015). Memory integration: Neural mechanisms and implications for behavior. Current Opinion in Behavioral Sciences, 1, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting M. L., Zeithamova D., Preston A. R. (2014). CA1 subfield contributions to memory integration and inference. Hippocampus, 24(10), 1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L., Hermans E. J., Joëls M., Roozendaal B. (2022). Mechanisms of memory under stress. Neuron, 110(9), 1450–1467. [DOI] [PubMed] [Google Scholar]
- Sherman B. E., Graves K. N., Turk-Browne N. B. (2020). The prevalence and importance of statistical learning in human cognition and behavior. Current Opinion in Behavioral Sciences, 32, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman B. E., Turk-Browne N. B. (2020). Statistical prediction of the future impairs episodic encoding of the present. Proceedings of the National Academy of Sciences, USA, 117(37), 22760–22770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D., Turk-Browne N. B. (2013). Mechanisms for widespread hippocampal involvement in cognition. Journal of Experimental Psychology: General, 142(4), 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D., Wagner A. D. (2008). Integrating memories in the human brain: Hippocampal-midbrain encoding of overlapping events. Neuron, 60(2), 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sias A. C., Morse A. K., Wang S., Greenfield V. Y., Goodpaster C. M., Wrenn T. M., Wikenheiser A., Holley S. M., Cepeda C., Levine M. S., Wassum K. M. (2021). A bidirectional corticoamygdala circuit for the encoding and retrieval of detailed reward memories. eLife, 10, Article e68617. 10.7554/eLife.68617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller-Pérez C., Serafín N., Prado-Alcalá R. A., Roozendaal B., Quirarte G. L. (2017). Glucocorticoid administration into the dorsolateral but not dorsomedial striatum accelerates the shift from a spatial toward procedural memory. Neurobiology of Learning and Memory, 141, 124–133. [DOI] [PubMed] [Google Scholar]
- Sladky R., Riva F., Rosenberger L. A., van Honk J., Lamm C. (2021). Basolateral and central amygdala orchestrate how we learn whom to trust. Communications Biology, 4(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. S., Graybiel A. M. (2013). A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron, 79(2), 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L. R. (1992). Declarative and nondeclarative memory: Multiple brain systems supporting learning and memory. Journal of Cognitive Neuroscience, 4(3), 232–243. [DOI] [PubMed] [Google Scholar]
- Starita F., Kroes M. C., Davachi L., Phelps E. A., Dunsmoor J. E. (2019). Threat learning promotes generalization of episodic memory. Journal of Experimental Psychology: General, 148(8), 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucevic J., Schapiro A. C. (2022). A neural network model of hippocampal contributions to category learning. bioRxiv. 10.1101/2022.01.12.47605 [DOI] [PMC free article] [PubMed]
- Terburg D., Scheggia D., Del Rio R. T., Klumpers F., Ciobanu A. C., Morgan B., Montoya E. R., Bos P. A., Giobellina G., van den Burg E. H., de Gelder B., Stein D. J., Stoop R., van Honk J. (2018). The basolateral amygdala is essential for rapid escape: A human and rodent study. Cell, 175(3), 723–735. 10.1016/j.cell.2018.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B. L., Erickson K., Schulkin J., Rosen J. B. (2004). Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behavioural Brain Research, 149(2), 209–215. [DOI] [PubMed] [Google Scholar]
- Thompson R., Steinmetz J. (2009). The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience, 162(3), 732–755. [DOI] [PubMed] [Google Scholar]
- Thorn C. A., Atallah H., Howe M., Graybiel A. M. (2010). Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron, 66(5), 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrailkill E. A., Trask S., Vidal P., Alcalá J. A., Bouton M. E. (2018). Stimulus control of actions and habits: A role for reinforcer predictability and attention in the development of habitual behavior. Journal of Experimental Psychology: Animal Learning and Cognition, 44(4), 370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman E. C. (1948). Cognitive maps in rats and men. Psychological Review, 55(4), 189–208. [DOI] [PubMed] [Google Scholar]
