Abstract
Objective
To determine whether longitudinal progression of small vessel disease in chronic stroke survivors is associated with longitudinal worsening of chronic aphasia severity.
Design
A longitudinal retrospective study. Severity of white matter hyperintensities (WMHs) as a marker for small vessel disease was assessed on fluid-attenuated inversion recovery (FLAIR) scans using the Fazekas scale, with ratings for deep WMHs (DWMHs) and periventricular WMHs (PVHs).
Setting
University research laboratories.
Participants
This study includes data from 49 chronic stroke survivors with aphasia (N=49; 15 women, 34 men, age range=32-81 years, >6 months post-stroke, stroke type: [46 ischemic, 3 hemorrhagic], community dwelling). All participants completed the Western Aphasia Battery-Revised (WAB) and had FLAIR scans at 2 timepoints (average years between timepoints: 1.87 years, SD=3.21 years).
Interventions
Not applicable.
Main Outcome Measures
Change in white matter hyperintensity severity (calculated using the Fazekas scale) and change in aphasia severity (difference in Western Aphasia Battery scores) were calculated between timepoints. Separate stepwise regression models were used to identify predictors of WMH severity change, with lesion volume, age, time between timepoints, body mass index (BMI), and presence of diabetes as independent variables. Additional stepwise regression models investigated predictors of change in aphasia severity, with PVH change, DWMH change, lesion volume, time between timepoints, and age as independent predictors.
Results
22.5% of participants (11/49) had increased WMH severity. Increased BMI was associated with increases in PVH severity (P=.007), whereas the presence of diabetes was associated with increased DWMH severity (P=.002). Twenty-five percent of participants had increased aphasia severity which was significantly associated with increased severity of PVH (P<.001, 16.8% variance explained).
Conclusion
Increased small vessel disease burden is associated with contributing to chronic changes in aphasia severity. These findings support the idea that good cardiovascular risk factor control may play an important role in the prevention of long-term worsening of aphasic symptoms.
Keywords: Aphasia, Cardiovascular risk factors, Rehabilitation, Small vessel disease, Stroke, White matter hyperintensities
There is growing evidence that recovery in the chronic stages of post-stroke aphasia may be more fluid than once thought,1, 2, 3 and although many individuals continue to recover into the chronic stages,1, 2, 3, 4, 5, 6 some experience worsening of aphasic symptoms months or even years after their stroke. Lesion size and location are good predictors of long-term recovery,7,8 but they only account for a portion of the variance in aphasia severity9,10 and may not explain why some individuals experience a worsening of symptoms over time.
Many stroke survivors have risk factors for cerebrovascular disease, such as increased age,11 diabetes,12 and hypertension.12 There is also evidence to suggest that cardiovascular risk factors are inadequately managed in stroke survivors,13 and that they may have higher rates of untreated hypertension and diabetes than individuals who have not experienced a stroke.14 Previous research has demonstrated that cardiovascular risk factors such as increased body mass index (BMI), hypertension, and diabetes,15,16 along with age17,18 are good predictors of small vessel disease severity in older age and are strongly associated with the presence of white matter hyperintensities (WMHs), a neuroimaging marker of small vessel disease.19 Both age20 and cardiovascular risk factors16,20, 21, 22, 23, 24 have been found to be predictive of increasing WMH volume over time and there is evidence that controlling vascular risk factors such as blood pressure control15,25 or increasing physical activity can slow the progression of WMHs in otherwise healthy participants26 and individuals with Alzheimer's disease.27,28
Severe WMHs are frequently associated with cognitive dysfunction in older adults29,30 and are a risk factor for further cognitive decline in typical aging.31, 32, 33, 34, 35 Moreover, a relationship between WMH load and working memory has been reported even in younger adults with low WMH severity,36,37 therefore it is likely that both the extent and spatial location of WMHs are important for progressive cognitive impairment.38 Previous research found an acceleration in WMH burden in those in the early, pre-symptomatic stages of mild cognitive impairment39 and postulated that higher WMH burden in mild cognitive impairment is a predictor of rapid cognitive decline40 and progression into more serious forms of impairment such as dementia,41, 42, 43, 44, 45, 46, 47 including Alzheimer's disease.47
The extent and location of WMHs may be of particular importance in stroke survivors as the integrity of the brain tissue spared by the stroke has a significant influence on stroke recovery.1,48,49 Indeed, recent years have seen a trend toward exploring the effect of small vessel disease on chronic stroke aphasia recovery, because small vessel disease is now recognized as a marker of poor50,51 and increased post-stroke cognitive decline.52, 53, 54, 55 Similarly, progression of WMH severity has been identified as a risk factor for cognitive decline in stroke survivors.21 Few studies have investigated the relationship with longitudinal changes in aphasia severity, but there is evidence that small vessel disease (measured at a single timepoint) is associated with suboptimal language recovery49,56 and thus increased WMHs burden may be associated with worsening aphasia severity in chronic stroke. Therefore, given previous research suggests that (a) stroke survivors typically have risk factors for cerebrovascular disease, (b) cardiovascular risk factors are associated with WMH severity, (c) WMH severity is associated with aphasia severity at a single timepoint, and (d) progression of WMH severity is a risk factor for cognitive decline, we hypothesized that longitudinal progression of small vessel disease in chronic stroke survivors is associated with longitudinal worsening of chronic aphasia severity. We tested this hypothesis in a cohort of post-stroke aphasic individuals with longitudinal imaging and behavioral assessments, controlling for age and lesion volume.
Methods
Participants
This retrospective multi-timepoint study included 49 community-dwelling participants (15 women, 34 men) in the chronic stage of recovery after a left hemisphere stroke (≥6 months post stroke, 46 ischemic, 3 hemorrhagic). Participants had been recruited for various studies at the lab.
