Abstract
The term “allergic diseases” encompasses several common, IgE-mediated conditions that range from being annoying to those that are life-threatening. Available treatments include active avoidance of the instigating allergen and the use of a variety of oral, inhaled, intranasal, intraocular and injected agents. While most individuals with allergies do well with existing therapies, there are still unmet therapeutic needs. Siglecs (sialic acid-binding, immunoglobulin-like lectins) are a family of single-pass transmembrane I-type lectins found on various subsets of cells, especially those of the immune system. All Siglecs have extracellular domains recognizing sialoside ligands, and most contain cytoplasmic domains with inhibitory signaling activity. This review focuses on Siglecs that likely play a role in regulating allergic and asthmatic responses, and how specific Siglecs, expressed on cells such as eosinophils and mast cells, are being targeted for therapeutic benefit.
1. Introduction
The seminal discovery and published description of anaphylaxis (meaning “backward protection” to distinguish it from prophylaxis) by Portier and Richet in 1902 for which they won the Nobel Prize (Portier and Richet, 1902), and the subsequent identification of so-called reaginic antibody that conferred such reactivity as IgE by Ishizaka et al. and Johansson et al. in the late 1960’s (Bennich et al., 1968) helped to clarify and galvanize the definition of allergy (originally allergie) as originally coined by von Pirquet (Von Pirquet, 1946). It is now abundantly evident that allergic reactions require IgE-mediated sensitization to typically innocuous proteins, glycans and drugs like pollens, danders, dust mites, molds, foods, alpha-gal (galactose-α1,3-galactose) and penicillin, just to name a few. Once an individual is sensitized by the binding of allergen-specific IgE to the alpha chain of the heterotrimeric high-affinity IgE receptor FcεRI on tissue mast cells and blood basophils, subsequent re-exposure to that allergen triggers an allergic response with reproducible characteristic features, signs, and symptoms.
Siglecs are a family of lectin receptors, expressed mainly but not exclusively on distinct subsets of leukocytes, that recognize different configurations of sialic acid decorating a range of glycoconjugates (Duan and Paulson, 2020). Most, but not all, possess cytoplasmic domains that confer inhibitory signaling activity. It should therefore not come as a surprise that there is growing interest in leveraging Siglecs both for their inhibitory activity in order to dampen unwanted immune responses and to eliminate immune evasion when triggered surreptitiously and nefariously, e.g., by sialoglycan-coated cancers to dampen beneficial immune responses (Bochner and Zimmermann, 2015; Duan and Paulson, 2020).
The goal of this review is to briefly outline the concept of allergy to facilitate a better understanding of its primary focus which is to summarize our current understanding of how Siglecs, along with their ligands, regulate cell survival and activation on cells involved in allergic reactions and allergic inflammation and how this knowledge is being leveraged to develop and test novel therapeutics for allergic and related diseases.
2. Allergy, type 2 immunity, and allergic diseases including asthma
As mentioned above, allergy is the result of the immune system’s response to a typically harmless foreign substance that results in the production of IgE recognizing that substance. Indeed, tests for allergy involve the measurement of allergen-specific IgE in serum or the assessment of allergen-specific reactivity through a bioassay. This bioassay consists of applying specific allergens epicutaneously or percutaneously to perform skin testing which, when positive due to the presence of allergen-specific IgE attached to FcεRI on cutaneous mast cells, results in mast cell activation within minutes causing a classic localized, histamine-dependent, itchy wheal and flare response. These IgE-dependent reactions are at the core of all allergic diseases and range from seasonal and perennial allergic rhinoconjunctivitis to food and drug allergy to atopic dermatitis to many forms of asthma to life-threatening anaphylaxis, a severe form of systemic allergic response (Greenberger et al., 2019; Komlosi et al., 2022).
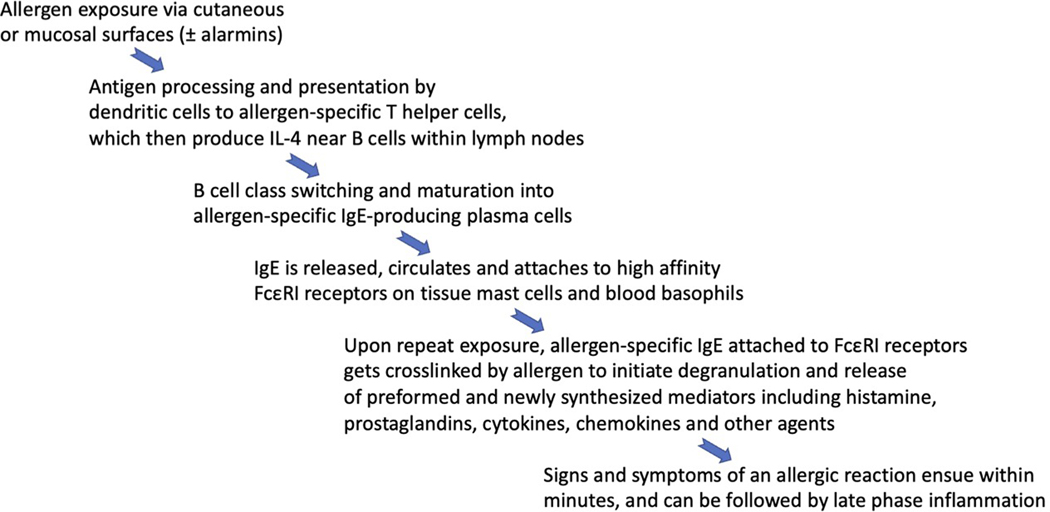
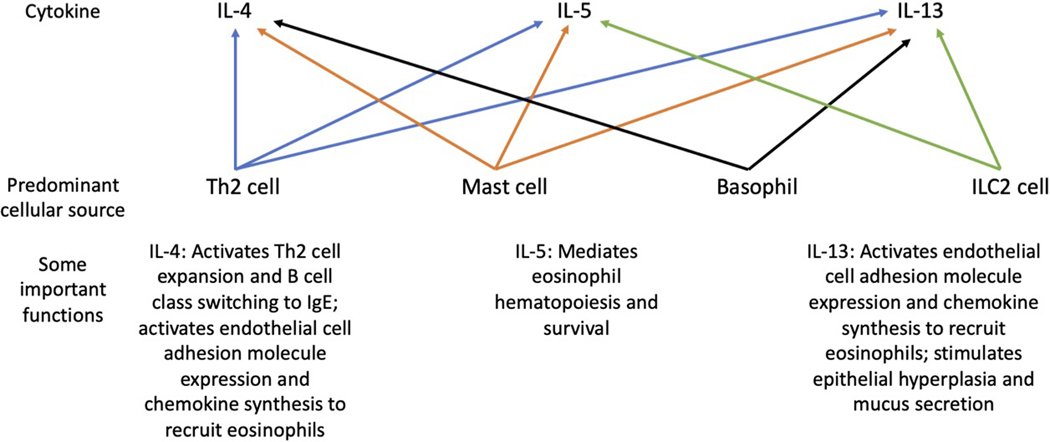
The production of IgE is the root cause of allergy, and the steps that lead to its production are complex yet well delineated (Fig. 1). Given the size constraints of this review, the interested reader is referred to textbooks and other more detailed sources of information on this topic. In brief, the production of IgE appears to stem from the arm of the immune system devoted to fighting off helminths and related parasites. Indeed, this so-called type 2 response includes a selective increase especially in the production of IgE antibody along with expansion of eosinophils and the so-called Th2 subset of T helper cells, as well as release of a distinct pattern of mediators including type 2 cytokines IL-4, IL-5, and IL-13 from these and other immune cells including mast cells, basophils, and type 2 innate lymphoid cells (ILC2s) (Fig. 2). But not all type 2 inflammatory diseases are IgE/allergen-mediated, even though many inflammatory aspects are similar, such as eosinophilic gastrointestinal disorders including eosinophilic esophagitis (Azouz and Rothenberg, 2019), chronic rhinosinusitis with nasal polyps (Schleimer, 2017) and chronic spontaneous urticaria (Kolkhir et al., 2021). Since mast cells and their histamine-containing blood counterpart, basophils, can be activated via receptors other than FcεRI (Dahlin et al., 2022; Galli et al., 2020; Kolkhir et al., 2021), allergic-like signs and symptoms can occur independent of IgE. Examples of the latter include acute reactions to radiocontrast dye infusions and various medications. In addition, not all forms of rhinitis, eczema and asthma are allergic in nature and are thus non-IgE mediated, underscoring the point that these diseases have subtypes and endotypes that are important to recognize during diagnosis because treatments for each differ depending on underlying mechanisms. Overall, for various forms of allergic and related disorders involving the airways, approved treatments include allergen immunotherapy (the goal of which is to induce immune tolerance to specific allergens), as well as mediator receptor antagonists (H1 antihistamines, leukotriene receptor antagonists), beta adrenergic agonists, topical and inhaled corticosteroids, and biologics targeting IgE, IL-4R⍺ (which blocks the activity of both IL-4 and IL-13), IL-5 and its receptor, and TSLP (thymic stromal lymphopoietin, an alarmin) (Brusselle and Koppelman, 2022; Greenberger et al., 2019; Lommatzsch et al., 2022). The fact that so many of these drugs are focused on mast cells or mast cell-derived mediators, eosinophils, and type 2 inflammatory cytokines underscores their crucial role in disease pathophysiology (Jacobsen et al., 2021). Targeting Siglecs on some of these same cells has the potential to dampen pathophysiologic pathways by directly inhibiting pathogenic cells that cause allergic diseases (Bochner, 2016; O’Sullivan et al., 2020); discussions that follow will expand upon this topic.
Figure 1.
Sequential events resulting in the development of allergic reactivity.
Figure 2.
Type 2 cytokines, their predominant sources, and examples of important functions in humans.