- Tompary A., Davachi L. (2017). Consolidation promotes the emergence of representational overlap in the hippocampus and medial prefrontal cortex. Neuron, 96(1), 228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth-Fáber E., Janacsek K., Szőllősi Á., Kéri S., Nemeth D. (2021). Regularity detection under stress: Faster extraction of probability-based regularities. PLOS ONE, 16(6), Article e0253123. 10.1371/journal.pone.0253123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne N. B. (2019). The hippocampus as a visual area organized by space and time: A spatiotemporal similarity hypothesis. Vision Research, 165, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne N. B., Scholl B. J., Johnson M. K., Chun M. M. (2010). Implicit perceptual anticipation triggered by statistical learning. The Journal of Neuroscience, 30(33), 11177–11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner K. M., Svegborn A., Langguth M., McKenzie C., Robbins T. W. (2022). Opposing roles of the dorsolateral and dorsomedial striatum in the acquisition of skilled action sequencing in rats. The Journal of Neuroscience, 42(10), 2039–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye K. M., Prakash R., Kim S.-Y., Fenno L. E., Grosenick L., Zarabi H., Thompson K. R., Gradinaru V., Ramakrishnan C., Deisseroth K. (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature, 471(7338), 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandael D., Wierda K., Vints K., Baatsen P., De Groef L., Moons L., Rybakin V., Gounko N. V. (2021). Corticotropin-releasing factor induces functional and structural synaptic remodelling in acute stress. Translational Psychiatry, 11(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandaele Y., Ottenheimer D. J., Janak P. H. (2021). Dorsomedial striatal activity tracks completion of behavioral sequences in rats. eNeuro, 8(6), Article ENEURO.0279-21.2021. 10.1523/ENEURO.0279-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer M. A., Johnson A., Schmitzer-Torbert N. C., Redish A. D. (2010). Triple dissociation of information processing in dorsal striatum, ventral striatum, and hippocampus on a learned spatial decision task. Neuron, 67(1), 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Honk J., Eisenegger C., Terburg D., Stein D. J., Morgan B. (2013). Generous economic investments after basolateral amygdala damage. Proceedings of the National Academy of Sciences, USA, 110(7), 2506–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikbladh O. M., Meager M. R., King J., Blackmon K., Devinsky O., Shohamy D., Burgess N., Daw N. D. (2019). Hippocampal contributions to model-based planning and spatial memory. Neuron, 102(3), 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanjia G., Favila S. E., Kim G., Molitor R. J., Kuhl B. A. (2021). Abrupt hippocampal remapping signals resolution of memory interference. Nature Communications, 12(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warlow S. M., Berridge K. C. (2021). Incentive motivation: ‘Wanting’ roles of central amygdala circuitry. Behavioural Brain Research, 411, Article 113376. 10.1016/j.bbr.2021.113376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum K. M., Izquierdo A. (2015). The basolateral amygdala in reward learning and addiction. Neuroscience & Biobehavioral Reviews, 57, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. M. (2009). Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behavioural Brain Research, 199(1), 3–23. [DOI] [PubMed] [Google Scholar]
- Wickens J. R., Horvitz J. C., Costa R. M., Killcross S. (2007). Dopaminergic mechanisms in actions and habits. The Journal of Neuroscience, 27(31), 8181–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer G. E., Shohamy D. (2012). Preference by association: How memory mechanisms in the hippocampus bias decisions. Science, 338(6104), 270–273. [DOI] [PubMed] [Google Scholar]
- Wood E. R., Dudchenko P. A., Robitsek R. J., Eichenbaum H. (2000). Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron, 27(3), 623–633. [DOI] [PubMed] [Google Scholar]
- Yamada K., McEwen B. S., Pavlides C. (2003). Site and time dependent effects of acute stress on hippocampal long-term potentiation in freely behaving rats. Experimental Brain Research, 152(1), 52–59. [DOI] [PubMed] [Google Scholar]
- Yassa M. A., Stark C. E. (2011). Pattern separation in the hippocampus. Trends in Neurosciences, 34(10), 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. H. (2010). The sensorimotor striatum is necessary for serial order learning. The Journal of Neuroscience, 30(44), 14719–14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A., Minney V. L., Shippenberg T. S. (2010). Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. The Journal of Neuroscience, 30(46), 15457–15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D., Bowman C. R. (2020). Generalization and the hippocampus: More than one story? Neurobiology of Learning and Memory, 175, Article 107317. 10.1016/j.nlm.2020.107317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Mano H., Ganesh G., Robbins T., Seymour B. (2016). Dissociable learning processes underlie human pain conditioning. Current Biology, 26(1), 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.-H., Zhang J.-Y., Holmes A., Pan B.-X. (2021). Amygdala circuit substrates for stress adaptation and adversity. Biological Psychiatry, 89(9), 847–856. [DOI] [PubMed] [Google Scholar]
- Zorawski M., Killcross S. (2003). Glucocorticoid receptor agonist enhances pavlovian appetitive conditioning but disrupts outcome-specific associations. Behavioral Neuroscience, 117(6), 1453–1457. [DOI] [PubMed] [Google Scholar]