Inclusion criteria for these studies comprised (i) aged between 21 and 85 years old, (ii) in the chronic stage of recovery (≥6 months) after a left hemisphere stroke to the middle cerebral artery, (iii) premorbid right-handed, (iv) presence of aphasia, as indicated by a Western Aphasia Battery (WAB) Aphasia Quotient (AQ) of <93.8, and (v) no magnetic resonance imaging (MRI) contraindications. For this retrospective study, a further inclusion criterion was a fluid attenuated inversion recovery (FLAIR) scan with behavioral data (WAB) collected within 0-3 days preceding the scan, at 2 separate timepoints. Exclusion criteria included a history of neurologic disorders, concomitant traumatic brain injury, or history of a developmental speech disorder. This study received approval from the local Institutional Review Board and all participants provided informed consent for study consent in accordance with the Declaration of Helsinki.
Participants were on average 53.03 years old at the time of stroke (SD=12.47), 55.74 years old when initially scanned (SD =11.77), and 2.65 years post stroke at the time of baseline (timepoint 1) assessment (SD =2.26). See table 1 for a full breakdown of demographic information.
Table 1.
Demographic information for participants
| Demographic Variables (N=49) | Mean ± SD |
|---|---|
| Stroke age | 53.03 (12.47) |
| Age (timepoint 1) | 55.74 (11.77) |
| Age (timepoint 2) | 57.61 (12.03) |
| Years post stroke (timepoint 1) | 2.65 (2.26) |
| Years post stroke (timepoint 2) | 4.51 (3.69) |
| Lesion volume (cubic millimeters) | 132.37 (82.18) |
| Sex (women:men) | 15:34 |
| BMI | 28.92 (7.21) |
| Diabetes (percent of participants) | 30.61% |
| WAB AQ (timepoint 1) | 57.63 (26.43) |
| WAB AQ (timepoint 2) | 55.83 (23.13) |
| Total Fazekas score (timepoint 1) | 2.91 (1.72) |
| Total Fazekas score (timepoint 2) | 3.14 (1.77) |
| PVH score (timepoint 1) | 2.00 (0.87) |
| PVH score (timepoint 2) | 2.11 (0.94) |
| DWMH score (timepoint 1) | 1.11 (0.95) |
| DWMH score (timepoint 2) | 1.13 (0.93) |
Behavioral and demographic data
Demographic data were collected from participants at timepoint 1, including age, BMI, and presence of diabetes (yes/no). Participants also completed the WAB at both timepoints to estimate aphasia type and severity. Changes in aphasia severity were calculated using WAB-AQ scores where WAB-AQ at timepoint 1 was subtracted from timepoint 2. Therefore, positive scores indicate increased WAB-AQ score (less severe aphasia), and negative scores indicate decreased WAB-AQ scores over time (more severe aphasia over time).
MRI data acquisition and preprocessing
Participants underwent high-resolution scanning on a Siemens Trio 3T scanner equipped with a 12-channel (Trio configuration) or 20-channel (following upfit to Prisma configuration) head coil. For all participants, we acquired a T1-weighted scan, a T2-FLAIR (for WMH identification) scan, and a T2-SPC (for lesion demarcation scan). T1-weighted imaging used a magnetization-prepared rapid gradient-echo sequence with 1 mm isotropic voxels, a 256 × 256 matrix size, a 9° flip angle, and a 92-slice sequence with repetition time (TR) =2250 ms, inversion time=925 ms, and echo time (TE) =4.11 ms. T2-weighted scans were acquired using the same angulation and volume center as the T1 scan. This 3D T2-weighted SPACE sequence used a resolution of 1 mm3 that was used with a field of view=256 × 256 mm, 160 sagittal slices, variable degree flip angle, TR =3200 ms, TE = 212 ms, x2 GRAPPA acceleration (80 reference lines). FLAIR scans were also acquired on the same scanner using the following parameters: TR =5000 ms, TE=387 ms, matrix = 256 × 256, field of view=230 × 230 × 173 mm2, slice thickness =1 mm, 160 sagittal slices.
Chronic stroke lesions were manually drawn using each participants T2-weighted image in native space. All lesion tracings were drawn by a neurologist (author L.B.) or by a trained study staff member who were blinded to behavioral data. Enantiomorphic segmentation-normalization was then employed using SPM12 and a series of custom MATLABa scripts57 that leverage multiple best-of-breed programs (SPM12b, FSL v6.0.3, ASLtbxc, and MRItrixd) in order to normalize and process MRI data acquired from individuals with lesioned brains. These scripts used enantiomorphic normalization.58 To create chimeric images (ie, “healed” brains) in which the damaged portion of the left hemisphere was temporarily replaced with the mirror image of intact areas from the healthy right hemisphere (using the SPM12s Clinical Toolbox which leverages SPM12s unified segmentation-normalization59 method to warp this chimeric image to standard (Montreal Neurological Institute) space, and the resulting spatial transform was then applied to the native-space T1 scan as well as the native-space versions of the hand-drawn lesion map. This additional step (enantiomorphic normalization) ensures that segmentation-normalization methods designed for intact brains do not incorrectly warp scans with large lesions to the left hemisphere. Lesion volume was measured by counting the number of voxels in the lesion mask and converting to cubic millimeters.