3. Siglecs relevant to effector cells in allergic responses
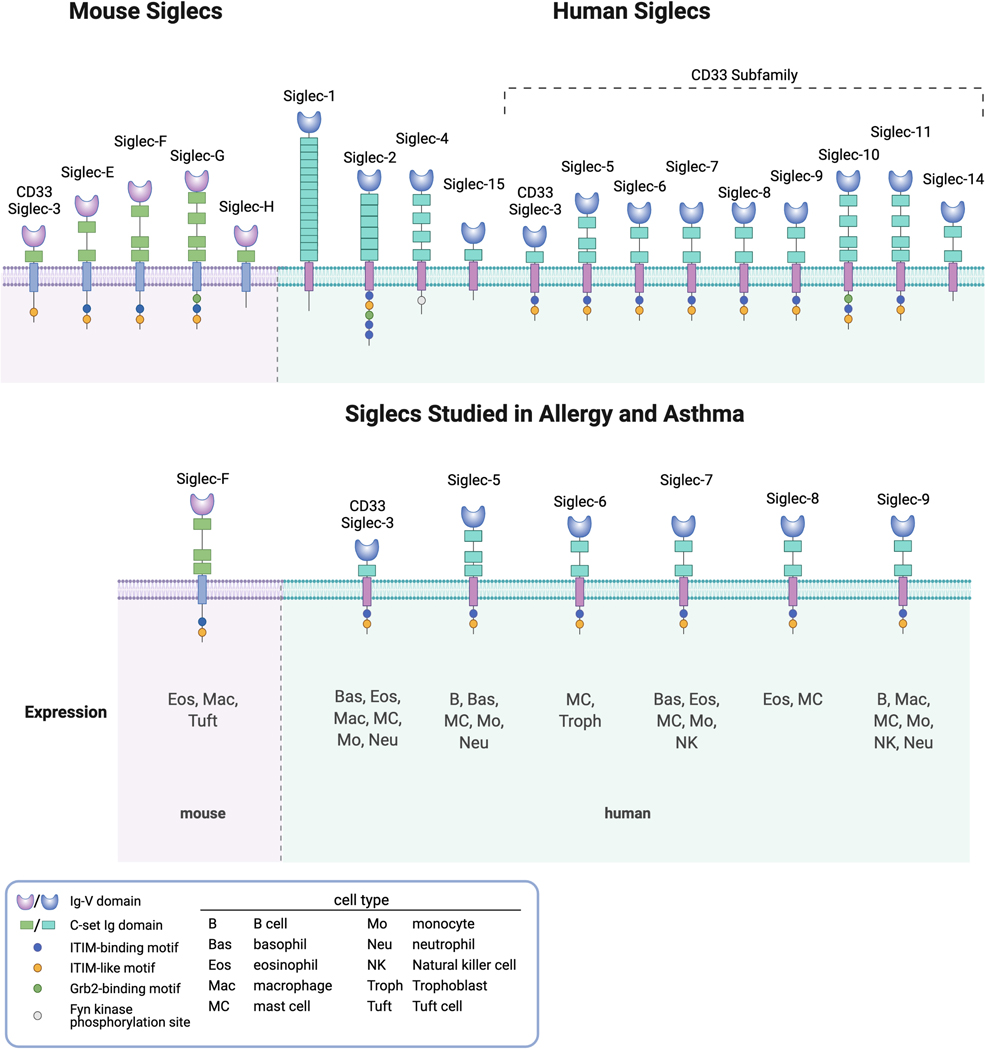
Siglecs are single-pass transmembrane receptors with N-terminal sialic acid-binding lectin domains predominantly expressed on the surface of leukocytes and are found on cells of virtually all vertebrate species. Most, but not all, contain cytoplasmic domains possessing immunoreceptor tyrosine-based inhibitory motifs (ITIMs) and immunoreceptor tyrosine-based switch motifs (ITSMs) that recruit phosphatases and thus facilitate inhibitory signaling. Siglecs are typically divided into two groups: those related to Siglec-3 (CD33) and all others, the latter group being more highly conserved as true orthologs, at least among mice and humans (Fig. 3). For the CD33-related subset of Siglecs, their genes tend to be clustered primarily in one location, such as on chromosome 19 in humans and non-human primates, chromosome 7 in mice and chromosome 1 in rats and dogs, suggesting that they evolved via gene duplication (Angata et al., 2004; Cao and Crocker, 2011; Cao et al., 2009). Some Siglecs, such as CD33, are highly conserved across species, while others are unique to humans and non-human primates, such as Siglec-8 (humans and chimpanzees) and Siglec-10 (humans, chimpanzees, and rhesus) (Cao et al., 2009; Hudson et al., 2011). For simplicity, clarity, and space constraints, as well as the fact that we know the most about them, what follows will focus on mouse and human Siglecs in allergy (Fig. 3). And given that the topic is allergies and asthma, most of the discussion will center around mast cells, basophils, eosinophils, and IgE-producing plasma cells. One final intriguing point worth mentioning is that ILC2 cells and CD4+ T cells, including the Th2 subset, do not appear to express any Siglecs.
Figure 3.
Mouse and human Siglecs, with a focus on those expressed on cells involved in allergy and asthma.
3.1. Mouse versus human Siglec expression patterns and allergy
When considering Siglecs relevant to mouse and human mast cells, eosinophils, basophils and plasma cells, there are more differences across species than similarities (Duan and Paulson, 2020; Varki et al., 2017), hence the motivation to make mice that express human Siglecs in ways that recapitulate their cellular expression patterns on human cells when the goal is to study human Siglec function in a preclinical model ((McCord and Macauley, 2022) and see below). For example, IgE-producing plasma cells in mice and humans have not specifically been studied for their expression of Siglecs, but these cells originate from B cells, which in humans express CD22, Siglec-5, Siglec-9 and Siglec-10 and in mice express CD22 and Siglec-G (Duan and Paulson, 2020). In other cases, expression patterns among mouse versus human cells are quite different. For instance, only CD33 has been detected on mouse mast cells (Akula et al., 2020) whereas human mast cells also express CD33 (note that mouse and human CD33 are considered orthologs but have different transmembrane and cytoplasmic domains and functions (Brinkman-Van der Linden et al., 2003; Duan et al., 2019)), but also express Siglec-6, Siglec-7, Siglec-8 and low levels of CD22, Siglec-5 and Siglec-10 (Florian et al., 2006; O’Sullivan et al., 2020; Yokoi et al., 2006). Human basophils express CD33, Siglec-5, Siglec-7, and low levels of CD22 and Siglec-8 (Florian et al., 2006; Mizrahi et al., 2014) but nothing is reported about mouse basophil Siglec expression. Human eosinophils express CD33, Siglec-7, Siglec-8 and Siglec-10 (Legrand et al., 2019b; O’Sullivan et al., 2020; Youngblood et al., 2021), while mouse eosinophils express CD33 and Siglec-F, the latter considered a functional paralog of Siglec-8 because of shared expression by eosinophils and some overlapping recognition of specific glycan ligand (Kiwamoto et al., 2015; Tateno et al., 2005). Siglec-F, unlike Siglec-8, is not expressed on mouse mast cells, and unlike human cells is expressed on alveolar macrophages and tuft cells of the gastrointestinal tract (Gicheva et al., 2016; Stevens et al., 2007). Put another way, there is no mouse ortholog of Siglec-5 or Siglec-6, while Siglec-9 is an ortholog of Siglec-E and Siglec-10 is an ortholog of Siglec-G, yet expression of orthologs vary between mice and humans. It is noteworthy that in humans, expression of Siglec-6 is particularly selective for mast cells (Plum et al., 2020; Robida et al., 2022) and trophoblastic cells of the placenta (Takei et al., 1997), while Siglec-8 is selectively expressed on mast cells and eosinophils (Bochner, 2016), and expression of Siglec-8 polymorphisms associate with asthma (Akula et al., 2020; Gao et al., 2010). Little is known about what controls this cellular selectivity at the promotor or epigenetic level other than a possible role for the transcription factor Olig2 in regulating Siglec-8 gene expression (Hwang et al., 2016) and the finding that leukemic cells can aberrantly express Siglec-6 (Jetani et al., 2021; Kovalovsky et al., 2021). Given the strong selectivity of expression of CD33, Siglec-6, Siglec-8, and mouse Siglec-F on allergic effector cells, the next two sections will focus exclusively on these receptors when reviewing what is known about their ligands.
3.2. Glycan ligands for CD33, Siglec-6, Siglec-8, and Siglec-F
By definition, Siglecs bind sialic acids, which exist as terminal sugars displayed on glycoproteins and glycolipids in α2,3, α2,6, or α2,8 linkages (Cummings and Pierce, 2014; Gonzalez-Gil and Schnaar, 2021; Schnaar, 2015, 2016). This binding occurs within the N-terminal lectin domain in a binding pocket that contains an arginine residue required for carbohydrate binding activity (Propster et al., 2016). In determining sialic acid binding specificities, numerous approaches have been employed. Among these, glycan arrays and related assays have been particularly fruitful (Blixt et al., 2004; Bochner et al., 2005; Bull et al., 2021; Cummings and Pierce, 2014; Rodrigues et al., 2020). Such approaches, which assay binding of Siglec constructs such as a Siglec-Ig fusion protein, have determined that CD33 and Siglec-6 prefer α2,6-linked sialic acid, while Siglec-8 and Siglec-F bind α2,3-linked sialic acid (Gonzalez-Gil and Schnaar, 2021). However, glycan interactions can be difficult to predict and involve numerous factors other than the sialic acid glycosidic linkage. For example, Siglec-8 binding also requires sulfation of the 6′ position on an adjacent galactose (this structure is also a ligand for Siglec-F); Siglec-8 glycan ligands such as 6’-sulfated sialyl Lewis X can be recognized by CD33 but not by other Siglecs; and Siglec-F recognizes some additional, multiantennary α2,3-linked sialic acid glycans that are not recognized by Siglec-8 (Bull et al., 2021; Kiwamoto et al., 2015; Propster et al., 2016). This knowledge has led to the development and testing of various glycans and glycomimetic agents, such as those that preferentially bind CD33 and Siglec-8 (Hudson et al., 2009; Kroezen et al., 2020; Nycholat et al., 2019; Rillahan et al., 2014). Implicit in these observations is the requirement for sialyl- and sulfotransferases necessary for the cellular synthesis of requisite glycan ligands. In mice, for example, animals lacking the St3gal3 gene product α2,3 sialyltransferase (ST3Gal-III) are incapable of making Siglec-F lung ligands (Guo et al., 2011). Similar approaches using specific sulfotransferase (e.g., keratan sulfate galactose 6-O-sulfotransferase (CHST1)) null mice (Kumagai et al., 2018; Patnode et al., 2013) or cell lines overexpressing sulfotransferases (Jung et al., 2021) have added to our knowledge of Siglec binding specificities and the requirement (or not) for sulfation for glycan binding to Siglec-F, Siglec-8 and CD33. Therefore, while glycan binding specificities for each Siglec can be overlapping or distinct, conformations of sialoside ligands confer this specificity.