Identifying white matter hyperintensities
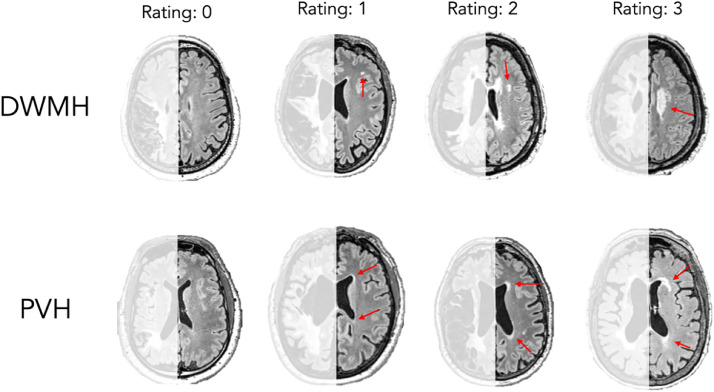
Given that the severity of WMHs is generally assumed to be symmetrical across hemispheres and that the stroke lesion may prevent an accurate estimation of WMHs, we adopted a method used in previous research in which we only scored WMHs in the intact, contralesional hemisphere.60,61 WMHs were identified based on FLAIR scans using the Fazekas scale,62 see figure 1 for ratings by 2 trained staff members. All scans were rated by both staff members who were blind to participant information. If there was disagreement, a third trained staff member who was also blind to participant information rated to achieve consensus. The Fazekas scale includes independent ratings (0-3) for periventricular hyperintensities (PVH) and deep WMHs (DWMH).
Fig 1.
Example of ratings using the Fazekas scale. Arrows point to regions of WMHs, blue arrows indicate DWMH, red indicate PVH. The rating scores were defined as follows for DWMH: 0= absent, 1= discrete diffuse lesions, 2= beginning of confluence of foci, 3= large confluent areas. The rating scores were defined as follows for PVH: 0= absent, 1= caps or pencil-thin lining around ventricles, 2= smooth halo around ventricles, 3= irregular PVH extending into the deep white matter. Left (lesioned) hemisphere is lightened as it was not used for rating.
We calculated change in WMH severity by subtracting the Fazekas scores at timepoint 2 from timepoint 1, separately for PVH and DWMH. For example, if Participant 1 had the following scores: timepoint 1: DWMH=1, PVH=2, timepoint 2: DWMH=2, PVH=2, their change scores would be: DWMH change=1, PVH change=0. Therefore, negative scores suggest reduced WMH severity at the second timepoint, and positive suggest increased WMH severity. The number of years between timepoint 1 and 2 was also recorded. See figure 2 for examples of participants WMH ratings.
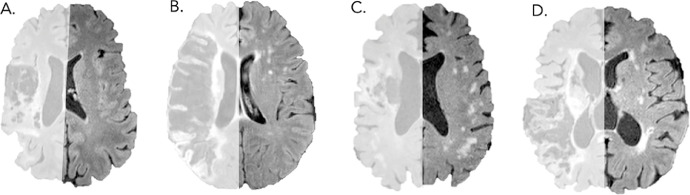
Fig 2.
Examples of participant FLAIR scans. Left hemispheres (where the stroke occurred) have been lightened as they were not used for ratings. Fazekas ratings are as follows: (A) PVH: 1, DWMH: 0; (B) PVH: 2, DWMH: 2; (C) PVH: 1, DWMH: 3; (D) PVH: 3, DWMH: 2.
Statistical analysis
Two separate stepwise regression models were used to identify significant predictors of change in WMH severity over time, 1 model for PVHs and 1 model for DWMH. Independent variables included lesion volume, age at timepoint 1, years between timepoints, BMI, and presence of diabetes. The dependent variable was PVH severity in the first model and DWMH severity in the second.
We conducted a third stepwise regression, where WAB-AQ change (between timepoints 1 and 2) was the dependent variable and the following were independent predictors: PVH change, DWMH change, lesion volume, years between timepoints 1 and 2, age at timepoint 1 and baseline aphasia severity. One additional stepwise regression was conducted only in participants who experienced increased aphasia severity (ie, declining WAB AQ scores) between timepoints. Statistical analyses were conducted in the statistical software R,e applied using R package NLME,63 and all figures were created using the GGPLOT2 package.64
Results
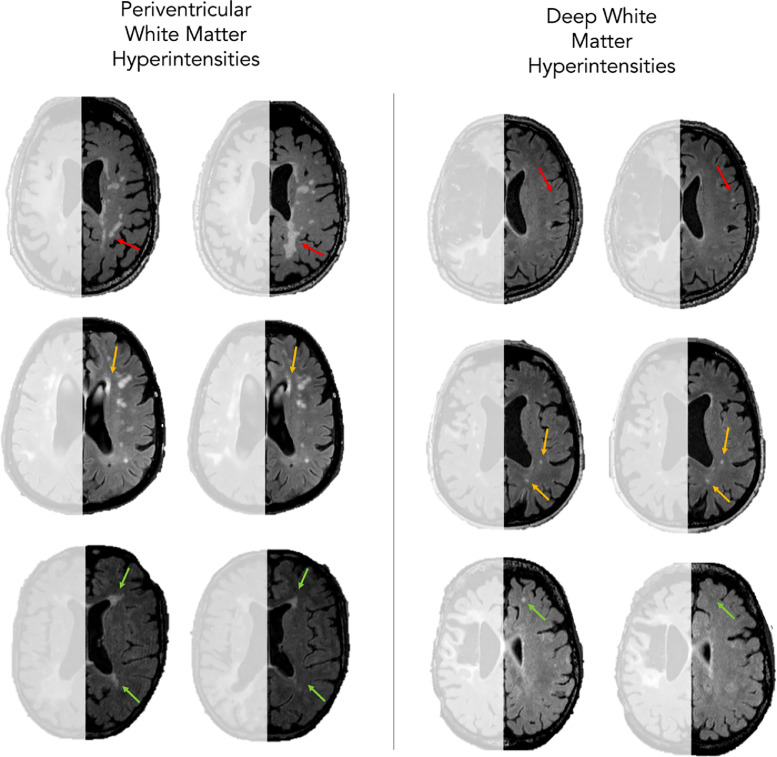
Between their first and last scan, 8 participants (16.33%) had a reduction in WMH severity, 30 participants (61.22%) had no change, and 11 participants (22.45%) had an increase in severity. In terms of specific changes in PVH, 7 participants (14.29%) had an improvement in PVH load (ie, reduction), 33 participants (67.35%) had no change, and 9 participants (18.67%) had an increase in PVH severity. Finally, 5 participants (10.20%) had a reduction in DWMH severity, 35 participants (71.43%) had no change, and 9 (18.37%) had an increase in severity, see figure 3.