3.3. Endogenous glycoprotein ligands for CD33, Siglec-6, Siglec-8, and Siglec-F
Unlike the approaches just mentioned above, different strategies are needed to identify the glycoprotein (most commonly) or glycolipid carrier of each glycan ligand, its so-called “natural ligand” (Varki, 2009). Here, tissues or biological fluids are typically screened using histochemistry or shotgun glycomics with Siglec-Ig fusion proteins or related scaffolds, along with western blotting, affinity chromatography, and mass spectrometry to identify endogenous ligands (Gonzalez-Gil and Schnaar, 2021; Park et al., 2020; Schnaar, 2016; Song et al., 2011). As a result, we now know that in mouse lung, Siglec-F ligands are carried mainly by the mucin Muc5b and to a much smaller extent by Muc4 (Kiwamoto et al., 2015). While reported ligands for Siglec-6 include leptin and glycodelin A (Lam et al., 2011; Patel et al., 1999), none of the above tissue-based or biological fluid-based assays have identified endogenous ligands. Similarly, despite some overlap between glycan binding specificities with Siglec-8 and CD33, little is known about endogenous ligands for CD33. In contrast, significant progress has been made in identifying Siglec-8 ligands, but with the surprising finding of marked tissue specificity: In the lower human airway, Siglec-8 ligands are carried by aggrecan on its keratan sulfate chains (Gonzalez-Gil et al., 2018), while in both the upper and lower airways, Siglec-8 ligand is also on keratan sulfate chains but on a subset of a different glycoprotein, DMBT1 (deleted in malignant brain tumor 1) (Gonzalez-Gil et al., 2021; Jia et al., 2015; Lee et al., 2021). Whether there are any allergic disease-related variations in the amounts or glycosylation patterns in DMBT1 in allergic diseases remains unknown but is under investigation. Finally, while not necessarily relevant to allergic diseases, yet another glycoprotein carries Siglec-8 and Siglec-F ligands in the brain, namely a minor subset of phosphacan, also known as receptor protein tyrosine phosphatase zeta (RPTPζ) that also binds to CD33 (Gonzalez-Gil et al., 2022).
4. Targeting Siglecs and their ligands to modulate allergic inflammation
The selective expression profile and immunoregulatory potential of Siglecs has led to numerous studies exploring the function and therapeutic opportunity of targeting Siglecs to dampen allergic inflammation. Two main approaches have been used to exploit the inhibitory nature of Siglecs in vivo and in vitro in allergic settings (Angata et al., 2015). One strategy has been to target Siglecs with antibodies to either reduce the numbers of Siglec-expressing cells, mediate inhibition through engagement of the Siglec, or both. The second approach has been to co-crosslink an activating receptor like FcεRI with a specific Siglec using liposomal nanoparticles displaying an allergen and a high-affinity glycan Siglec ligand. Both strategies have yielded strongly positive proof-of-concept data and further support Siglecs as viable therapeutic targets in allergic disease. Indeed, antibodies against Siglec-8 and Siglec-6 are currently under clinical and pre-clinical development, respectively for the treatment of allergic and inflammatory diseases.
4.1. Mouse models in wild type and genetically modified mice
The role of Siglecs in allergic inflammation has been studied in vivo using diverse methodology due to the selective expression pattern and species specificity of mouse and human Siglecs. Given its predominant expression on mouse eosinophils, Siglec-F has been widely studied in models of allergic inflammation using knockout (KO) mice or antibody targeting. The first insight into the biological function of Siglec-F came from mice lacking Siglec-F. In a model of allergic asthma, Siglec-F KO mice had enhanced lung, blood, and bone marrow eosinophilia, perhaps due to reduced death of eosinophils compared to wild type mice (Zhang et al., 2007) suggesting Siglec-F functions as an inhibitory receptor on eosinophils. Similar airway eosinophilia findings were noted in lung allergen challenge models using mice incapable of generating lung ligands for Siglec-F due to a lack of the St3gal3 enzyme, a specific sialyl transferase (Kiwamoto et al., 2014; Kiwamoto et al., 2015; Suzukawa et al., 2013). Other studies in normal or IL-5 transgenic mice demonstrated that Siglec-F antibody administration selectively reduced blood and tissue eosinophils (Zimmermann et al., 2008). Similarly, Siglec-F antibody treatment significantly reduced circulating and tissue eosinophils and improved disease severity in multiple models of allergen-induced eosinophilic inflammation (Camilleri et al., 2021; Rubinstein et al., 2011; Song et al., 2009a; Song et al., 2009b). But a word of caution is warranted when interpreting these results because Siglec-F antibody-mediated depletion can be incomplete or relatively ineffective given that Siglec-F engagement, unlike Siglec-8 engagement, is a poor inducer of mouse eosinophil death (Knuplez et al., 2020; Mao et al., 2013). It therefore remains possible that antibodies targeting Siglec-F could be having effects due to effects on cells beyond just eosinophils, or by blocking Siglec-F ligand binding to endogenous ligands. Despite these lingering questions, this literature highlights the potential of targeting an eosinophil Siglec to attenuate allergic inflammation.
Because Siglec-F appears to regulate eosinophilic inflammation, significant effort has been made to better understand the biology of human Siglecs and their potential role in modulating allergic responses in mice. Since most of these human Siglecs are not conserved in lower species, mice have been genetically engineered to express them on relevant immune cells that contribute to allergic inflammation, including mast cells and eosinophils (McCord and Macauley, 2022). To study the role of Siglec-3 and Siglec-8 in modulating mast cell-mediated anaphylaxis in vivo, transgenic mice were generated using Cre-specific strategies to specifically express these human Siglecs on mouse mast cells (Duan et al., 2021; Duan et al., 2019; Wei et al., 2018). In these mice, liposomal nanoparticles displaying both allergen and a high-affinity glycan ligand for either Siglec-3 (CD33) or Siglec-8 profoundly suppressed IgE-mediated mast cell activation in vitro and in vivo, including systemic and cutaneous anaphylaxis (Duan et al., 2021; Duan et al., 2019). In addition to transgenic mice, humanized mice expressing human stem cell factor, granulocyte-macrophage colony stimulating factor (GM-CSF) and IL-3 engrafted with human thymus, liver, and hematopoietic stem cells have been shown to be a useful model to study Siglec function on human mast cells in mice in the context of allergy. These mice give rise to functional human mast cells in tissues that express human Siglec-3, −6, and −8 and can undergo mast cell-mediated systemic anaphylaxis (Bryce et al., 2016), as can other types of humanized mast cell mice (Dispenza et al., 2020). The administration of a Siglec-8 monoclonal antibody (mAb) significantly protected humanized mice from IgE-mediated systemic anaphylaxis (Youngblood et al., 2019b). Similarly, treatment with a Siglec-6 mAb completely protected against IgE-mediated systemic anaphylaxis and reduced mast cell-derived mediators in humanized mice (Schanin et al., 2022). These findings demonstrate that human Siglec-3, −6, and −8 can potently and effectively inhibit IgE-activated mast cells in vivo through ligand or antibody-targeting strategies.
To better understand the role of Siglec-8 in chronic allergic inflammation, a Siglec-8 transgenic mouse that bears the human SIGLEC8 gene, including the putative promoter and regulator elements, was generated. This mouse shows human-relevant expression of Siglec-8 on both mouse eosinophils and mast cells that is not dependent on a Cre-promoter (Youngblood et al., 2019a). In a model of allergen-induced eosinophilic gastroenteritis, Siglec-8 mAb treatment significantly reduced blood, gastric, and duodenal eosinophils as well as local and systemic type-2 inflammatory cytokines and chemokines (Youngblood et al., 2019a). Siglec-8 mAb treatment also reduced the infiltration of gastrointestinal mast cells, consistent with mast cell inhibition. Subsequently, the effects of Siglec-8 mAb treatment have been evaluated in a model of mast cell-driven IL-33-induced inflammation. IL-33 is an alarmin that can contribute to allergic inflammation by activating eosinophils, mast cells, basophils and ILC2s through its receptor ST2 (Chan et al., 2019). Siglec-8 transgenic mice injected with IL-33 and dosed with a Siglec-8 mAb had significantly reduced neutrophils, eosinophils, and inflammatory mediators, including IL-6, CCL2, and IL-13 (Schanin et al., 2020). Transcriptomic profiling of mast cells ex vivo revealed that Siglec-8 mAb treatment normalized the IL-33-activated mast cell transcriptome, indicative of Siglec-8-mediated mast cell inhibition. Collectively, these studies demonstrate that Siglec-8 induces inhibition of both IgE- and IL-33-mediated mast cell activation.
Respiratory viral infections cause asthmatic exacerbations by triggering the recruitment of inflammatory cells into the lungs via multiple mechanisms, including toll-like receptor (TLR) activation (Jackson and Johnston, 2010). While eosinophils and mast cells are recognized as pathogenic drivers in allergic asthma, their contribution to viral inflammation is not well established. The activity of a Siglec-8 mAb was evaluated in a model of TLR-mediated viral inflammation using polyinosinic:polycytidylic acid (poly I:C) as well as a respiratory viral infection model in Siglec-8 transgenic mice. In both settings, Siglec-8 mAb treatment reduced overall airway inflammation and protected against virus-induced pathology (Gebremeskel et al., 2021), suggesting Siglec-8 and antibodies that target this receptor can modulate viral inflammation in the lung.
Finally, while not necessarily a treatment for existing allergic diseases, at least one strategy involving Siglecs has been used to prevent production of IgE by plasma cells. By targeting both CD22 (Siglec-2) and the B cell receptor recognizing peanut allergen with liposomes, IgE-mediated sensitization to peanut was blocked. This might have been due to the elimination of B cells capable of becoming plasma cells that would have made peanut specific IgE (Orgel et al., 2017).
4.2. Human cells in vitro
In vitro experimentation has been instrumental in characterizing the functions and signaling cascades initiated by Siglec receptors on human eosinophils, mast cells, and basophils. Among the human Siglecs most comprehensively studied in vitro on these cell types is Siglec-8, particularly on eosinophils. The extensive crosslinking of Siglec-8 on peripheral blood eosinophils or the antibody engagement of Siglec-8 on eosinophils following cytokine (e.g., IL-5, GM-CSF, or IL-33) priming leads to cell death (Na et al., 2012; Nutku et al., 2003; Nutku-Bilir et al., 2008), although the mechanisms leading to cell death appear to differ somewhat between these conditions. In response to extensive crosslinking, non-cytokine-primed eosinophils undergo cell death that is caspase-dependent and involves reactive oxygen species (ROS) production and mitochondrial membrane potential (MMP) dissipation (Nutku et al., 2003; Nutku et al., 2005), whereas Siglec-8 antibody engagement on IL-5-primed eosinophils accelerates MMP dissipation and leads to caspase-independent cell death that remains dependent on ROS (Nutku-Bilir et al., 2008). Ligation of Siglec-8 on IL-5-primed eosinophils with the high-avidity ligand 6′-sulfo-sialyl Lewis X-decorated polyacrylamide likewise induces eosinophil death (Hudson et al., 2009).
The signaling and cellular events preceding cell death have also been explored in vitro in human eosinophils. Siglec-8 ligation on IL-5-primed eosinophils leads to the upregulation and conformational activation of CD11b/CD18 integrin, and CD11b/CD18-mediated adhesion is necessary for ROS generation and cell death (Carroll et al., 2018). PI3K, p38, and JNK1 are also necessary for cell death induction, and their activation is augmented by integrin-mediated adhesion. The concurrent stimulation of eosinophils with IL-5 and anti-Siglec-8 leads to cell death in a manner that is dependent on MEK/ERK signaling only after ROS production, implicating these enzymes in a later stage of the cell death process (Kano et al., 2013). Only recently have the early signaling events connecting Siglec-8 ligation to integrin upregulation been elucidated. In IL-5-primed eosinophils, Siglec-8 ligation initiates a signaling cascade involving a SFK, Syk, PI3K, PAK1, Rac-1, MEK1, ERK1/2, PLC, and PKC that leads to CD11b upregulation (Carroll et al., 2021). The enzyme Btk is involved in the conformational activation of CD11b. Actin filament depolymerization is necessary and sufficient for CD11b upregulation, but actin polymerization is necessary for ROS production following integrin involvement.