Fig 3.
Examples of progression of white matter hyperintensities. The left column focuses on periventricular white matter hyperintensities and the right on deep white matter hyperintensities. Red arrows indicate a worsening (ie, increased severity) of white matter hyperintensities over time, orange indicate similar severity over time, and green show improvement over time.
Predicting changes in PVH
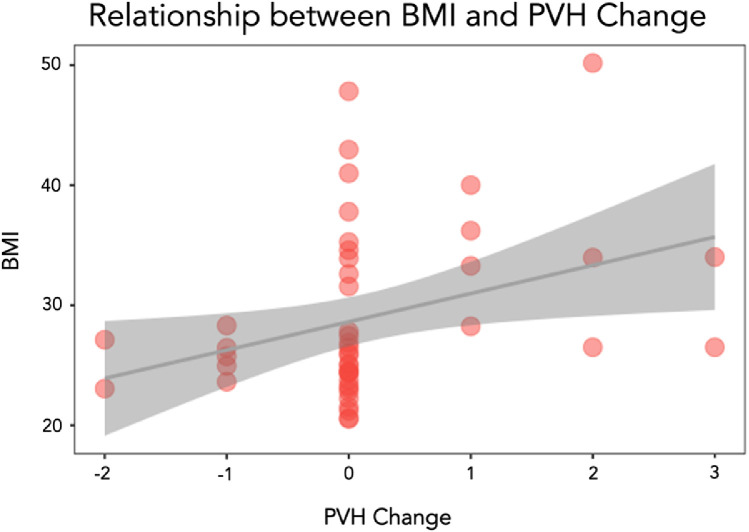
Starting with 5 factors which may be good predictors of changes in PVH over time, a stepwise regression model was able to reduce them to 1 significant predictor which was BMI as a predictor of increased PVH (P=.007), indicating that participants with higher BMI were more likely to have an increased in PVH between the 2 timepoints, see figure 4. Lesion volume (P=.994), years between timepoints (P=.593), age (P=.902), and diabetes (P=.105) were not included in the final model.
Fig 4.
Scatterplot to show the relation between BMI and PVH change.
Predicting changes in DWMH
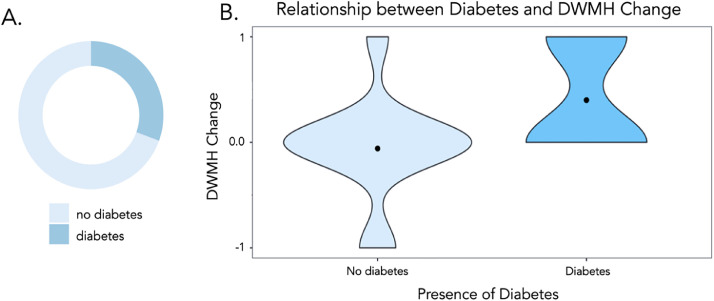
Starting with 5 factors which may be good predictors of changes in DWMH over time, a stepwise regression model was able to reduce them to 1 significant predictor which was diabetes as a predictor of increased PVH (P=.002), indicating that participants with diabetes were more likely to have an increased in DWMH between the 2 timepoints, see figure 5. Lesion volume (P=.505), years between timepoints (P=.181), age (P=.474), and BMI (P=.470) were not included in the final model.
Fig 5.
Relation between diabetes and DWMH change. (A) The proportion of participants with diabetes and (B) a violin plot to show the pattern of DWMH change in participants with and without diabetes.
Predicting changes in WAB AQ
Starting with 7 factors which maybe be good predictors of change in WAB AQ over time, a stepwise regression model was able to reduce them to 1 significant predictor which was change in PVH severity (P<.001), indicating that increased severity of PVH was associated with a decrease in WAB AQ (ie, more severe aphasia), see figure 6. Lesion volume (P=.571), age (P=.215), years between timepoints (P=.833), years post stroke at baseline (P=.409), baseline aphasia severity (P=.268), and change in DWMH severity (P=.176) were not included in the final model.
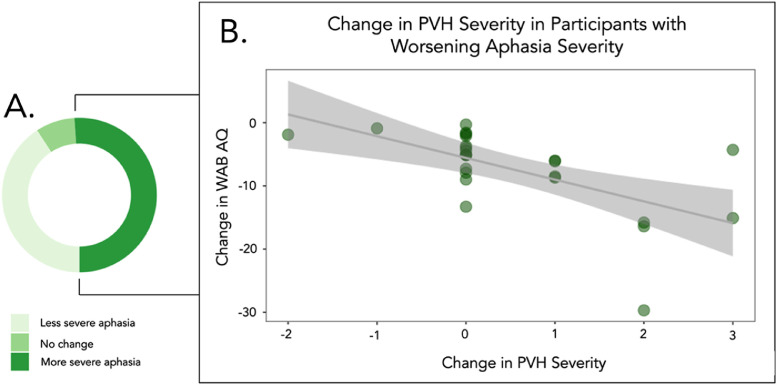
Fig 6.
Scatterplot to show the relation between change in WAB AQ and corresponding change in PVH severity. (A) The proportion of participants who had worse WAB AQ scores (ie, more severe aphasia) between timepoints. (B) In those participants, the relation between change in PVH severity and aphasia severity.
Twenty-five percent of participants displayed worse aphasia severity at timepoint 2 than baseline. Within these participants, a final stepwise regression revealed that change in PVH severity (P<.001) was the only factor included in the final model. Lesion volume (P=.726), age (P=.973), years between timepoints (P=.972), years post stroke at baseline (P=.289), baseline aphasia severity (P=.723), and change in DWMH severity (P=.068) were not included in the final model.
Discussion
Increased BMI was associated with increases in PVH severity, while the presence of diabetes was associated with increased DWMH severity. Finally, a quarter of participants had increased aphasia severity between timepoints which was partially explained by increasing PVH severity.
Changes in WMHs over time
Although it is typically reported that WMH severity increases over time, dynamic changes have also been described. For example, Kim et al65 found that most participants in their study (80%) had WMH progression and approximately 20% displayed WMH reduction over a 3-year period. Our results revealed a similar proportion of WMH reduction (16%). A reduction in WMH severity is often noted but unexplored in the literature66, 67, 68 as the reasons for WMH reduction are unclear, although Wardlaw et al postulate that it may be that signal change on FLAIR scans may be partially due to shifts in water content and not just permanent myelin loss.69 They also suggest that it might be due to improving vascular risk factors and speculate that if some WMHs are areas of tissue edema, reductions in tissue edema would both reduce WMH severity and decrease brain volume.69 Therefore, future studies could investigate changes in brain volume alongside changes in WMH severity.
In the current study, progression changes were more variable with approximately 22% displaying progression and the remaining 61% showing no change. This larger proportion of individuals showing no change may reflect the different time gaps between first and last testing timepoints. While the average time between first and last scans was approximately 2 years, the time between scans was much shorter for several individuals (range=0.5-12.32). It is possible that more time may be needed to demonstrate sufficient changes in WMH severity. Another possibility is that the Fazekas scale is too broad of a measure of WMH change to capture more subtle increases in WMH load. For example, a PVH rating of 1 would be given to an individual who has a single PVH, or multiple PVH (but no confluence). Therefore, subtle changes are unlikely to be detected. Future studies could incorporate volumetric measures of WMH load to elucidate smaller changes in severity.
Role of diabetes on DWMH progression
The presence of diabetes was a predictor of DWMH severity, suggesting that those with diabetes have an increased progression of DWMHs compared with those without diabetes. Previous research has associated diabetes with lowered cognition after stroke,70 and interactions with diabetes have also been found to influence behavioral outcomes in stroke. Johnson et al found an interaction between diabetes and exercise in participants with stroke aphasia and diabetes, where increased exercise improved behavioral outcomes for individuals with diabetes.1 In addition, Roth et al found that individuals who had diabetes alongside stroke aphasia had fewer treatment gains compared to those without diabetes.71 They describe a possible explanation for this, where the integrity of the structural brain network was associated with aphasia treatment gains in those without diabetes, but not in those with diabetes, highlighting the importance of the structural white matter integrity.71 Indeed, similar research has found individuals with diabetes have been found to have lower gray matter density and disrupted white matter networks.72, 73, 74, 75 Our results support this idea; if the presence of diabetes influences the severity or rate of progression of DWMHs, this would result in more widespread white matter network disruption.
Role of BMI on PVH progression
Being overweight or obese (high BMI) has previously been highlighted as an important contributor to the presence of WMHs in the elderly.76, 77, 78, 79 Indeed, Kim et al found in a large sample of otherwise healthy participants that those with WMHs had a higher BMI than those without WMHs.80 However, these studies focus on WMHs generally (ie, both DWMH and PVH). Our results extend this notion, suggesting that a high BMI is specifically predictive of increased PVH severity. Future research could further investigate this relation in healthy older adults.
Relation with behavior
The relation between WMH severity and cognitive decline is well-documented throughout the literature21,30,65; however, less research has focused on the role of WMH in stroke aphasia. Given that the integrity of the tissue spared by the stroke is likely important for recovery, it follows that presence of WMHs, a marker of poorer brain health, may be related to suboptimal outcomes in stroke aphasia. Some studies have found that severe WMHs predict suboptimal language recovery between the acute-subacute stage81 and that WMH severity predicts response to language therapy.49,56 However, most of these studies use 1 global measure of WMH severity, rather than separately considering PVH and DWMHs. Our results showing that PVH severity - but not DWMH severity - is associated with increased aphasia severity suggests that the 2 may have differential effects on behavior. This may be due to the location of the white matter damage, as PVH and DWMH will affect different white matter tracts which may, in turn, have different behavioral outcomes. There may also be a relation between the lesion size or location and the type or quantity of WMHs found, which would result in an interaction with behavioral deficits.
Limitations
There are several limitations with the current study. As it encompassed retrospective data from multiple studies, each participant had data collected at different timepoints and different lengths of time between testing. While this enabled us to have a larger sample size at multiple timepoints, a well-controlled study is necessary to fully elucidate the role of different risk factors on changes in WMH severity over time. Similarly, despite the literature indicating that there is no significant learning effect when completing the WAB multiple times,82,83 it is possible that some of the change we found in WAB-AQ could be attributed to participants completing the assessment multiple times.
Furthermore, our measure of diabetes was a binary present/absent measure. To better understand the nature of the relation between diabetes and WMH severity, future research should include more detailed information related to diabetes, such as type, length of time since diagnosis, and so on. This may help to better understand the role of diabetes on DWMH severity. Similarly, we used the Fazekas scale as our measure of WMH severity which may not be as sensitive as volumetric measures. Therefore, future studies could incorporate more specific volumetric measures of WMH to assess progression.
Conclusions
In conclusion, for stroke survivors, the integrity of the remaining cortical tissue is likely integral to recovery trajectories. The presence of WMHs is indicative of poorer brain health and reduced cortical integrity. Although age is a well-reported predictor of WMH severity, age alone does not predict the rate of progression of WMH severity in a stroke population. The results from this study corroborate previous research suggesting that WMH changes over time are dynamic, with possible reduction and progression.