In vitro assays have also been used to establish that Siglec-8 is internalized into human eosinophils in a manner that requires actin rearrangement and the activities of a tyrosine kinase and PKC (O’Sullivan et al., 2018). This process can also be exploited to deliver therapeutic or toxic cargo to eosinophils, e.g., under conditions in which Siglec-8 ligation alone would not consistently lead to cell death.
Beyond in vivo studies, the signaling and function of Siglec-F on mouse eosinophils has also been studied in vitro. Siglec-F ligation induces receptor internalization (Tateno et al., 2007) as well as cell death of mouse eosinophils, but to a minimal extent compared with its closest functional paralog in humans, Siglec-8 (Knuplez et al., 2020; Mao et al., 2013). This pathway also differs from that initiated by Siglec-8 engagement in that it is independent of both SFK activity and ROS and is not associated with CD11b upregulation (Knuplez et al., 2020; Mao et al., 2013). Siglec-F antibody ligation exerts additional—and unanticipated—effects, such as the potentiation of IL-33-induced activation of and IL-4, IL-13, CCL3, and CCL4 secretion from mouse bone marrow-derived eosinophils (Westermann et al., 2022).
Human eosinophils additionally express Siglec-7 (Munitz et al., 2006). The engagement of Siglec-7, unlike Siglec-8, does not lead to cell death but rather to the reduction of GM-CSF-stimulated release of EPX, TNF-α, and IL-8 from human eosinophils (Legrand et al., 2019b). This is accompanied by the phosphorylation of SHP-1 and the inhibition of ERK1/2 and p38 phosphorylation.
Siglec-8 and Siglec-7 have been investigated in vitro on human mast cells as well. The ligation of Siglec-8 on human CD34+ cell-derived mast cells was found to inhibit FcεRI-mediated Ca2+ flux and release of histamine and PGD2 as well as reduce IgE-mediated human bronchial ring smooth muscle contraction (Yokoi et al., 2008). Co-crosslinking of FcεRIα and Siglec-8 has additionally been shown to inhibit the activation and degranulation of mouse bone marrow-derived and human CD34+ cell-derived mast cells as well as ex vivo human lung tissue mast cells (Kerr et al., 2020; Korver et al., 2022). Phospho-proteomic profiling of FcεRI-activated mast cells revealed Siglec-8 mAb treatment globally inhibited proximal and downstream kinases, leading to attenuated mast cell activation and degranulation. In fact, Siglec-8 was found to directly interact with FcεRI signaling molecules. Siglec-8 inhibition was dependent on both cytoplasmic ITIMs that interact with the SH2 containing protein phosphatase Shp-2 upon Siglec-8 phosphorylation. These findings support a model in which Siglec-8 regulates proximal FcεRI-induced phosphorylation events through phosphatase recruitment and interaction with FcεRI that results mast cell inhibition upon Siglec-8 mAb engagement (Korver et al., 2022). Ligation of Siglec-8 with an antibody has also been shown to inhibit IgE-independent mast cell activation in vitro, including IL-33-mediated neutrophil migration (Schanin et al., 2020). Although Siglec-8 engagement itself does not induce cell death of human mast cells, the Siglec-8 endocytic pathway can be exploited to deliver toxic cargo to cause the death of even human mast cell lines, like HMC-1.2 (O’Sullivan et al., 2018).
Co-crosslinking Siglec-7 with FcεRI on human CD34+ cell-derived mast cells inhibits degranulation and the release of the PGD2 and GM-CSF (Mizrahi et al., 2014). This inhibition requires co-crosslinking with FcεRI and is accompanied by the recruitment and phosphorylation of SHP-1, implicating this phosphatase in inhibitory signaling. Co-crosslinking of Siglec-7 with FcεRI on human basophils isolated from peripheral blood also inhibits degranulation, although this effect is weaker than observed on mast cells (Mizrahi et al., 2014). This diminished inhibitory effect may be related to its reduced expression on basophils relative to mast cells. The functions of other Siglecs on human basophils have not been characterized.
CD33 plays a similar inhibitory role upon co-crosslinking with FcεRI. Co-crosslinking using antigenic liposomes bearing a lipid-modified CD33 glycan ligand inhibits calcium flux and degranulation of the human mast cell line LAD2 as well as the IgE-mediated bronchoconstriction of human lung sections in vitro (Duan et al., 2019).
Siglec-6 also exerts inhibitory effects on mast cell activation and mediator release. Ligation of Siglec-6 concurrent with stimulation through FcεRI, C5aR, or MRGPRX2 reduces activation of human CD34+ cell- or skin tissue-derived mast cells (Robida et al., 2022; Yu et al., 2018). Furthermore, the co-crosslinking of Siglec-6 with FcεRI potentiates its inhibitory effects on mast cell degranulation and cytokine/chemokine secretion and reduces activating phosphorylation of ERK1/2 and p38 (Robida et al., 2022). Although Siglec-6 undergoes endocytosis on human mast cells, it is maintained at the cell surface for longer periods than Siglec-8 on the same cells.
4.3. Lirentelimab (AK002) pharmacology and clinical activity
The selective targeting of eosinophils and mast cells by Siglec-8 provides a promising approach for the treatment of a wide range of allergic and inflammatory diseases. Lirentelimab (AK002) is a humanized non-fucosylated IgG1 kappa antibody that was generated by the humanization of the mouse Siglec-8 mAb 2E2 clone (its precursor) (Nutku et al., 2003; Youngblood et al., 2019b). Lirentelimab was developed as a non-fucosylated IgG1 antibody to enhance its binding affinity to FcγRIIIA, (CD16a), an Fc-receptor that mediates ADCC (antibody-dependent cellular cytotoxicity) (Falconer et al., 2018). Lirentelimab or its chimeric precursor mAb depletes human blood eosinophils in vitro via a mechanism that is dependent on its afucosylated backbone, consistent with ADCC-mediated activity (Legrand et al., 2019a; Youngblood et al., 2019b). In addition to ADCC, lirentelimab, like mouse anti-Siglec-8 mAbs (Nutku-Bilir et al., 2008), induces cell death of IL-5-activated eosinophils isolated from peripheral blood, highlighting an additional mechanism for eosinophil depletion within tissues where eosinophils may be primed by cytokines. When using dissociated human lung tissues from multiple donors, lirentelimab significantly reduced tissue eosinophil numbers and inhibited IgE-mediated mast cell activation (Kerr et al., 2020; Youngblood et al., 2019b). Interestingly, mast cells were not reduced. The lack of ADCC activity against tissue mast cells by lirentelimab is most likely due to the preponderance of the less cytotoxic NK cell population (CD16Lo CD56Hi) in most human tissues compared to the more cytotoxic, ADCC-inducing NK cell population (CD16Hi CD56Lo) found in the blood (Stabile et al., 2017). In support of this, co-culturing human mast cells from systemic mastocytosis patients with blood NK cells (CD16Hi CD56Lo ) reduced mast cell numbers via ADCC in the presence of lirentelimab (Falahati et al., 2015). Collectively, the targeting Siglec-8 of with lirentelimab provides a selective therapeutic option for the treatment of allergic and inflammatory diseases associated with eosinophils and mast cells.
Lirentelimab has been evaluated in multiple allergic and inflammatory diseases, including indolent systemic mastocytosis (ISM), chronic urticaria (CU), severe allergic conjunctivitis (SAC), and eosinophilic gastrointestinal diseases (EGIDs) (Table 3). The first-in-human study with lirentelimab was an open-label phase 1 study in patients with ISM. ISM is a rare disease characterized by clonal, constitutively activated mast cells that can release excessive mediators contributing to an array of debilitating symptoms and substantial quality of life impairment. Study subjects received monthly infusions of lirentelimab over 6 months, with a starting dose of 1 mg/kg and subsequent doses of 1, 3, 6, or 10 mg/kg. Lirentelimab was well tolerated and produced consistent and substantial improvements across all ISM symptom domains, including skin, gastrointestinal, neurological, and musculoskeletal, as measured by patient-reported outcome questionnaires (Siebenhaar et al., 2019; Youngblood et al., 2021).
Table 3:
Clinical studies with Lirentelimab
| Study Number | Indication or Population | Study Design | Planned Enrollment | Study Status as of March 2022 |
|---|---|---|---|---|
| AK002-001 NCT02808793 |
Indolent systemic mastocytosis | Phase 1, first-in-human, open label, dose escalation, single and repeat-dose of lirentelimab IV | 25 | Study completed (Siebenhaar et al., 2019) |
| AK002-002 NCT02859701 |
Healthy volunteers | Phase 1, placebo-controlled, dose escalation, single and repeat dose of lirentelimab IV | 51 total 15 placebo 36 lirentelimab |
Study completed (Rasmussen et al., 2018) |
| AK002-003 (ENIGMA 1) NCT03496571 |
Eosinophilic gastritis and/or Eosinophilic gastroenteritis | Phase 2, randomized, double-blind placebo-controlled, repeat dose of lirentelimab IV | 65 total 22 placebo 43 lirentelimab |
Study completed (Dellon et al., 2020) |
| AK002-005 NCT03379311 |
Severe allergic conjunctivitis | Phase 1, open label, repeat dose of lirentelimab IV | 27 | Study completed (Anesi et al., 2022) |
| AK002-006 NCT03436797 |
Chronic urticaria | Phase 2, open label, repeat dose of lirentelimab IV | 47 | Study completed (Altrichter et al., 2021) |
| AK002-011 | Mast cell gastritis and gastroenteritis | Phase 1, open label, repeat dose of lirentelimab IV | 10-15 | Study completed |
| AK002-014 (KRYPTOS) NCT04322708 |
Eosinophilic esophagitis | Phase 2/3, randomized, double-blind placebo-controlled, repeat dose of lirentelimab IV | 300 245 |
Double-blind period completed Extension period in progress |
| AK002-016 (ENIGMA 2) NCT04322604 |
Eosinophilic gastritis and/or Eosinophilic duodenitis | Phase 3, randomized, double-blind placebo-controlled, repeat dose of lirentelimab IV | 160 159 |
Double-blind period completed Extension period in progress |
| AK002-017 NCT04324268 |
Healthy volunteers | Phase 1 study to evaluate safety, tolerability, and bioavailability of lirentelimab SC | 66 total 36 lirentelimab SC 12 Placebo SC 18 lirentelimab IV |
Study completed |
| AK002-018 NCT05155085 |
Atopic dermatitis | Phase 2, placebo-controlled, double-blind, randomized repeat dose of lirentelimab SC | 130 total 65 placebo 65 lirentelimab |
Study in progress |
| AK002-021 NCT04856891 |
Eosinophilic duodenitis | Phase 3, placebo-controlled, double-blind, randomized repeat dose of lirentelimab IV | 94 | Study in progress |
Abbreviations: IV, intravenous; SC, subcutaneous
Chronic urticaria, including chronic spontaneous (CSU) and chronic inducible urticaria (CIndU), is a mast cell-driven disease, characterized by recurrent pruritic hives and itching, angioedema, or both for >6 weeks. These patients have debilitating symptoms, and many do not respond to approved therapies, such as antihistamines and omalizumab (Maurer et al., 2011). Lirentelimab demonstrated beneficial activity in an open-label phase 2a trial that enrolled patients with CSU (omalizumab naïve and refractory) and CIndU (such as cholinergic and symptomatic dermographism) (Altrichter et al., 2021). Patients received six monthly intravenous doses of lirentelimab (0.3, 1 and up to 3 mg/kg) with a primary efficacy endpoint of change in urticaria control test (UCT) score at week 22. Urticaria activity score (UAS7) was assessed in patients with CSU while CholUAS7 scores were used for CholU patients. The study enrolled 45 patients in 4 cohorts, including omalizumab-naïve CSU, omalizumab-refractory CSU, cholinergic urticaria, and symptomatic dermographism. UCT scores improved with lirentelimab across cohorts, with mean changes at week 22 of 11.1±4.1, 4.8±7.0, 6.5±6.2, and 3.4±4.1 with complete response rates (UCT ≥12) of 92%, 36%, 82%, and 40%, respectively. In addition, disease activity improved at week 22 in omalizumab-naïve and –refractory CSU patients (mean UAS7 change: −73% and −47%, respectively), with UAS7 response rates (≥50% reduction) of 77% and 45%, respectively. In symptomatic dermographism patients, 50% and 40% had complete itch and hive resolution by FricTest, respectively, and 100% of CholU patients had negative responses to pulse-controlled ergometry exercise test. Lirentelimab was generally well-tolerated with no treatment-related serious adverse events reported.
In addition to ISM and chronic urticaria, lirentelimab demonstrated clinical activity in relieving the signs and symptoms of allergic conjunctivitis, including reducing ocular inflammation and symptoms of concomitant atopic dermatitis, asthma, and rhinitis (Anesi et al., 2022). In this open-label phase 1b study patients with chronic, severely symptomatic atopic keratoconjunctivitis (AKC), vernal keratoconjunctivitis (VKC) and perennial allergic conjunctivitis (PAC), and who had a history of topical or systemic corticosteroid use, were enrolled to receive up to six monthly lirentelimab infusions (Dose 1: 0.3 mg/kg, Dose 2: 1 mg/kg, subsequent doses: 1 or 3 mg/kg). Lirentelimab improved ocular signs and symptoms across AKC, VKC, and PAC groups as measured by investigator- and patient-reported outcome questionnaires. In addition, patients with atopic comorbidities, including atopic dermatitis, asthma, and rhinitis demonstrated meaningful improvement, suggesting lirentelimab treatment may be effective in treating a broad spectrum of allergic diseases. Consistent with the improvement in signs and symptoms, decreased levels of type-1, type-2, and type-17 cytokines and chemokines were observed post-lirentelimab treatment in patient tears, including CCL3, CCL5, IL-4, IL-13, CCL11, CCL26, and IL-17A and most of these mediators returned to baseline levels after infusions were stopped.
Eosinophilic gastrointestinal disorders are clinicopathologic conditions characterized by chronic gastrointestinal symptoms and elevated eosinophil numbers in gastrointestinal tissues. For example, biopsies from patients with eosinophilic esophagitis (EoE) and eosinophilic gastritis/duodenitis (EoG/EoD) have been shown to have elevated and activated eosinophils and mast cells (Ben-Baruch Morgenstern et al., 2022; Youngblood et al., 2019a). The randomized, double-blind, placebo-controlled, multicenter Phase 2 ENIGMA trial evaluated lirentelimab for the treatment of EoG and EoD (Dellon et al., 2020). The study enrolled 65 patients with EoG, EoD, or both. A daily patient-reported outcome questionnaire and central pathology reading were used to assess efficacy. Patients received 4 monthly IV infusions of either 1 or 3 mg/kg lirentelimab or placebo. The primary endpoint was the change in eosinophil count from baseline to 2 weeks after the final dose. Secondary endpoints included treatment response (>30% reduction in total symptom score and >75% reduction in gastrointestinal eosinophil count) and change in total symptom score (TSS).
In the intention-to-treat analysis of the primary endpoint, the mean percentage change in gastrointestinal eosinophil count was −86% in the combined lirentelimab groups and 9% in the placebo group (p<0.001). Treatment response was observed in 63% of patients treated with lirentelimab and 5% of patients treated with placebo (p<0.001). The mean change in TSS was −48% in the lirentelimab group and −22% in the placebo group (p=0.004). Similar statistically significant results were seen in the pre-specified per-protocol analyses. Lirentelimab was generally well-tolerated. The most common adverse event was mild-to-moderate infusion-related reactions (IRRs) including flushing, a feeling of warmth, headache, nausea, or dizziness. IRRs were reported in 60% of all patients treated with lirentelimab and 23% of patients in the placebo group. Other adverse events were similar among the groups. Serious adverse events occurred in 9% of patients treated with lirentelimab and 14% of patients receiving placebo. Similar efficacy and safety outcomes were seen in an open-label extension study.
Subsequently, lirentelimab has been administered in a Phase 3 EoG/EoD study as well as a Phase 2/3 EoE study. To date, the results of these studies have not been published, however the sponsor has announced that lirentelimab met the histologic co-primary endpoint for both studies but did not meet statistical significance in the symptomatic co-primary endpoints for both studies (https://investor.allakos.com/news-releases/news-release-details/allakos-announces-topline-phase-3-data-enigma-2-study-and-phase).
5. Summary and conclusions
Allergic effector cells, including eosinophils, mast cells and basophils, are centrally involved in the pathophysiology of allergy and asthma. Their activation results in the release of numerous preformed and newly synthesized mediators that contribute to the signs and symptoms of these disorders. There are several drugs approved for use or in development, ranging from small molecules to biologics, that target many of the substances released by, or that act on, these cells. But those that eliminate these cells or deliver signals to dampen or prevent their activation, are sorely lacking. Siglecs, by virtue of these functional inhibitory activities, show great promise as novel targets for asthma and allergies.
Table 1.
Summary of the effects of Siglec targeting in mouse models of disease.
| Disease Model | Siglec Targeted | Mouse Strain | Effect |
|---|---|---|---|
| Anaphylaxis | Human CD33 (Siglec-3) | hCD33 knock-in | Suppresses mast cell activation and protects against anaphylaxis (Duan et al., 2019) |
| Siglec-6 | Humanized | Protects against anaphylaxis and reduces mast cell-derived mediators (Schanin et al., 2022) | |
| Siglec-8 | Siglec-8 knock-in and humanized | Suppresses mast cell activation and protects against anaphylaxis (Duan et al., 2021; Youngblood et al., 2019b) | |
| Allergic asthma | Siglec-F | Wild type vs. Siglecf−/−, St3gal3−/−, Muc5bΔ/Δ | Lack of Siglec-F or its ligands exacerbate blood, bone marrow, and airway eosinophilia (Kiwamoto et al., 2014; Kiwamoto et al., 2015; Suzukawa et al., 2013; Zhang et al., 2007) |
| Allergen-induced eosinophilic inflammation | Siglec-F | Wild type | Anti-Siglec-F Ab administration decreases blood and tissue eosinophil numbers and ameliorates disease severity (Camilleri et al., 2021; Rubinstein et al., 2011; Song et al., 2009a; Song et al., 2009b) |
| EGIDs | Siglec-8 | Siglec-8 transgenic | Reduces blood and tissue eosinophil numbers, diminishes type-2 inflammatory cytokines and chemokines, reduces mast cell GI infiltration (Youngblood et al., 2019a) |
| IL-33-mediated inflammation | Siglec-8 | Siglec-8 transgenic | Reduces neutrophil and eosinophil infiltration, decreases inflammatory mediators (Schanin et al., 2020) |
| TLR-mediated inflammation, viral infection | Siglec-8 | Siglec-8 transgenic | Reduces airway inflammation and protects against virus-induced pathology (Gebremeskel et al., 2021) |
Table 2.
Summary of the effects of Siglec engagement in vitro on eosinophils, mast cells, and basophils.
| Cell Type | Siglec Targeted | Effect |
|---|---|---|
| Eosinophil | Siglec-7 | Inhibition of GM-CSF-induced degranulation and cytokine secretion (Legrand et al., 2019b) |
| Siglec-8 | Cell death (upon extensive crosslinking or antibody ligation after cytokine priming) (Na et al., 2012; Nutku et al., 2003; Nutku-Bilir et al., 2008) | |
| Siglec-F (mouse eosinophils) | Slight induction of ROS- and SFK-independent cell death (Mao et al., 2013); enhancement of IL-33-induced cellular activation and cytokine/chemokine secretion (Westermann et al., 2022) | |
| Mast cell | Siglec-3 | Inhibition of LAD2 cell degranulation, inhibition of IgE-mediated bronchoconstriction in vitro (with FcεRI co-crosslinking) (Duan et al., 2019) |
| Siglec-6 | Inhibition of degranulation and cytokine/chemokine secretion (enhanced by co-crosslinking with FcεRI) (Robida et al., 2022; Yu et al., 2018) | |
| Siglec-7 | Inhibition of degranulation and cytokine/chemokine secretion (upon co-crosslinking with FcεRI) (Mizrahi et al., 2014) | |
| Siglec-8 | Inhibition of calcium flux, degranulation, and cytokine/chemokine secretion (upon antibody ligation or co-crosslinking with FcεRI); inhibition of IL-33-mediated cytokine/chemokine secretion (Korver et al., 2022; Yokoi et al., 2008) | |
| Basophil | Siglec-7 | Inhibition of degranulation (Mizrahi et al., 2014) |
Funding
This research was funded by The Mastocytosis Society and the American Academy of Allergy, Asthma and Immunology (J.A.O.); the National Heart, Lung, and Blood Institute (P01 HL107151 to B.S.B.); and the National Institute of Allergy and Infectious Diseases (U19 AI136443 and R21 AI159586 to B.S.B., and U19 AI070535 subaward 107905120 to J.A.O.).