Diabetes was predictive of DWMH progression, while higher BMI was associated with PVH progression and, in turn, PVH severity was indicative of increased aphasia severity over time. Future research could investigate the mechanistic explanations for the role of cardiovascular risk factors on WMH progression, and whether certain aphasia types or symptoms are particularly associated with WMH load or progression.
Suppliers
-
a.
MATLAB: R2017b; The MathWorks.
-
b.
SPM12; Functional Imaging Laboratory, Wellcome Trust Centre for Neuroimaging, Institute of Neurology [www.fil.ion.ucl.ac.uk/spm].
-
c.
ASLtbx.[https://www.nitrc.org/projects/asltbx/].
-
d.
Mrtrix. [https://www.mrtrix.org].
-
e.
R; R Core Team.
Footnotes
This work was supported by a grant from the National Institute on Deafness and Other Communication Disorders (P50 DC014664).
Disclosures: The authors report no competing interests.
References
- 1.Johnson L, Basilakos A, Yourganov G, et al. Progression of aphasia severity in the chronic stages of stroke. Am J Speech Lang Pathol. 2019;28:639–649. doi: 10.1044/2018_AJSLP-18-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland A, Fromm D, Forbes M, MacWhinney B. Long-term recovery in stroke accompanied by aphasia: a reconsideration. Aphasiology. 2017;31:152–165. doi: 10.1080/02687038.2016.1184221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hope TMH, Leff AP, Prejawa S, et al. Right hemisphere structural adaptation and changing language skills years after left hemisphere stroke. Brain. 2017;140:1718–1728. doi: 10.1093/brain/awx086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridriksson J, Bonilha L, Baker JM, Moser D, Rorden C. Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cereb Cortex. 2010;20:1013–1019. doi: 10.1093/cercor/bhp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. Neuroimage. 2012;60:854–863. doi: 10.1016/j.neuroimage.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitenstein C, Grewe T, Flöel A, et al. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet. 2017;389:1528–1538. doi: 10.1016/S0140-6736(17)30067-3. [DOI] [PubMed] [Google Scholar]
- 7.Fridriksson J, Yourganov G, Bonilha L, Basilakos A, Den Ouden DB, Rorden C. Revealing the dual streams of speech processing. Proc Natl Acad Sci U S A. 2016;113:15108–15113. doi: 10.1073/pnas.1614038114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Døli H, Andersen Helland W, Helland T, Specht K. Associations between lesion size, lesion location and aphasia in acute stroke. Aphasiology. 2021;35:745–763. [Google Scholar]
- 9.Osa García A, Brambati SM, Brisebois A, et al. Predicting early post-stroke aphasia outcome from initial aphasia severity. Front Neurol. 2020;11:120. doi: 10.3389/fneur.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridriksson J, den Ouden DB, Hillis AE, et al. Anatomy of aphasia revisited. Brain. 2018;141:848–862. doi: 10.1093/brain/awx363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly-Hayes M. Influence of age and health behaviors on stroke risk: lessons from longitudinal studies. J Am Geriatr Soc. 2010;58(Suppl 2):S325–S328. doi: 10.1111/j.1532-5415.2010.02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etherton MR, Wu O, Rost NS. Recent advances in leukoaraiosis: white matter structural integrity and functional outcomes after acute ischemic stroke. Curr Cardiol Rep. 2016;18:1–13. doi: 10.1007/s11886-016-0803-0. [DOI] [PubMed] [Google Scholar]
- 13.Kopunek SP, Michael KM, Shaughnessy M, et al. Cardiovascular risk in survivors of stroke. Am J Prev Med. 2007;32:408–412. doi: 10.1016/j.amepre.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner DA, Zweifler RM, Gomez CR, et al. Awareness, treatment, and control of vascular risk factors among stroke survivors. J Stroke Cerebrovasc Dis. 2010;19:311–320. doi: 10.1016/j.jstrokecerebrovasdis.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufouil C, de Kersaint–Gilly A, Besançon V, et al. Longitudinal study of blood pressure and white matter hyperintensities. Neurology. 2001;56:921. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- 16.Lin Q, Huang WQ, Ma QL, et al. Incidence and risk factors of leukoaraiosis from 4683 hospitalized patients: a cross-sectional study. Medicine (Baltimore) 2017;96:e7682. doi: 10.1097/MD.0000000000007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grueter BE, Schulz UG. Age-related cerebral white matter disease (leukoaraiosis): a review. Postgrad Med J. 2012;88:79–87. doi: 10.1136/postgradmedj-2011-130307. [DOI] [PubMed] [Google Scholar]
- 18.Raz N, Yang Y, Dahle CL, Land S. Volume of white matter hyperintensities in healthy adults: contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim Biophys Acta. 2012;1822:361–369. doi: 10.1016/j.bbadis.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scharf EL, Graff-Radford J, Przybelski SA, et al. Cardiometabolic health and longitudinal progression of white matter hyperintensity. Stroke. 2019;50:3037–3044. doi: 10.1161/STROKEAHA.119.025822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang J, Yao K, Huang X, Zhang Y, Shen F, Weng S. Longitudinal white matter hyperintensity changes and cognitive decline in patients with minor stroke. Aging Clin Exp Res. 2022;34:1047–1054. doi: 10.1007/s40520-021-02024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coskun O, Yildiz H, Emre U, et al. Leukoaraiosis in stroke patients. Int J Neurosci. 2003;113:915–922. doi: 10.1080/00207450390220349. [DOI] [PubMed] [Google Scholar]
- 23.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 24.Inzitari D, Diaz F, Fox A, et al. Vascular risk factors and leuko-araiosis. Arch Neurol. 1987;44:42–47. doi: 10.1001/archneur.1987.00520130034014. [DOI] [PubMed] [Google Scholar]
- 25.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 26.Fleischman DA, Yang J, Arfanakis K, et al. Physical activity, motor function, and white matter hyperintensity burden in healthy older adults. Neurology. 2015;84:1294–1300. doi: 10.1212/WNL.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richard E, Gouw AA, Scheltens P, Van Gool WA. Vascular care in patients with Alzheimer disease with cerebrovascular lesions slows progression of white matter lesions on MRI: the evaluation of vascular care in Alzheimer's disease (EVA) study. Stroke. 2010;41:554–556. doi: 10.1161/STROKEAHA.109.571281. [DOI] [PubMed] [Google Scholar]
- 28.Mendez Colmenares A, Voss MW, Fanning J, et al. White matter plasticity in healthy older adults: the effects of aerobic exercise. Neuroimage. 2021;239 doi: 10.1016/j.neuroimage.2021.118305. [DOI] [PubMed] [Google Scholar]