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- AKC
atopic keratoconjunctivitis
- Btk
Bruton’s tyrosine kinase
- C5aR
receptor for the complement protein C5a
- CCL
CC chemokine ligand
- CholU
cholinergic urticaria
- CholUAS7
cholinergic urticaria UAS7
- CIndU
chronic inducible urticaria
- CSU
chronic spontaneous urticaria
- DMBT1
deleted in malignant brain tumor 1
- EGID
eosinophilic gastrointestinal disorder
- EoD
eosinophilic duodenitis
- EoE
eosinophilic esophagitis
- EoG
eosinophilic gastritis
- EPX
eosinophil peroxidase
- ERK
extracellular signal-regulated kinase
- FcεRI
high affinity IgE receptor
- GM-CSF
granulocyte-macrophage colony stimulating factor
- IgE
immunoglobulin E
- IL
interleukin
- IRR
infusion-related reaction
- IV
intravenous
- ISM
indolent systemic mastocytosis
- JNK1
c-Jun N-terminal kinase
- KO
knock out
- mAb
monoclonal antibody
- MEK
mitogen-activated protein kinase kinase
- MMP
mitochondrial membrane potential
- MRGPRX2
Mas-related G protein-coupled receptor X2
- p38
p38 mitogen-activated protein kinase
- PAC
perennial allergic conjunctivitis
- PAK1
serine/threonine p21-activated kinase 1
- PGD
prostaglandin D
- PI3K
phosphatidylinositol-3 kinase
- PKC
protein kinase C
- PLC
phospholipase C
- Rac-1
Ras-related C3 botulinum toxin substrate 1
- ROS
reactive oxygen species
- RPTPζ
receptor protein tyrosine phosphatase zeta
- SAC
severe allergic conjunctivitis
- SC
subcutaneous
- SFK
Src family non-receptor tyrosine kinase
- SHP
Src homology 2 domain-containing protein phosphatase
- Siglec
sialic acid-binding immunoglobulin-like lectin
- Syk
spleen tyrosine kinase
- Th2
type 2 helper T cell
- TLR
toll like receptor
- TNF
tumor necrosis factor
- TSLP
thymic stromal lymphopoietin
- TSS
total symptom score
- UAS7
urticaria activity score over 7 days
- UCT
urticaria control test
- VKC
vernal keratoconjunctivitis
Footnotes
Conflict of interest statement
B.S.B. receives remuneration for serving on the scientific advisory board of Allakos, Inc. and owns stock in Allakos. He receives publication-related royalty payments from Elsevier and UpToDate. He is a co-inventor on existing Siglec-8-related patents and thus may be entitled to a share of royalties received by Johns Hopkins University during development and potential sales of such products. B.S.B. is also a co-founder of Allakos, Inc. which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies. J.A.O. has nothing to disclose. A.T.C and B.A.Y. are current employees of and/or own stock and/or stock options from Allakos, Inc.
References
- Akula S, Paivandy A, Fu Z, Thorpe M, Pejler G, Hellman L, 2020. Quantitative in-depth analysis of the mouse mast cell transcriptome reveals organ-specific mast cell heterogeneity. Cells 9 (1), 211. doi: 10.3390/cells9010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altrichter S, Staubach P, Pasha M, Singh B, Chang AT, Bernstein JA, Rasmussen HS, Siebenhaar F, Maurer M, 2021. An open-label, proof-of-concept study of lirentelimab for antihistamine-resistant chronic spontaneous and inducible urticaria. J. Allergy Clin. Immunol. doi: 10.1016/j.jaci.2021.12.772. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Anesi SD, Tauber J, Nguyen QD, Chang P, Berdy GJ, Lin CC, Chu DS, Levine HT, Fernandez AD, Roy N, Asbell PA, Kantor AM, Chang AT, Singh B, Youngblood BA, Jeng BH, Jhanji V, Rasmussen HS, Foster CS, 2022. Lirentelimab for severe and chronic forms of allergic conjunctivitis. J. Allergy Clin. Immunol. doi: 10.1016/j.jaci.2022.03.021. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Angata T, Margulies EH, Green ED, Varki A, 2004. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc. Natl. Acad. Sci. USA 101 (36), 13251–13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angata T, Nycholat CM, Macauley MS, 2015. Therapeutic targeting of siglecs using antibody- and glycan-based approaches. Trends Pharmacol. Sci. 36 (10), 645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz NP, Rothenberg ME, 2019. Mechanisms of gastrointestinal allergic disorders. J. Clin. Invest. 129 (4), 1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Baruch Morgenstern N, Ballaban AY, Wen T, Shoda T, Caldwell JM, Kliewer K, Felton JM, Abonia JP, Mukkada VA, Putnam PE, Bolton SM, Dwyer DF, Barrett NA, Rothenberg ME, 2022. Single-cell RNA sequencing of mast cells in eosinophilic esophagitis reveals heterogeneity, local proliferation, and activation that persists in remission. J. Allergy Clin. Immunol. doi: 10.1016/j.jaci.2022.02.025. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennich HH, Ishizaka K, Johansson SG, Rowe DS, Stanworth DR, Terry WD, 1968. Immunoglobulin E: a new class of human immunoglobulin. Immunology 15 (3), 323–324. [PMC free article] [PubMed] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC, 2004. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. USA 101 (49), 17033–17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS, 2016. “Siglec”ting the allergic response for therapeutic targeting. Glycobiology 26 (6), 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL, 2005. Glycan array screening reveals a candidate ligand for Siglec-8. J. Biol. Chem. 280 (6), 4307–4312. [DOI] [PubMed] [Google Scholar]
- Bochner BS, Zimmermann N, 2015. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J. Allergy Clin. Immunol. 135 (3), 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman-Van der Linden EC, Angata T, Reynolds SA, Powell LD, Hedrick SM, Varki A, 2003. CD33/Siglec-3 binding specificity, expression pattern, and consequences of gene deletion in mice. Mol. Cell Biol. 23 (12), 4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusselle GG, Koppelman GH, 2022. Biologic therapies for severe asthma. N. Engl. J. Med. 386 (2), 157–171. [DOI] [PubMed] [Google Scholar]
- Bryce PJ, Falahati R, Kenney LL, Leung J, Bebbington C, Tomasevic N, Krier RA, Hsu CL, Shultz LD, Greiner DL, Brehm MA, 2016. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J. Allergy Clin. Immunol. 138 (3), 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Nason R, Sun L, Van Coillie J, Madriz Sorensen D, Moons SJ, Yang Z, Arbitman S, Fernandes SM, Furukawa S, McBride R, Nycholat CM, Adema GJ, Paulson JC, Schnaar RL, Boltje TJ, Clausen H, Narimatsu Y, 2021. Probing the binding specificities of human Siglecs by cell-based glycan arrays. Proc. Natl. Acad. Sci. U S A 118 (17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri AE, Nag S, Russo AR, Stiles KM, Crystal RG, Pagovich OE, 2021. Gene therapy for a murine model of eosinophilic esophagitis. Allergy 76 (9), 2740–2752. [DOI] [PubMed] [Google Scholar]
- Cao H, Crocker PR, 2011. Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation? Immunology 132 (1), 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, de Bono B, Belov K, Wong ES, Trowsdale J, Barrow AD, 2009. Comparative genomics indicates the mammalian CD33rSiglec locus evolved by an ancient large-scale inverse duplication and suggests all Siglecs share a common ancestral region. Immunogenetics 61 (5), 401–417. [DOI] [PubMed] [Google Scholar]
- Carroll DJ, Cao Y, Bochner BS, O’Sullivan JA, 2021. Siglec-8 signals through a non-canonical pathway to cause human eosinophil death in vitro. Front. Immunol. 12, 737988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll DJ, O’Sullivan JA, Nix DB, Cao Y, Tiemeyer M, Bochner BS, 2018. Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating beta2-integrin-dependent function in human eosinophils. J. Allergy Clin. Immunol. 141 (6), 2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BCL, Lam CWK, Tam LS, Wong CK, 2019. IL33: Roles in allergic inflammation and therapeutic perspectives. Front. Immunol. 10, 364. doi: 10.3389/fimmu.2019.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings RD, Pierce JM, 2014. The challenge and promise of glycomics. Chem. Biol. 21 (1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin JS, Maurer M, Metcalfe DD, Pejler G, Sagi-Eisenberg R, Nilsson G, 2022. The ingenious mast cell: Contemporary insights into mast cell behavior and function. Allergy 77 (1), 83–99. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Peterson KA, Murray JA, Falk GW, Gonsalves N, Chehade M, Genta RM, Leung J, Khoury P, Klion AD, Hazan S, Vaezi M, Bledsoe AC, Durrani SR, Wang C, Shaw C, Chang AT, Singh B, Kamboj AP, Rasmussen HS, Rothenberg ME, Hirano I, 2020. Anti-Siglec-8 antibody for eosinophilic gastritis and duodenitis. N. Engl. J. Med. 383 (17), 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dispenza MC, Krier-Burris RA, Chhiba KD, Undem BJ, Robida PA, Bochner BS, 2020. Bruton’s tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis. J. Clin. Invest. 130 (9), 4759–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Arlian BM, Nycholat CM, Wei Y, Tateno H, Smith SA, Macauley MS, Zhu Z, Bochner BS, Paulson JC, 2021. Nanoparticles displaying allergen and Siglec-8 ligands suppress IgE-FcεRI-mediated anaphylaxis and desensitize mast cells to subsequent antigen challenge. J. Immunol. 206 (10), 2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Koziol-White CJ, Jester WF Jr., Nycholat CM, Macauley MS, Panettieri RA Jr., Paulson JC, 2019. CD33 recruitment inhibits IgE-mediated anaphylaxis and desensitizes mast cells to allergen. J. Clin. Invest. 129 (3), 1387–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Paulson JC, 2020. Siglecs as immune cell checkpoints in disease. Annu. Rev. Immunol. 38, 365–395. [DOI] [PubMed] [Google Scholar]
- Falahati R, Bright J, Dorenbaum A, Bebbington C, Tomasevic N, Lidke D, George TI, Gotlib J, 2015. A recombinant antibody to Siglec-8 shows selective ADCC activity against mast cells from systemic mastocytosis patients. Blood 126, 4092 (abstract). [Google Scholar]
- Falconer DJ, Subedi GP, Marcella AM, Barb AW, 2018. Antibody fucosylation lowers the FcɣRIIIa/CD16a affinity by limiting the conformations sampled by the N162-Glycan. ACS Chem. Biol. 13 (8), 2179–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian S, Sonneck K, Czerny M, Hennersdorf F, Hauswirth AW, Buhring HJ, Valent P, 2006. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy 61 (9), 1054–1062. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Gaudenzio N, Tsai M, 2020. Mast cells in inflammation and disease: Recent progress and ongoing concerns. Annu. Rev. Immunol. 38, 49–77. [DOI] [PubMed] [Google Scholar]