- 29.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MMB. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56:1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- 30.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol. 2007;20:390–397. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- 31.Waldstein SR, Brown JRP, Maier KJ, Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Ann Behav Med. 2005;29:174–180. doi: 10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- 32.Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes. 2014;63:2244–2252. doi: 10.2337/db14-0348. [DOI] [PubMed] [Google Scholar]
- 33.Sadanand S, Balachandar R, Bharath S. Memory and executive functions in persons with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2016;32:132–142. doi: 10.1002/dmrr.2664. [DOI] [PubMed] [Google Scholar]
- 34.Olaya B, Moneta MV, Bobak M, Haro JM, Demakakos P. Cardiovascular risk factors and memory decline in middle-aged and older adults: the English Longitudinal Study of Ageing. BMC Geriatr. 2019;19:1–10. doi: 10.1186/s12877-019-1350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyle PA, Yu L, Fleischman DA, et al. White matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old age. Ann Clin Transl Neurol. 2016;3:791–800. doi: 10.1002/acn3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garnier-Crussard A, Bougacha S, Wirth M, et al. White matter hyperintensities across the adult lifespan: relation to age, Aβ load, and cognition. Alzheimers Res Ther. 2020;12:127. doi: 10.1186/s13195-020-00669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busby N, Newman-Norlund S, Sayers S, et al. White matter hyperintensity load is associated with premature brain aging. Aging (Albany NY) 2022;14:9458. doi: 10.18632/aging.204397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silbert LC, Dodge HH, Perkins LG, et al. Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology. 2012;79:741–747. doi: 10.1212/WNL.0b013e3182661f2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tosto G, Zimmerman ME, Carmichael OT, Brickman AM. Predicting aggressive decline in mild cognitive impairment: the importance of white matter hyperintensities. JAMA Neurol. 2014;71:872–877. doi: 10.1001/jamaneurol.2014.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brickman AM, Provenzano FA, Muraskin J, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol. 2012;69:1621–1627. doi: 10.1001/archneurol.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Debette S, Beiser A, Decarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 44.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 45.West NA, Windham BG, Knopman DS, Shibata DK, Coker LH, Mosley TH. Neuroimaging findings in midlife and risk of late-life dementia over 20 years of follow-up. Neurology. 2019;92:e917–e923. doi: 10.1212/WNL.0000000000006989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garde E, Lykke Mortensen E, Rostrup E, Paulson OB. Decline in intelligence is associated with progression in white matter hyperintensity volume. J Neurol Neurosurg Psychiatry. 2005;76:1289–1291. doi: 10.1136/jnnp.2004.055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang YL, Chen W, Cai WJ, et al. Associations of white matter hyperintensities with cognitive decline: a longitudinal study. J Alzheimer Dis. 2020;73:759–768. doi: 10.3233/JAD-191005. [DOI] [PubMed] [Google Scholar]
- 48.Chang AJ, Wilmskoetter J, Fridriksson J, et al. Cortical microstructural changes associated with treated aphasia recovery. Ann Clin Transl Neurol. 2021;8:1884–1894. doi: 10.1002/acn3.51445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basilakos A, Stark BC, Johnson L, et al. Leukoaraiosis is associated with a decline in language abilities in chronic aphasia. Neurorehabil Neural Repair. 2019;33:718–729. doi: 10.1177/1545968319862561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liou LM, Chen CF, Guo YC, et al. Cerebral white matter hyperintensities predict functional stroke outcome. Cerebrovasc Dis. 2010;29:22–27. doi: 10.1159/000255970. [DOI] [PubMed] [Google Scholar]
- 51.Arsava EM, Rahman R, Rosand J, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology. 2009;72:1403–1410. doi: 10.1212/WNL.0b013e3181a18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mok VCT, Wong A, Lam WWM, et al. Cognitive impairment and functional outcome after stroke associated with small vessel disease. J Neurol Neurosurg Psychiatry. 2004;75:560–566. doi: 10.1136/jnnp.2003.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang Y, Chen YK, Liu YL, et al. Cerebral small vessel disease burden is associated with accelerated poststroke cognitive decline: a 1-year follow-up study. J Geriatr Psychiatry Neurol. 2019;32:336–343. doi: 10.1177/0891988719862630. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Ren W, Shao B, et al. Leukoaraiosis is associated with worse short-term functional and cognitive recovery after minor stroke. Neurol Med Chir (Tokyo) 2017;57:136–143. doi: 10.2176/nmc.oa.2016-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Podgorska A, Hier DB, Pytlewski A, Czlonkowska A. Leukoaraiosis and stroke outcome. J Stroke Cerebrovasc Dis. 2002;11:336–340. doi: 10.1053/jscd.2002.130123. [DOI] [PubMed] [Google Scholar]
- 56.Varkanitsa M, Peñaloza C, Charidimou A, Caplan D, Kiran S. White matter hyperintensities predict response to language treatment in poststroke aphasia. Neurorehabil Neural Repair. 2020;34:945–953. doi: 10.1177/1545968320952809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rorden C, McKinnon E, Hanayik T, Yourganov G, Reddy D. nii_preprocess: Zenodo DOI release (v1.1). Zenodo 10.5281/zenodo.4027711, 2020 [DOI]
- 58.Nachev P, Coulthard E, Jäger HR, Kennard C, Husain M. Enantiomorphic normalization of focally lesioned brains. Neuroimage. 2008;39:1215–1226. doi: 10.1016/j.neuroimage.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 60.Pantoni L. Leukoaraiosis: from an ancient term to an actual marker of poor prognosis. Stroke. 2008;39:1401–1403. doi: 10.1161/STROKEAHA.107.505602. [DOI] [PubMed] [Google Scholar]
- 61.Wilmskoetter J, Marebwa B, Basilakos A, et al. Long-range fibre damage in small vessel brain disease affects aphasia severity. Brain. 2019;142:3190–3201. doi: 10.1093/brain/awz251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 63.Pinheiro J, Bates D, DebRoy S, Sarkar D. R Development Core Team, nlme: Linear and Nonlinear Mixed Effects Models, Published online 2013. https://CRAN.R-project.org/package=nlme.