- Gao PS, Shimizu K, Grant AV, Rafaels N, Zhou LF, Hudson SA, Konno S, Zimmermann N, Araujo MI, Ponte EV, Cruz AA, Nishimura M, Su SN, Hizawa N, Beaty TH, Mathias RA, Rothenberg ME, Barnes KC, Bochner BS, 2010. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Eur. J. Hum. Genet. 18 (6), 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremeskel S, Schanin J, Coyle KM, Butuci M, Luu T, Brock EC, Xu A, Wong A, Leung J, Korver W, Morin RD, Schleimer RP, Bochner BS, Youngblood BA, 2021. Mast cell and eosinophil activation are associated with COVID-19 and TLR-mediated viral inflammation: Implications for an anti-Siglec-8 antibody. Front. Immunol. 12 (641), 650331. doi: 10.653389/fimmu.652021.650331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gicheva N, Macauley MS, Arlian BM, Paulson JC, Kawasaki N, 2016. Siglec-F is a novel intestinal M cell marker. Biochem. Biophys. Res. Commun. 479 (1), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gil A, Li TA, Porell RN, Fernandes SM, Tarbox HE, Lee HS, Aoki K, Tiemeyer M, Kim J, Schnaar RL, 2021. Isolation, identification, and characterization of the human airway ligand for the eosinophil and mast cell immunoinhibitory receptor Siglec-8. J. Allergy Clin. Immunol. 147 (4), 1442–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gil A, Porell RN, Fernandes SM, Maenpaa E, August Li T, Li T, Wong PC, Aoki K, Tiemeyer M, Yu ZJ, Orsburn BC, Bumpus NN, Matthews RT, Schnaar RL, 2022. Human brain sialoglycan ligand for CD33, a microglial inhibitory Siglec implicated in Alzheimer’s disease. J. Biol. Chem, 101960. doi: 10.101016/j.jbc.102022.101960. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gil A, Porell RN, Fernandes SM, Wei Y, Yu H, Carroll DJ, McBride R, Paulson JC, Tiemeyer M, Aoki K, Bochner BS, Schnaar RL, 2018. Editor’s Choice: Sialylated keratan sulfate proteoglycans are Siglec-8 ligands in human airways. Glycobiology 28 (10), 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gil A, Schnaar RL, 2021. Siglec ligands. Cells 10 (5), 1260; doi.org/1210.3390/cells10051260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger PA, Saltoun CA, Grammer LC, 2019. Northwestern University Allergy-Immunology Syllabus. Allergy Asthma Proc. 40 (6), 1–361. [DOI] [PubMed] [Google Scholar]
- Guo JP, Brummet ME, Myers AC, Na HJ, Rowland E, Schnaar RL, Zheng T, Zhu Z, Bochner BS, 2011. Characterization of expression of glycan ligands for Siglec-F in normal mouse lungs. Am. J. Respir. Cell Mol. Biol. 44 (2), 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson SA, Bovin N, Schnaar RL, Crocker PR, Bochner BS, 2009. Eosinophil-selective binding and pro-apoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6’-sulfated sialyl Lewis X. J. Pharmacol. Exp. Ther. 330 (2), 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson SA, Herrmann H, Du J, Cox P, Haddad el B, Butler B, Crocker PR, Ackerman SJ, Valent P, Bochner BS, 2011. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil Siglec-8 expression. J. Clin. Immunol. 31 (6), 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SM, Uhm TG, Lee SK, Kong SK, Jung KH, Binas B, Chai YG, Park SW, Chung IY, 2016. Olig2 is expressed late in human eosinophil development and controls Siglec-8 expression. J. Leukoc. Biol. 100 (4), 711–723. [DOI] [PubMed] [Google Scholar]
- Jackson DJ, Johnston SL, 2010. The role of viruses in acute exacerbations of asthma. J. Allergy Clin. Immunol. 125 (6), 1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen EA, Jackson DJ, Heffler E, Mathur SK, Bredenoord AJ, Pavord ID, Akuthota P, Roufosse F, Rothenberg ME, 2021. Eosinophil knockout humans: Uncovering the role of eosinophils through eosinophil-directed biological therapies. Annu. Rev. Immunol. 39, 719–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetani H, Navarro-Bailon A, Maucher M, Frenz S, Verbruggen C, Yeguas A, Vidriales MB, Gonzalez M, Rial Saborido J, Kraus S, Mestermann K, Thomas S, Bonig H, Luu M, Monjezi R, Mougiakakos D, Sauer M, Einsele H, Hudecek M, 2021. Siglec-6 is a novel target for CAR T-cell therapy in acute myeloid leukemia. Blood 138 (19), 1830–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, Vajn K, Stevens WW, Peters AT, Bochner BS, Kern RC, Schleimer RP, Schnaar RL, 2015. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J. Allergy Clin. Immunol. 135 (3), 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Enterina JR, Bui DT, Mozaneh F, Lin PH, Nitin, Kuo CW, Rodrigues E, Bhattacherjee A, Raeisimakiani P, Daskhan GC, St Laurent CD, Khoo KH, Mahal LK, Zandberg WF, Huang X, Klassen JS, Macauley MS, 2021. Carbohydrate sulfation as a mechanism for fine-tuning Siglec ligands. ACS Chem. Biol. 16 (11), 2673–2689. [DOI] [PubMed] [Google Scholar]
- Kano G, Almanan M, Bochner BS, Zimmermann N, 2013. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: role for reactive oxygen species-enhanced MEK/ERK activation. J. Allergy Clin. Immunol. 132 (2), 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr SC, Gonzalez JR, Schanin J, Peters MC, Lambrecht BN, Brock EC, Charbit A, Ansel KM, Youngblood BA, Fahy JV, 2020. An anti-Siglec-8 antibody depletes sputum eosinophils from asthmatic subjects and inhibits lung mast cells. Clin. Exp. Allergy 50 (8), 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiwamoto T, Brummet ME, Wu F, Motari MG, Smith DF, Schnaar RL, Zhu Z, Bochner BS, 2014. Mice deficient in the St3gal3 gene product α2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. J. Allergy Clin. Immunol. 133 (1), 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA, Zhu Z, Tiemeyer M, Bochner BS, 2015. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J. Allergy Clin. Immunol. 135 (5), 1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuplez E, Krier-Burris R, Cao Y, Marsche G, O’Sullivan J, Bochner BS, 2020. Frontline Science: Superior mouse eosinophil depletion in vivo targeting transgenic Siglec-8 instead of endogenous Siglec-F: Mechanisms and pitfalls. J. Leukoc. Biol. 108 (1), 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolkhir P, Elieh-Ali-Komi D, Metz M, Siebenhaar F, Maurer M, 2021. Understanding human mast cells: lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. doi: 10.1038/s41577-021-00622-y. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Komlosi ZI, van de Veen W, Kovacs N, Szucs G, Sokolowska M, O’Mahony L, Akdis M, Akdis CA, 2022. Cellular and molecular mechanisms of allergic asthma. Mol. Aspects Med. 85, 100995. [DOI] [PubMed] [Google Scholar]
- Korver W, Wong A, Gebremeskel S, Negri GL, Schanin J, Chang K, Leung J, Benet Z, Luu T, Brock EC, Luehrsen K, Xu A, Youngblood BA, 2022. The inhibitory receptor Siglec-8 interacts with FcεRI and globally inhibits intracellular signaling in primary mast cells upon activation. Front. Immunol. 13, 833728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D, Yoon JH, Cyr MG, Simon S, Voynova E, Rader C, Wiestner A, Alejo J, Pittaluga S, Gress RE, 2021. Siglec-6 is a target for chimeric antigen receptor T-cell treatment of chronic lymphocytic leukemia. Leukemia 35 (9), 2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroezen BS, Conti G, Girardi B, Cramer J, Jiang X, Rabbani S, Muller J, Kokot M, Luisoni E, Ricklin D, Schwardt O, Ernst B, 2020. A potent mimetic of the Siglec-8 ligand 6’-sulfo-Sialyl Lewis(x). ChemMedChem 15 (18), 1706–1719. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Kiwamoto T, Brummet ME, Wu F, Aoki K, Zhu Z, Bochner BS, Tiemeyer M, 2018. Airway glycomic and allergic inflammatory consequences resulting from keratan sulfate galactose 6-O-sulfotransferase (CHST1) deficiency. Glycobiology 28 (6), 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KK, Chiu PC, Lee CL, Pang RT, Leung CO, Koistinen H, Seppala M, Ho PC, Yeung WS, 2011. Glycodelin-A protein interacts with Siglec-6 protein to suppress trophoblast invasiveness by down-regulating extracellular signal-regulated kinase (ERK)/c-Jun signaling pathway. J. Biol. Chem. 286 (43), 37118–37127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Gonzalez-Gil A, Drake V, Li TA, Schnaar RL, Kim J, 2021. Induction of the endogenous sialoglycan ligand for eosinophil death receptor Siglec-8 in chronic rhinosinusitis with hyperplastic nasal polyposis. Glycobiology 31 (8), 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand F, Cao Y, Wechsler JB, Zhu X, Zimmermann N, Rampertaap S, Monsale J, Romito K, Youngblood BA, Brock EC, Makiya MA, Tomasevic N, Bebbington C, Maric I, Metcalfe DD, Bochner BS, Klion AD, 2019a. Sialic acid-binding immunoglobulin-like lectin (Siglec) 8 in patients with eosinophilic disorders: Receptor expression and targeting using chimeric antibodies. J. Allergy Clin. Immunol. 143 (6), 2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand F, Landolina N, Zaffran I, Emeh RO, Chen E, Klion AD, Levi-Schaffer F, 2019b. Siglec-7 on peripheral blood eosinophils: Surface expression and function. Allergy 74 (7), 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Brusselle GG, Canonica GW, Jackson DJ, Nair P, Buhl R, Virchow JC, 2022. Disease-modifying anti-asthmatic drugs. Lancet 399 (10335), 1664–1668. [DOI] [PubMed] [Google Scholar]
- Mao H, Kano G, Hudson SA, Brummet M, Zimmermann N, Zhu Z, Bochner BS, 2013. Mechanisms of Siglec-F-induced eosinophil apoptosis: a role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PLoS. One. 8 (6), e68143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Weller K, Bindslev-Jensen C, Gimenez-Arnau A, Bousquet PJ, Bousquet J, Canonica GW, Church MK, Godse KV, Grattan CE, Greaves MW, Hide M, Kalogeromitros D, Kaplan AP, Saini SS, Zhu XJ, Zuberbier T, 2011. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy 66 (3), 317–330. [DOI] [PubMed] [Google Scholar]
- McCord KA, Macauley MS, 2022. Transgenic mouse models to study the physiological and pathophysiological roles of human Siglecs. Biochem. Soc. Trans. BST20211203. doi: 10.1042/BST20211203 Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Mizrahi S, Gibbs BF, Karra L, Ben-Zimra M, Levi-Schaffer F, 2014. Siglec-7 is an inhibitory receptor on human mast cells and basophils. J. Allergy Clin. Immunol. 134 (1), 230–233. [DOI] [PubMed] [Google Scholar]
- Munitz A, Bachelet I, Eliashar R, Moretta A, Moretta L, Levi-Schaffer F, 2006. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood 107 (5), 1996–2003. [DOI] [PubMed] [Google Scholar]
- Na HJ, Hudson SA, Bochner BS, 2012. IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine 57 (1), 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutku E, Aizawa H, Hudson SA, Bochner BS, 2003. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 101 (12), 5014–5020. [DOI] [PubMed] [Google Scholar]
- Nutku E, Hudson SA, Bochner BS, 2005. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem. Biophys. Res. Commun. 336 (3), 918–924. [DOI] [PubMed] [Google Scholar]
- Nutku-Bilir E, Hudson SA, Bochner BS, 2008. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am. J. Respir. Cell Mol. Biol. 38 (1), 121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nycholat CM, Duan S, Knuplez E, Worth C, Elich M, Yao A, O’Sullivan J, McBride R, Wei Y, Fernandes SM, Zhu Z, Schnaar RL, Bochner BS, Paulson JC, 2019. A sulfonamide sialoside analogue for targeting Siglec-8 and -F on immune cells. J. Am. Chem. Soc. 141 (36), 14032–14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan JA, Carroll DJ, Cao Y, Salicru AN, Bochner BS, 2018. Leveraging Siglec-8 endocytic mechanisms to kill human eosinophils and malignant mast cells. J. Allergy Clin. Immunol. 141 (5), 1774–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan JA, Chang AT, Youngblood BA, Bochner BS, 2020. Eosinophil and mast cell Siglecs: From biology to drug target. J. Leukoc. Biol. 108 (1), 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel KA, Duan S, Wright BL, Maleki SJ, Wolf JC, Vickery BP, Burks AW, Paulson JC, Kulis MD, Macauley MS, 2017. Exploiting CD22 on antigen-specific B cells to prevent allergy to the major peanut allergen Ara h 2. J. Allergy Clin. Immunol. 139 (1), 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Jung J, Rodrigues E, Kitova EN, Macauley MS, Klassen JS, 2020. Mass spectrometry-based shotgun glycomics for discovery of natural ligands of glycan-binding proteins. Anal. Chem. 92 (20), 14012–14020. [DOI] [PubMed] [Google Scholar]
- Patel N, Brinkman-Van der Linden EC, Altmann SW, Gish K, Balasubramanian S, Timans JC, Peterson D, Bell MP, Bazan JF, Varki A, Kastelein RA, 1999. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J. Biol. Chem. 274 (32), 22729–22738. [DOI] [PubMed] [Google Scholar]
- Patnode ML, Cheng CW, Chou CC, Singer MS, Elin MS, Uchimura K, Crocker PR, Khoo KH, Rosen SD, 2013. Galactose 6-O-sulfotransferases are not required for the generation of Siglec-F ligands in leukocytes or lung tissue. J. Biol. Chem. 288 (37), 26533–26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum T, Wang X, Rettel M, Krijgsveld J, Feyerabend TB, Rodewald HR, 2020. Human mast cell proteome reveals unique lineage, putative functions, and structural basis for cell ablation. Immunity 52 (2), 404–416. [DOI] [PubMed] [Google Scholar]
- Portier P, Richet C, 1902. De l’action anaphylactique de certains venins. C. R. Soc. Biol. (Paris) 54, 170–172. [Google Scholar]
- Propster JM, Yang F, Rabbani S, Ernst B, Allain FH, Schubert M, 2016. Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc. Natl. Acad. Sci. USA 113 (29), E4170–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HS, Chang AT, Tomasevic N, Bebbington C, 2018. A randomized, double-blind, placebo-controlled, ascending dose phase 1 study of AK002, a novel Siglec-8 selective monoclonal antibody, in healthy subjects. J. Allergy Clin. Immunol. 141, AB403 (abstr). [Google Scholar]
- Rillahan CD, Macauley MS, Schwartz E, He Y, McBride R, Arlian BM, Rangarajan J, Fokin VV, Paulson JC, 2014. Disubstituted sialic acid ligands targeting Siglecs CD33 and CD22 associated with myeloid leukaemias and B cell lymphomas. Chem. Sci. 5 (6), 2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robida PA, Rische CH, Morgenstern NB-B, Janarthanam R, Cao Y, Krier-Burris RA, Korver W, Xu A, Luu T, Schanin J, Leung J, Rothenberg ME, Wechsler JB, Youngblood BA, Bochner BS, O’Sullivan JA, 2022. Functional and phenotypic characterization of Siglec-6 on human mast cells. Cells 11 (7), 1138. doi: 10.3390/cells11071138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues E, Jung J, Park H, Loo C, Soukhtehzari S, Kitova EN, Mozaneh F, Daskhan G, Schmidt EN, Aghanya V, Sarkar S, Streith L, St Laurent CD, Nguyen L, Julien JP, West LJ, Williams KC, Klassen JS, Macauley MS, 2020. A versatile soluble Siglec scaffold for sensitive and quantitative detection of glycan ligands. Nat. Commun. 11 (1), 5091. doi: 10.1038/s41467-41020-18907-41466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein E, Cho JY, Rosenthal P, Chao J, Miller M, Pham A, Aceves SS, Varki A, Broide DH, 2011. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 53 (4), 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanin J, Gebremeskel S, Korver W, Falahati R, Butuci M, Haw TJ, Nair PM, Liu G, Hansbro NG, Hansbro PM, Evensen E, Brock EC, Xu A, Wong A, Leung J, Bebbington C, Tomasevic N, Youngblood BA, 2020. A monoclonal antibody to Siglec-8 suppresses non-allergic airway inflammation and inhibits IgE-independent mast cell activation. Mucosal Immunol. 14 (2), 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanin J, Luu T, Wouter Korver W, Brock EC, Benet Z, Xu A, Chang K, Wong A, Butuci M, McEwen LM, Coyle K, Leung J, Youngblood BA, 2022. An agonistic monoclonal antibody against Siglec-6 selectively inhibits and reduces human tissue mast cells. Allergy (abstract in press). [Google Scholar]
- Schleimer RP, 2017. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu. Rev. Pathol. 12, 331–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar RL, 2015. Glycans and glycan-binding proteins in immune regulation: A concise introduction to glycobiology for the allergist. J. Allergy Clin. Immunol. 135 (3), 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar RL, 2016. Glycobiology simplified: diverse roles of glycan recognition in inflammation. J. Leukoc. Biol. 99 (6), 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenhaar F, Bonnekoh H, Hawro T, Hawro M, Michaelis E, Rasmussen H, Singh B, Kantor A, Chang A, Maurer M, 2019. Safety and efficacy data of AK002, an anti-Siglec-8 monoclonal antibody, in patients with indolent systemic mastocytosis (ISM): Results from a first-in-human, open-label phase 1 study. Allergy 74, 910–911 (abstr).30515838 [Google Scholar]
- Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH, 2009a. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J. Immunol. 183 (8), 5333–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, Broide DH, 2009b. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin. Immunol. 131, 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Lasanajak Y, Xia B, Heimburg-Molinaro J, Rhea JM, Ju H, Zhao C, Molinaro RJ, Cummings RD, Smith DF, 2011. Shotgun glycomics: a microarray strategy for functional glycomics. Nat. Methods 8 (1), 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabile H, Fionda C, Gismondi A, Santoni A, 2017. Role of distinct natural killer cell subsets in anticancer response. Front. Immunol. 8, 293. doi: 10.3389/fimmu.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ, 2007. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Methods 327 (1–2), 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa M, Miller M, Rosenthal P, Cho JY, Doherty TA, Varki A, Broide D, 2013. Sialyltransferase ST3Gal-III regulates Siglec-F ligand formation and eosinophilic lung inflammation in mice. J. Immunol. 190 (12), 5939–5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Sasaki S, Fujiwara T, Takahashi E, Muto T, Nakamura Y, 1997. Molecular cloning of a novel gene similar to myeloid antigen CD33 and its specific expression in placenta. Cytogenet. Cell Genet. 78 (3–4), 295–300. [DOI] [PubMed] [Google Scholar]
- Tateno H, Crocker PR, Paulson JC, 2005. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6’-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology 15 (11), 1125–1135. [DOI] [PubMed] [Google Scholar]
- Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, Paulson JC, 2007. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol. Cell. Biol. 27 (16), 5699–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, 2009. Natural ligands for CD33-related Siglecs? Glycobiology 19 (8), 810–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Schnaar RL, Crocker PR, 2017. Chapter 35. I-type Lectins, in: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH (Eds.), Essentials of Glycobiology, 3rd edition, 2nd ed, Cold Spring Harbor; (NY: ), p. DOI: 10.1101/glycobiology.1103e.1035. [DOI] [Google Scholar]
- Von Pirquet C, 1946. Allergie. Ann. Allergy 4 (5), 388–390. [PubMed] [Google Scholar]
- Wei Y, Chhiba KD, Zhang F, Ye X, Wang L, Zhang L, Robida PA, Moreno-Vinasco L, Schnaar RL, Roers A, Hartmann K, Lee CM, Demers D, Zheng T, Bochner BS, Zhu Z, 2018. Mast cell-specific expression of human Siglec-8 in conditional knock-in mice. Int. J. Mol. Sci. 20 (1), 19. doi: 10.3390/ijms20010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann S, Dietschmann A, Doehler D, Castiglione K, Bochner BS, Voehringer D, Radtke D, 2022. Siglec-F promotes IL-33-induced cytokine release from bone marrow-derived eosinophils independently of the itim and itim-like motif phosphorylation. J. Immunol. 208 (3), 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, Ryu SD, von Gunten S, Bickel CA, Hudson SA, Macglashan DW Jr., Bochner BS, 2008. Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J. Allergy Clin. Immunol. 121 (2), 499–505. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Myers A, Matsumoto K, Crocker PR, Saito H, Bochner BS, 2006. Alteration and acquisition of Siglecs during in vitro maturation of CD34+ progenitors into human mast cells. Allergy 61 (6), 769–776. [DOI] [PubMed] [Google Scholar]
- Youngblood BA, Brock EC, Leung J, Falahati R, Bochner BS, Rasmussen HS, Peterson K, Bebbington C, Tomasevic N, 2019a. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight 4 (19), e126219. doi: 10.121172/jci.insight.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood BA, Brock EC, Leung J, Falahati R, Bryce PJ, Bright J, Williams J, Shultz LD, Greiner DL, Brehm MA, Bebbington C, Tomasevic N, 2019b. AK002, a humanized sialic acid-binding immunoglobulin-like lectin-8 antibody that induces antibody-dependent cell-mediated cytotoxicity against human eosinophils and inhibits mast cell-mediated anaphylaxis in mice. Int. Arch. Allergy Immunol. 180, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood BA, Leung J, Falahati R, Williams J, Schanin J, Brock EC, Singh B, Chang AT, O’Sullivan JA, Schleimer RP, Tomasevic N, Bebbington CR, Bochner BS, 2021. Discovery, function, and therapeutic targeting of Siglec-8. Cells 10 (1), 19. doi: 10.3390/cells10010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Blokhuis BRJ, Diks MAP, Keshavarzian A, Garssen J, Redegeld FA, 2018. Functional inhibitory Siglec-6 is upregulated in human colorectal cancer-associated mast cells. Front. Immunol. 9, 2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A, 2007. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood 109 (10), 4280–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS, 2008. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 63 (9), 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]