- 64.Wickham H. ggplot2: Elegant Graphics for Data Analysis, 2016, Springer-Verlag; New York. https://ggplot2.tidyverse.org.
- 65.Kim SJ, Lee DK, Jang YK, et al. The effects of longitudinal white matter hyperintensity change on cognitive decline and cortical thinning over three years. J Clin Med. 2020;9:2663. doi: 10.3390/jcm9082663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MMB. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 67.Schmidt R, Enzinger C, Ropele S, Schmidt H, Fazekas F. Progression of cerebral white matter lesions: 6-year results of the Austrian Stroke Prevention Study. Lancet. 2003;361:2046–2048. doi: 10.1016/s0140-6736(03)13616-1. [DOI] [PubMed] [Google Scholar]
- 68.Prins ND, Van Straaten ECW, Van Dijk EJ, et al. Measuring progression of cerebral white matter lesions on MRI. Neurology. 2004;62:1533–1539. doi: 10.1212/01.wnl.0000123264.40498.b6. [DOI] [PubMed] [Google Scholar]
- 69.Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What are white matter hyperintensities made of?: relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. Prevalence of diabetes and its effects on stroke outcomes: a meta-analysis and literature review. J Diabetes Investig. 2019;10:780–792. doi: 10.1111/jdi.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roth R, Busby N, Wilmskoetter J, et al. Diabetes, brain health, and treatment gains in post-stroke aphasia. Cereb Cortex. 2023;33:8557–8564. doi: 10.1093/cercor/bhad140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Elderen SGC, de Roos A, de Craen AJM, et al. Progression of brain atrophy and cognitive decline in diabetes mellitus. Neurology. 2010;75:997–1002. doi: 10.1212/WNL.0b013e3181f25f06. [DOI] [PubMed] [Google Scholar]
- 73.Musen G, In KL, Sparks CR, et al. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes. 2006;55:326–333. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- 74.Hughes TM, Ryan CM, Aizenstein HJ, et al. Frontal gray matter atrophy in middle aged adults with type 1 diabetes is independent of cardiovascular risk factors and diabetes complications. J Diabetes Complicat. 2013;27:558–564. doi: 10.1016/j.jdiacomp.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang YF, Gu P, Zhang J, et al. Deteriorated functional and structural brain networks and normally appearing functional–structural coupling in diabetic kidney disease: a graph theory-based magnetic resonance imaging study. Eur Radiol. 2019;29:5577–5589. doi: 10.1007/s00330-019-06164-1. [DOI] [PubMed] [Google Scholar]
- 76.Yamashiro K, Tanaka R, Tanaka Y, et al. Visceral fat accumulation is associated with cerebral small vessel disease. Eur J Neurol. 2014;21:667–673. doi: 10.1111/ene.12374. [DOI] [PubMed] [Google Scholar]
- 77.García-García I, Michaud A, Jurado MÁ, Dagher A, Morys F. Mechanisms linking obesity and its metabolic comorbidities with cerebral grey and white matter changes. Rev Endocr Metab Disord. 2022;23:833–843. doi: 10.1007/s11154-021-09706-5. [DOI] [PubMed] [Google Scholar]
- 78.Gustafson DR, Steen B, Skoog I. Body mass index and white matter lesions in elderly women. An 18-year longitudinal study. Int Psychogeriatr. 2004;16:327–336. doi: 10.1017/s1041610204000353. [DOI] [PubMed] [Google Scholar]
- 79.Vuorinen M, Solomon A, Rovio S, et al. Changes in vascular risk factors from midlife to late life and white matter lesions: a 20-year follow-up study. Dement Geriatr Cogn Disord. 2011;31:119–125. doi: 10.1159/000323810. [DOI] [PubMed] [Google Scholar]
- 80.Kim KW, Seo H, Kwak MS, Kim D. Visceral obesity is associated with white matter hyperintensity and lacunar infarct. Int J Obes. 2017;41:683–688. doi: 10.1038/ijo.2017.13. [DOI] [PubMed] [Google Scholar]
- 81.Wright A, Tippett D, Saxena S, et al. Leukoaraiosis is independently associated with naming outcome in poststroke aphasia. Neurology. 2018;91:e526–e532. doi: 10.1212/WNL.0000000000005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bond B, Jackson ST, Heidrick L, Gatts J, Mumy AP. The test-retest reliability of the Western Aphasia Battery-Revised. Published online May 31, 2019. Available at: https://kuscholarworks.ku.edu/handle/1808/30075. Accessed September 4, 2023.
- 83.Kertesz A. The Western Aphasia Battery: a systematic review of research and clinical applications. Aphasiology. 2020;36:21–50. [Google Scholar]